Introduction
Hepatic epithelioid hemangioendothelioma (HEH) is a
rare vascular tumor of endothelial origin with low- to
intermediate-grade malignancy (1).
HEH has a prevalence of 1 per 100,000 population (2). HEH may present as a solitary liver
nodule or, more frequently, as multifocal liver nodules. Previous
studies have revealed that HEH tends to coalesce into diffuse
lesions in the late stages of the disease (3). Conventional treatments of HEH include
surgical resection or liver transplantation. Previously, certain
novel systemic drugs have been used in the treatment of HEH,
including thalidomide and sorafenib (4,5). The
prognosis for patients with HEH is considered much more favorable
compared with that of other hepatic malignancies, with a 5-year
survival rate of 43–55% (6).
Previously, HEH was frequently misdiagnosed as
metastasis or, more rarely, as primary tumor of the liver, based on
imaging and pathology (7).
Pathological studies have indicated that myxoid, hyaloplasm and
fiber compositions are present in the central part of HEH, which
determine the appearance of the computed tomography (CT) and
magnetic resonance imaging (MRI) scans and the enhancement patterns
of the tumor (6,8). The peripheral region of the lesion,
which is rich in tumor cells, demonstrates rim enhancement on the
arterial phase, while the central part, which is rich in fiber
composition, demonstrated delayed enhancement (9). Similar enhancement patterns have
frequently been identified in cholangiocarcinoma (10). In addition, the fibrous contraction in
the central region of the lesion caused the adjacent capsular
contraction (3). The myxoid and
hyaloplasm composition in the central region of HEH demonstrates no
enhancement and the peripheral region demonstrates rim enhancement,
termed the ‘black target sign’ or ‘bulls eye sign’, which may be
frequently identified in metastatic tumors and hepatic abscesses
(6).
Clinical presentation and history may be useful for
the differential diagnosis of HEH, metastatic tumors and abscesses.
The peripheral regions of certain hemangiosarcomas and atypical
hemangiomas often demonstrate progressive enhancement, while the
central necrosis or cystic regions demonstrate no enhancement
(11). Differentiating HEH from the
aforementioned tumors is challenging. At present, with the
development and popularity of CT and MRI techniques, increasing
numbers of characteristics associated with HEH have been extracted
from CT and MRI images, including the ‘target sign’ and ‘lollipop
sign’ (12). In addition, apparent
diffusion coefficient (ADC) maps may be useful in revealing the
malignant potential of the tumor (13). The final diagnosis of HEH depends on
the pathology.
Thus, it is important to increase awareness of the
imaging characteristics of HEH. In the current study, the CT and
MRI findings of 14 cases of histopathologically confirmed HEH were
retrospectively evaluated.
Materials and methods
Patients
Data from 14 cases of HEH, treated between 2010 and
2014 in the Chinese People's Liberation Army General Hospital
(Beijing, China), were retrospectively collected. Informed consent
from the patients was not required for this retrospective study as
patient privacy was maintained. The diagnoses of 2 cases were
determined by surgery, and 12 cases were confirmed by needle
biopsy, with hematoxylin and eosin and immunohistochemical staining
(BenchMark; Ventana Medical Systems, Inc., Tucson, AZ, USA). The
immunohistochemical assessment was positive for cluster of
differentiation 34 (CD34) and factor VIII-related antigen (12). The male:female ratio was 1:1, and the
mean age of the patients was 43.5 years (range, 24–70 years). The
percentage of asymptomatic cases was 50% (7 cases), whilst 21% (3
cases) presented with right upper quadrant pain, 28.5% (4 cases)
presented with weight loss, and 7.1% (1 case) presented with
jaundice and 7.1% (1 case) with fever. Only 1 case had a history of
lung cancer resection. In 1 case, the level of carcinoembryonic
antigen (CEA) was increased slightly (6.14 µg/l; normal range,
0.1–5 µg/l). The level of carbohydrate antigen 125 (CA125) was also
increased in 1 case (63 U/ml; normal range: <5 units/ml).
Scan protocol
Non-contrast and two-phase dynamic
contrast-enhancement CT scans were performed in 7 cases using a
Siemens Sensation Cardiac 64 CT scanner (Siemens AG, Munich,
Germany). In addition, 9 patients were examined by MRI (Signa
Excite HD 1.5T; GE Healthcare Life Sciences, Shanghai, China).
Patients were imaged in the supine position with a surface
phased-array coil. For the complete evaluation of liver lesions,
breath-hold transverse T2-weighted fast spin-echo sequences were
initially performed, followed by transverse T1-weighted dual-echo
in-phase and out-phase sequences, with a 5-mm slice thickness and
1-mm interspace. Three-dimensional fat-saturated T1-weighted
dynamic contrast-enhanced sequences were performed during suspended
respiration. Gadopentetate-dimeglumine (Gd-DTPA; 0.1 mmol/kg; GE
Healthcare Life Sciences) was injected intravenously at a rate of 2
ml/sec by a power injector. Dynamic contrast-enhanced MRI was
performed in the transverse plane with a 3-mm slice thickness and
no interspace at baseline (pre-contrast), followed by the hepatic
arterial-dominant (20–25 sec), portal venous (30–35 sec) and delay
(300–360 sec) phases after contrast injection. Before dynamic
contrast-enhanced imaging, transverse respiratory-triggered
diffusion-weighted single-shot echo-planar imaging sequence was
performed with tri-directional diffusion gradients by using two
b-values of 0 and 800 sec/mm2.
Image analysis
All images were analyzed separately by two
radiologists. Two reviewers analyzed all images with regard to the
following aspects: Number, location and size of lesions;
morphological features; intensity; and characteristics of dynamic
contrast-enhanced images. By consensus through a joint review of
the recorded images, each case was finally classified as one of the
following types: (i) Solitary nodular type, a solitary lesion that
has a diameter of <30 mm; (ii) multifocal type, multifocal
lesions that may be separated from one another; or (iii) diffuse
type, coalescent multifocal or diffuse lesions that have no clear
margins between each other.
Results
Solitary nodular type
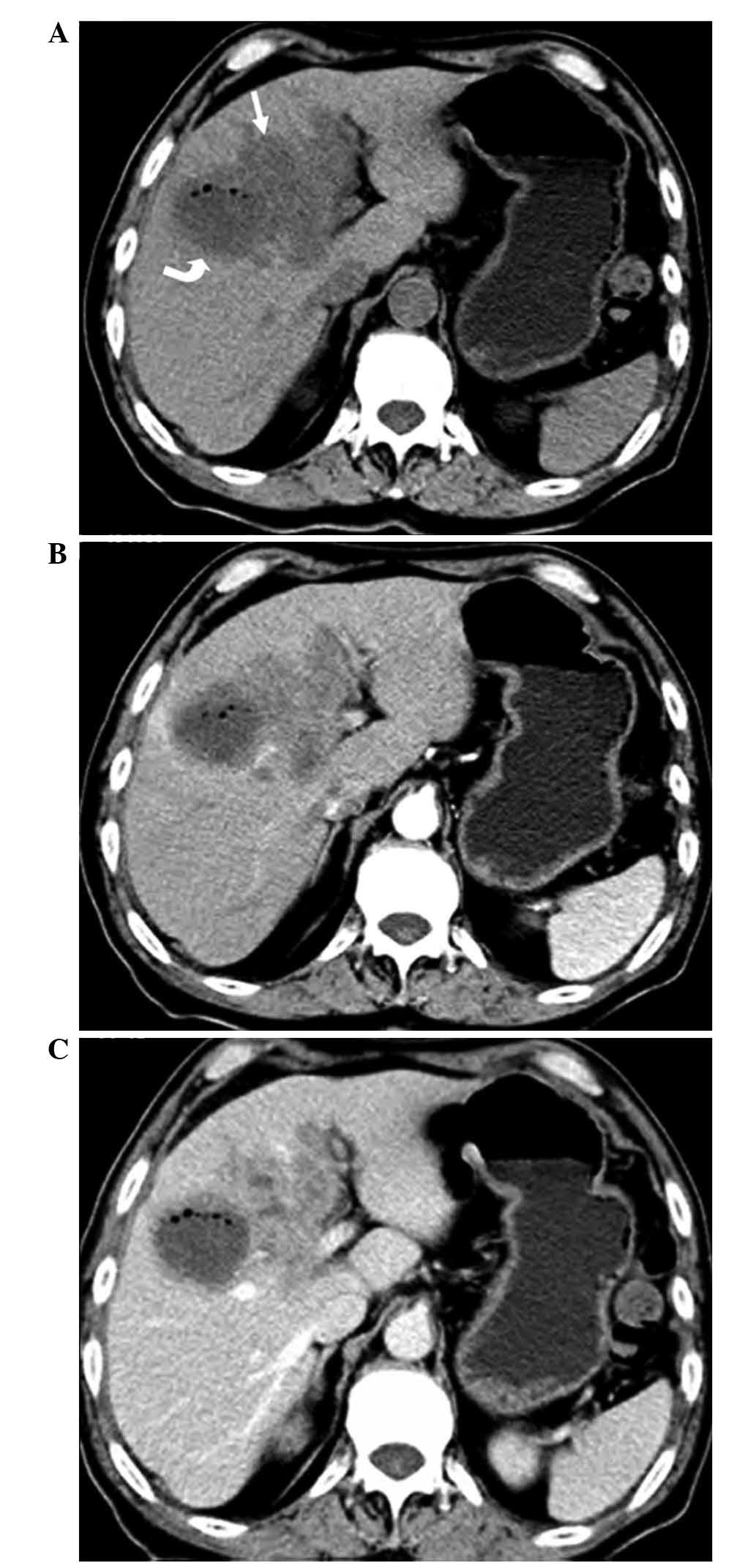
In 1 case, a solitary, low-density nodular lesion
with an irregular shape was observed on non-contrast CT imaging,
with slightly progressive enhancement on contrast-enhanced imaging
(Fig. 1).
Multifocal nodular type
Number, size and distribution of lesions
Multifocal lesions were detected in 11 cases: 46
lesions were detected by CT in 4 cases, and 178 lesions were
detected by MRI in 7 cases. In 4 cases (28.5%), the lesions were
located in the right lobe of the liver, whilst in 1 case (7%)
lesions were in the left lobe, and 6 cases (43%) involved the whole
liver. The lesions were predominantly located in submarginal areas
of the liver. Coalescent lesions were detected in a number of
cases. The hepatic or portal veins were involved in 6 cases (42%).
The diameters of the lesions ranged from 5 to 105 mm.
Patterns of lesion signal intensity
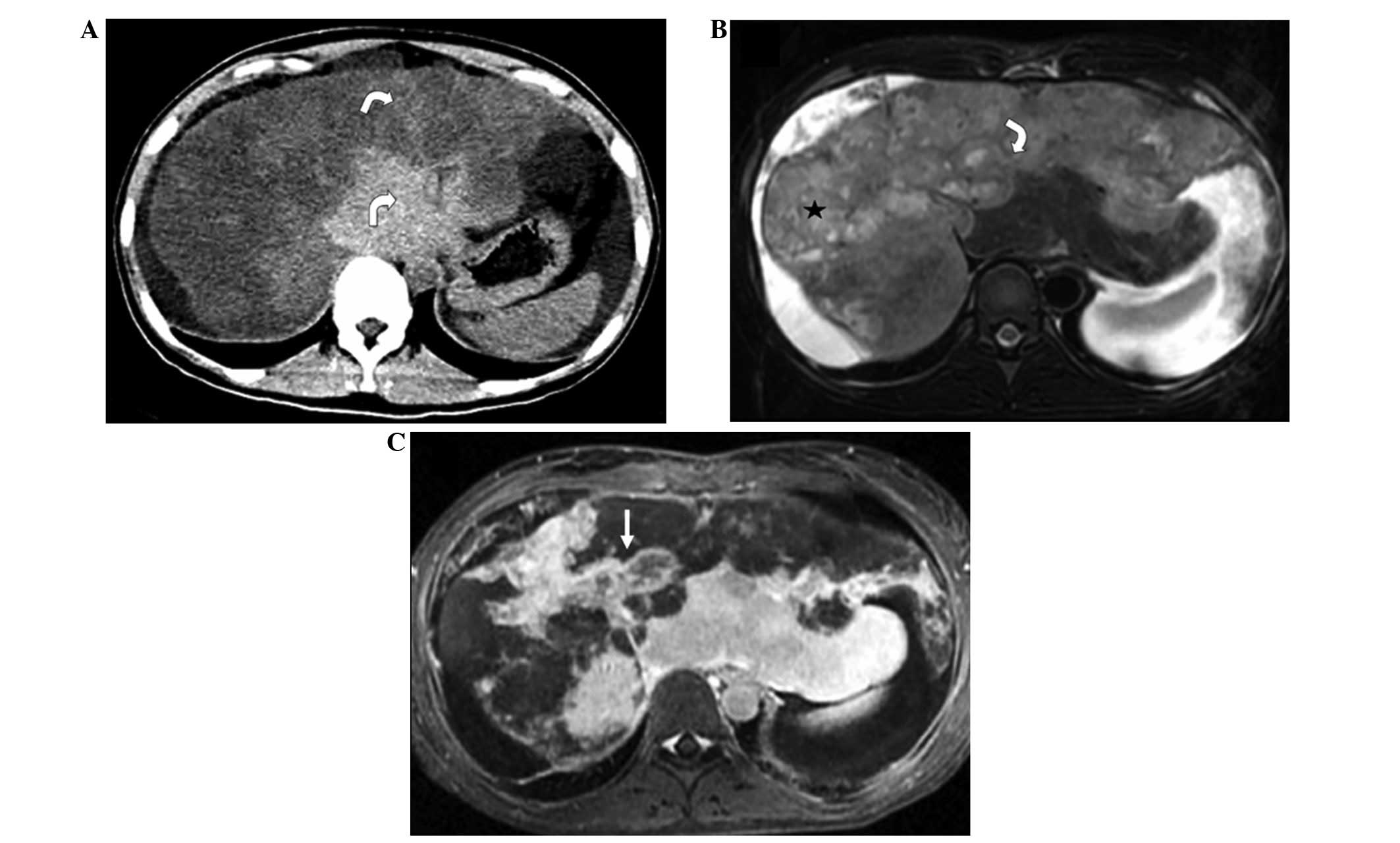
The lesions exhibited low density with clear margins
on CT non-contrast imaging, and slight centripetal enhancement from
the arterial to portal phases on contrast-enhanced CT imaging. On
T1-weighted imaging (T1WI), the lesions exhibited low signal
intensity. On T2-weighted imaging (T2WI), the lesions showed high
signal intensity relative to the liver parenchyma, and the
scattered or coalescent lesions, located in submarginal areas,
showed a ‘white target-like’ sign or ‘strip-like’ sign; the
two-layered ‘target-like’ appearance was formed by the high-signal
intensity core and peripheral slightly hyperintense halo. On
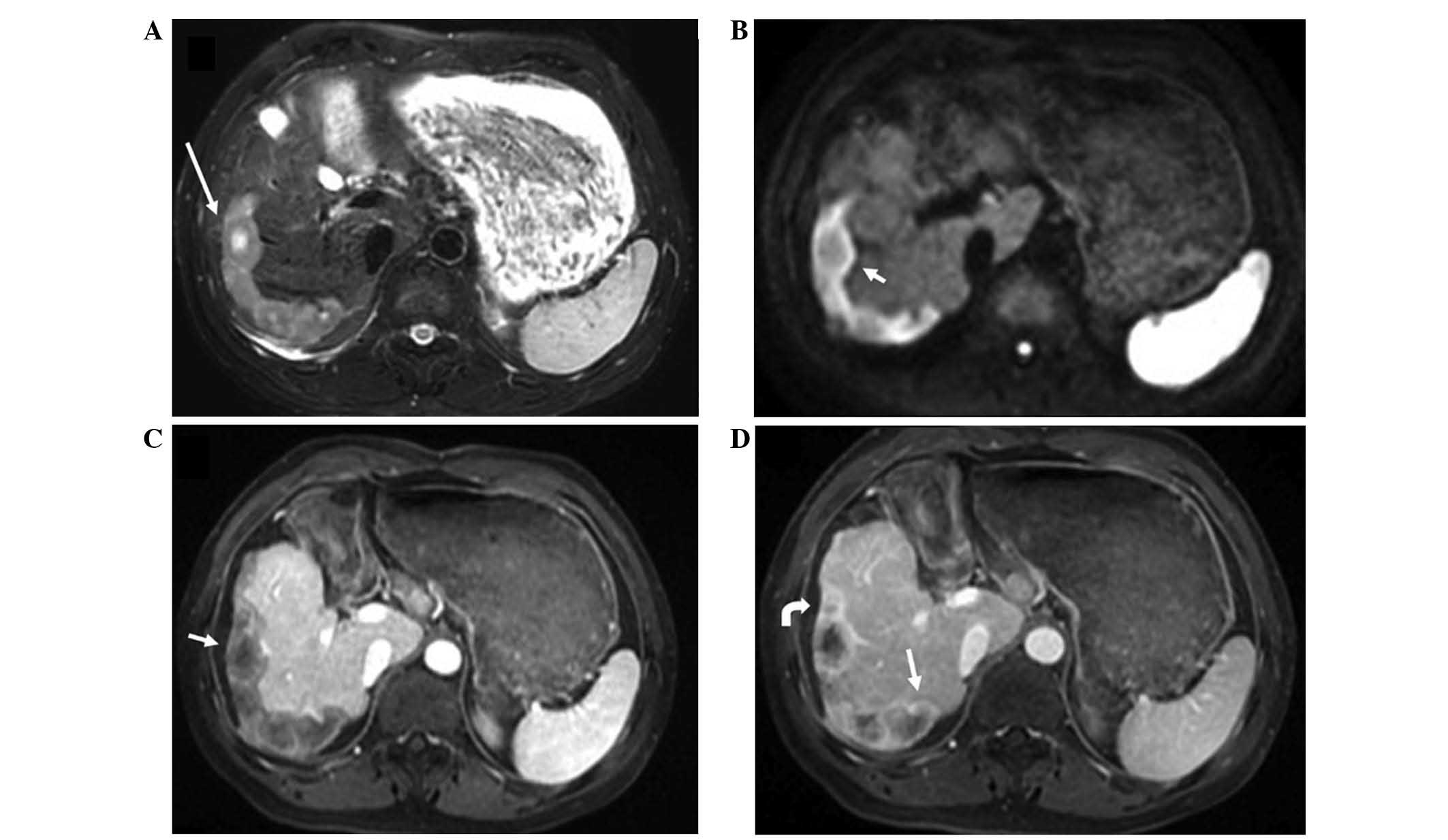
diffusion-weighted imaging (DWI), the lesions showed a high-signal
intensity halo outside of the central slightly high-signal
intensity core. ‘Target-like’ configurations were detected in 141
cases (60.5%) (Figs. 2 and 3). On dynamic contrast-enhanced MRI scans,
the enhancement features varied with different blood supply
patterns. In total, 160 lesions (70%) exhibited gradual peripheral
ring-like enhancement patterns, with central low signal intensity
in the arterial to portal venous and delay phases, and 51 cases
(24%) showed peripheral ring-like enhancement patterns with central
low signal intensity in arterial phase, and the enhanced lesions
were surrounded by a thin hypointense ring in the portal venous or
delay phases (‘black target-like’ sign) (Fig. 3). Liver capsular flattening or
retraction was observed in 60 lesions (27%), and 1 case (4%)
exhibited ‘strip-like’ coalescence of multifocal nodules (Fig. 3). Nodular enhancement along the
central vessels (‘lollipop’ sign) was observed in 10 lesions (4%)
(Fig. 4). Heterogeneous-intensity
masses surrounded by several nodules were present in 5 cases (36%);
the masses exhibited peripheral nodular enhancement in the arterial
phase and gradual homogeneous enhancement in portal venous phase.
The transient abnormal perfusion of the peripheral liver parenchyma
was visualized in the arterial phase and disappeared in portal
venous phase (Fig. 4).
Diffuse type
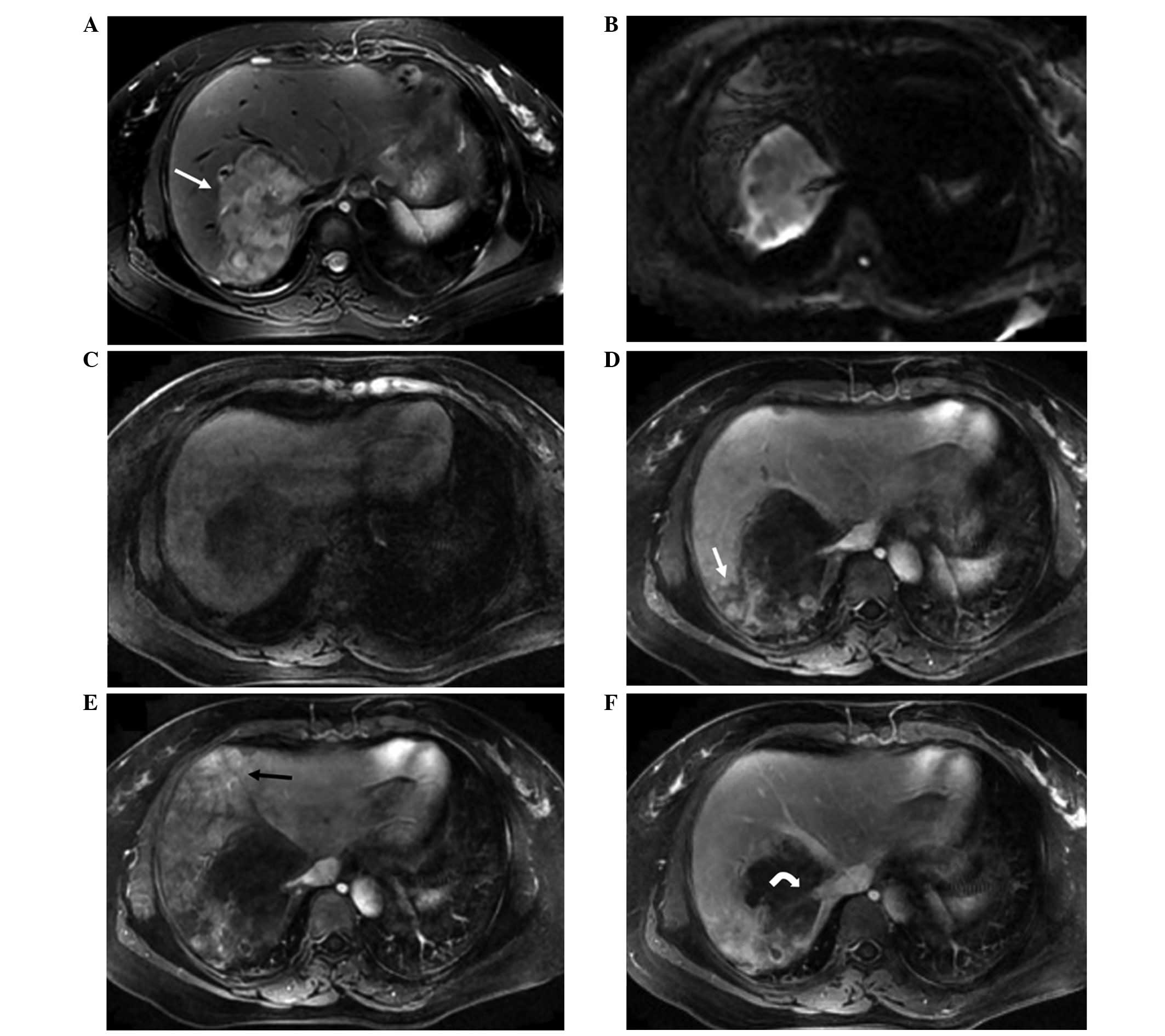
Diffuse lesions throughout the whole liver were
observed in 2 cases. The CT features included diffuse low-density
lesions with minimal residual areas of normal liver parenchyma, in
addition to nodular or irregular enhancement in the arterial phase
and gradual enhancement in portal venous phase. The MRI features
included heterogeneous low signal intensity on T1WI and high signal
intensity on T2WI, with patchy uniform signal intensity within the
lesions. Nodules and masses coexisted in the 2 cases. Nodules
exhibited ‘target-like’ signs on non-contrast imaging and
heterogeneous enhancement on contrast-enhanced imaging, while
masses showed ‘strip-like’ enhancement along central vessels in the
arterial phase and gradual enhancement in the portal venous and
delay phases. Stenosis or occlusion of the portal and hepatic veins
were also detected (Fig. 5).
Other findings
Metastatic tumors in the lung, spleen, thoracic
vertebra, ilium or thoracic wall were detected in 4 cases (28.5%),
a liver abscess was observed in 1 case (7%), and ascites was
present in 1 case (7%). The hepatic or portal veins were involved
in 6 cases (42%).
Methods of definitive diagnosis
All cases were misdiagnosed prior to surgery or
needle biopsy: 2 cases were diagnosed as malignant tumor of the
liver, 4 cases were diagnosed as cholangiocellular carcinoma, 4
cases were diagnosed as metastatic tumors, 1 case was diagnosed as
hepatic fibrosis and 2 hypovascular cases were undetermined.
Discussion
The present study investigated the CT and MRI
characteristics of HEH. The findings revealed a number of typical
CT and MRI signs associated with HEH, most notably the ‘white
target-like’ sign on contrast-enhanced MRI.
HEH is a rare malignant tumor of vascular origin. It
was described as being characterized by the presence of
‘epithelioid’ or ‘histiocytoid’ endothelial cells in 1982 by Weiss
and Enzinger (14), who used the term
‘epithelioid hemangioendothelioma’ to designate these biologically
‘borderline’ neoplasms. HEH appears to have a clinical course
between benign hemangioma and angiosarcoma. The World Health
Organization classifies HEH as a malignant tumor (10). In the majority of patients, both lobes
of the liver are involved, and lung, peritoneum, lymph nodes and
bone are the most common sites of simultaneous extra-hepatic
involvement (1,15). HEH predominantly occurs in adult
females; the mean age of patients is ~41.7 years, and the
female:male ratio is 1.6–2.0:1. The etiology of HEH remains
unknown; however, it may be associated with oral contraceptive use,
exposure to polyethylene, trauma or viral hepatitis (2,6,16).
In the current study, a total of 229 lesions were
detected, most of which were located in the submarginal areas of
the liver, and 56% of which had capsular flattening or retraction;
this was lower than that reported by Miller et al (3) and Paolantonio et al (17), but similar to that reported by Zhao
et al (18), in which capsular
retraction was observed in 59.5% of HEH in Chinese patients. MRI
has been demonstrated to have advantages over CT in the detection
of submarginal and small size lesions. Certain studies indicated
that submarginal nodular lesions may be an earlier form of HEH, as
they later gradually transform into the diffuse type (3,19,20).
In the current study, HEH was classified into three
types according to the number of lesions: Solitary nodule,
multifocal nodule and diffuse types. The percentage of solitary
nodular-type cases (7%) was markedly lower than that in previous
reports (2,4). This may be due to the fact that the
imaging features of the solitary nodular-type disease are
relatively non-specific (21).
On the contrary, multifocal nodular and diffuse
types had a more typical appearance on CT and MRI. On non-contrast
CT scans, tumors appeared as low density lesions with clear
margins. On T1WI, the lesions exhibited low signal intensity
whilst, on non-contrast T2-weighted fat-suppressed imaging, the
lesions exhibited heterogeneously high signal intensity relative to
the adjacent normal liver parenchyma. On DWI, the lesions appeared
with slightly high-signal intensity cores and a high-signal
intensity halo; these two-layered ‘target-like’ configurations were
detected in 141 lesions (60.5%).
On contrast-enhanced MRI, the enhancement features
varied with different blood supply patterns. Multifocal lesions
were classified into four categories according to different
enhancement patterns: (i) Slightly irregular homogeneous
enhancement; (ii) peripheral enhancement with central low signal
intensity in the arterial phase, and enhanced lesions surrounded by
a thin hypointense ring in the portal venous and delay phases
(‘black target-like’ sign); (iii) nodular enhancement in the
central part of the lesion in the arterial phase surrounded by
ring-like enhancement in the portal venous and delay phases (‘white
target-like’ sign); and (iv) peripheral nodular enhancement in the
arterial phase and centripetal enhancement in the portal venous and
delay phases, an enhancement pattern that is more commonly
indicated in hemangioma.
In the current study, 79% of lesions exhibited a
‘target-like’ sign on T2WI, and 70% of lesions exhibited the ‘black
target-like’ sign on contrast-enhancement scans. This was
consistent with previous reports (9,22–25). In the study by Fan et al
(23), the HEH was revealed to
possess slightly increased signal intensity with increased signal
intensity centers on the T2-weighted image, and ‘ring-like’
peripheral enhancement on the post-contrast enhanced MRI. In the
study by Chen et al (9), HEH
presented as two types (the multifocal and diffuse types), and
96.4% of the two types presented as the ‘target sign’ with a
progressive enhancement rim on the contrast-enhanced multiple-phase
MRI. The study by Bruegel et al (24) indicated that HEH showed a
‘target-like’ sign on T2, DWI and ADC maps, and the lesions also
showed a variable degree of peripheral rim enhancement Notably, the
‘white target-like’ sign on contrast-enhanced imaging had not
previously been described; we hypothesize that this unique sign may
have value with regard to the diagnosis of HEH. As the majority of
cases were confirmed by needle biopsy, the relationship between the
‘white target-like’ sign and histopathological features remains
unknown. As the core of the lesion had high signal intensity on
T2WI and low signal intensity on contrast-enhanced T1WI, the ‘white
target-like’ sign may correlate with central necrosis in the
lesion. A thin, hypointense ring outside of a peripherally enhanced
halo (‘black target-like’ sign) in the portal venous or delay
phases may correlate to a layer of fibrous tissue between the
lesion and the normal liver parenchyma (3). Certain previous studies have revealed
that, during the hepatobiliary phase, the lesions may exhibit a
contrast-enhanced core surrounded by a low-signal intensity halo;
the central enhancement was indicated to be due to ‘entrapment’ of
contrast agent in the central fibrous stroma, and the peripheral
hypointense halo due to the lack of hepatobiliary enhancement in
the peripheral tumor zone (17,21).
In the present study, a ‘lollipop’ sign was also
observed, as reported by Alomari (12). The detection of the involvement of the
portal and hepatic veins on the CT and MRI scans were
satisfactorily consistent with the pathology, as the scans were
able to detect all involved portal and hepatic veins that were
proven by pathological methods. In a study by Makhlouf et al
(6), calcification was detected in
20% of lesions; however, none was observed in the current study,
probably due to the insensitivity to calcification of MRI (3,26,27). With regard to diffuse-type HEH,
similar CT features to the present study were reported by Baron
et al (28).
In the current study, one case was misdiagnosed as
hepatic fibrosis with a sub-marginal ‘strip-like’ sign; this sign
may indicate the coalescence of multifocal nodules. None of the 14
cases in the study were diagnosed correctly based on CT and MRI
findings, as the imaging characteristics of HEH were not widely
recognized at the time of diagnosis, particularly for the solitary
nodular-type cases. It is difficult to distinguish solitary HEH
from metastatic tumors or cholangiocellular carcinoma (6).
The clinical symptoms of HEH vary greatly, and the
disease may be asymptomatic or present with non-specific symptoms.
Typically, HEH is present for a long time before a definitive
diagnosis is made (2). For example,
in the present study, hepatic lesions were found in 1 patient ~4
years prior to a definitive diagnosis. In the present study, 7
cases (50%) had non-specific complaints, 3 cases (21%) had right
upper quadrant pain, 3 cases (28.5%) had weight loss, 1 case (7%)
had jaundice attributable to the tumors, 1 case (7%) had fever, and
1 case (7%) had nausea. This was consistent with previous reports
(1,6).
Makhlouf et al (6) collected
137 cases of HEH with nonspecific symptoms, including right upper
quadrant pain or weight loss. In the study by Ishak et al
(1), 12.5% of cases were
asymptomatic. With regard to biochemical examinations, the
concentrations of serum bilirubin, alkaline phosphatase and
aspartate aminotransferase are typically significantly increased
(2). In addition, the majority of
cases are negative results for presence of tumor markers, and only
a few cases exhibit slightly increased levels of CEA (26). In the present study, CEA levels were
marginally increased in 1 case (6.14 µg/l), CA125 levels were
increased in 1 case (63 U/ml), and tumor markers were negative in
the other cases. Thus, the tumor markers most commonly used in
clinical practice have no significant value for the diagnosis of
HEH.
Histologically, HEH is composed of dendritic and
epithelioid cells that often contain vacuoles representing
intracellular lumina. Furthermore, at least one of the endothelial
markers (factor VIII-related antigen, CD34 and/or CD31) is
positively expressed (6,29). Microscopically, characteristic
spindle- and oval-shaped cells with abundant eosinophilic cytoplasm
are observed (30). Commonly, biopsy
is able to confirm a diagnosis of HEH; however, an insufficient
size of biopsy specimen may results in failure to diagnose to HEH,
and laparoscopic liver biopsy is preferred in order to obtain
adequate specimens for analysis (31). Fortunately, in the current study,
definitive diagnosis could be made with needle biopsy in the
majority of cases.
Management strategies for HEH include liver
resection, liver transplantation, transcatheter arterial
chemoembolization and palliative treatment (2,32). Recent
studies have suggested that immediate treatment may not be the
optimal strategy (33). HEH often
follows an indolent course. In a previous study, there was no
difference of 5-year survival in patients with unilateral or
bilateral lesions, localized or metastatic disease, even with an
initial treatment regimen of surgery (33) Therefore, the biological behavior of
the tumor may be associated, in part, with its matrix, which may
exhibit inflammation, dense sclerosis and calcification. Assessment
of disease behavior may better stratify treatment options (33). For unresectable HEH, liver
transplantation is the best option; studies have demonstrated that
the long-term survival time is very good, and much better than in
HCC patients (29). One study
indicated that 62.5% of patients with HEH survived for over 5
years, and one patient succumbed 28 years subsequent to the initial
diagnosis (1).
The limitations of the present study include its
retrospective nature, the fact that it was conducted in a single
center, and the small sample size. Further studies are required to
identify more novel signs associated with HEH.
In conclusion, HEH is a rare tumor that may present
certain typical imaging features, including the ‘white
target-like’, ‘black target-like’, ‘lollipop’ and ‘strip-like’
signs, capsular contraction, submarginal distribution. These
features may contribute significantly to the definitive diagnosis
of HEH. Use of a variety of MRI sequences may provide more
information for the differential diagnosis of HEH.
References
|
1
|
Ishak KG, Sesterhenn IA, Goodman ZD, Rabin
L and Stromeyer FW: Epithelioid hemangioendothelioma of the liver:
A clinicopathologic and follow-up study of 32 cases. Hum Pathol.
15:839–852. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehrabi A, Kashfi A, Fonouni H, Schemmer
P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H,
Buchler MW and Schmidt J: Primary malignant hepatic epithelioid
hemangioendothelioma: A comprehensive review of the literature with
emphasis on the surgical therapy. Cancer. 107:2108–2121. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller WJ, Dodd GD III, Federle MP and
Baron RL: Epithelioid hemangioendothelioma of the liver: Imaging
findings with pathologic correlation. AJR Am J Roentgenol.
159:53–57. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salech F, Valderrama S, Nervi B, Rodriguez
JC, Oksenberg D, Koch A, Smok G, Duarte I, PerezAyuso RM, Jarufe N,
Martinez J, Soza A, Arrese M and Riquelme A: Thalidomide for the
treatment of metastatic hepatic epithelioid hemangioendothelioma: a
case report with a long term follow-up. Ann Hepatol.
2011.10:99–102. PubMed/NCBI
|
|
5
|
Sangro B, Inarrairaegui M and
Fernandez-Ros N: Malignant epithelioid hemangioendothelioma of the
liver successfully treated with Sorafenib. Rare Tumors. 2012.4:e34
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makhlouf HR, Ishak KG and Goodman ZD:
Epithelioid hemangioendothelioma of the liver: A clinicopathologic
study of 137 cases. Cancer. 85:562–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neofytou K, Chrysochos A, Charalambous N,
Dietis M, Petridis C, Andreou C and Petrou A: Hepatic epithelioid
hemangioendothelioma and the danger of misdiagnosis: Report of a
case. Case Rep Oncol Med. 2013:2439392013.PubMed/NCBI
|
|
8
|
Azzam RI, Alshak NS and Pham HP: AIRP best
cases in radiologic-pathologic correlation: Hepatic epithelioid
hemangioendothelioma. Radiographics. 32:789–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Yu RS, Qiu LL, Jiang DY, Tan YB
and Fu YB: Contrast-enhanced multiple-phase imaging features in
hepatic epithelioid hemangioendothelioma. World J Gastroenterol.
17:3544–3553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamilton SR and Aaltonen LA: Tumours of
the Liver and Intrahepatic Bile Ducts In: World Health Organization
Classification of Tumours. Pathology and Genetics of Tumours of the
Digestive System (Lyon). IARC Press. 1582000.
|
|
11
|
Klotz T, Montoriol PF, Da Ines D,
Petitcolin V, Joubert-Zakeyh J and Garcier JM: Hepatic haemangioma:
Common and uncommon imaging features. Diagn Interv Imaging.
94:849–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alomari AI: The lollipop sign: A new
cross-sectional sign of hepatic epithelioid hemangioendothelioma.
Eur J Radiol. 59:460–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okano H, Nakajima H, Tochio T, Suga D,
Kumazawa H, Isono Y, Tanaka H, Matsusaki S, Sase T, Saito T, et al:
A case of a resectable single hepatic epithelioid
hemangioendothelioma with characteristic imaging by ADC map. Clin J
Gastroenterol. 8:406–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss SW and Enzinger FM: Epithelioid
hemangioendothelioma: A vascular tumor often mistaken for a
carcinoma. Cancer. 50:970–981. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verbeken E, Beyls J, Moerman P, Knockaert
D, Goddeeris P and Lauweryns JM: Lung metastasis of malignant
epithelioid hemangioendothelioma mimicking a primary intravascular
bronchioalveolar tumor. A histologic, ultrastructural and
immunohistochemical study. Cancer. 55:1741–1746. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Idilman R, Dokmeci A, Beyler AR, Bastemir
M, Ormeci N, Aras N, Ekinci C, Uzunalimoglu O, De Maria N and Van
Thiel DH: Successful medical treatment of an epithelioid
hemangioendothelioma of liver. Oncology. 54:171–175. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paolantonio P, Laghi A, Vanzulli A,
Grazioli L, Morana G, Ragozzino A and Colagrande S: MRI of hepatic
epithelioid hemangioendothelioma (HEH). J Magn Reson Imaging.
40:552–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao XY, Rakhda MI, Habib S, Bihi A,
Muhammad A, Wang TL and Jia JD: Hepatic epithelioid
hemangioendothelioma: A comparison of Western and Chinese methods
with respect to diagnosis, treatment and outcome. Oncol Let.
7:977–983. 2014.
|
|
19
|
Furui S, Itai Y, Ohtomo K, Yamauchi T,
Takenaka E, Iio M, Ibukuro K, Shichijo Y and Inoue Y: Hepatic
epithelioid hemangioendothelioma: Report of five cases. Radiology.
171:63–68. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Beers B, Roche A, Mathieu D, Menu Y,
Delos M, Otte JB, Lalonde L and Pringot J: Epithelioid
hemangioendothelioma of the liver: MR and CT findings. J Comput
Assist Tomogr. 16:420–424. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EH, Rha SE, Lee YJ, Yoo IR, Jung ES
and Byun JY: CT and MR imaging findings of hepatic epithelioid
hemangioendotheliomas: Emphasis on single nodular type. Abdom
Imaging. 40:500–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J and Ji Y: CT and MRI diagnosis of
hepatic epithelioid hemangioendothelioma. Hepatobiliary Pancreat
Dis Int. 9:154–158. 2010.PubMed/NCBI
|
|
23
|
Fan F, Yang X, Zhu B and Zhang Y: Clinical
and radiological characteristics of Chinese patients with hepatic
epithelioid hemangioendothelioma. Ann Saudi Med. 33:334–338.
2013.PubMed/NCBI
|
|
24
|
Bruegel M, Muenzel D, Waldt S, Specht K
and Rummeny EJ: Hepatic epithelioid hemangioendothelioma: Findings
at CT and MRI including preliminary observations at
diffusion-weighted echo-planar imaging. Abdom Imaging. 36:415–424.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lyburn ID, Torreggiani WC, Harris AC,
Zwirewich CV, Buckley AR, Davis JE, Chung SW, Scudamore CH and Ho
SG: Hepatic epithelioid hemangioendothelioma: Sonographic, CT and
MR imaging appearances. AJR Am J Roentgenol. 180:1359–1364. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Earnest FT IV and Johnson CD: Case: 96
Hepatic epithelioid hemangioendothelioma. Radiology. 240:295–298.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartolozzi C, Cioni D, Donati F and
Lencioni R: Focal liver lesions: MR imaging-pathologic correlation.
Eur Radiol. 11:1374–1388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baron PW, Amankonah T, Cubas RF, Kore AH,
Elihu A, de Vera ME and Perez MC: Diffuse hepatic epithelioid
hemangioendothelioma developed in a patient with hepatitis C
cirrhosis. Case Rep Transplant. 2014:6949032014.PubMed/NCBI
|
|
29
|
Remiszewski P, Szczerba E, Kalinowski P,
Gierej B, Dudek K, Grodzicki M, Kotulski M, Paluszkiewicz R,
Patkowski W, Zieniewicz K and Krawczyk M: Epithelioid
hemangioendothelioma of the liver as a rare indication for liver
transplantation. World J Gastroenterol. 20:11333–11339. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubota S, Baba H, Kumamoto K, Hatano S,
Amano K, Ohsawa T, Okada T, Kumagai Y, Ishibashi K, Haga N, et al:
A case of multiple hepatic epithelioid hemangioendothelioma
mimicking metastatic hepatic tumor. Gan To Kagaku Ryoho.
39:2012–2014. 2012.(In Japanese). PubMed/NCBI
|
|
31
|
Deng Y, Zhou Y and Cheng N: Laparoscopic
liver biopsy in the diagnosis of hepatic epithelioid
hemangioendothelioma: A case report. Oncol Let. 8:1317–1319.
2014.
|
|
32
|
Mistry AM, Gorden DL, Busler JF, Coogan AC
and Kelly BS: Diagnostic and therapeutic challenges in hepatic
epithelioid hemangioendothelioma. J Gastrointest Cancer.
43:521–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thomas RM, Aloia TA, Truty MJ, Tseng WH,
Choi EA, Curley SA, Vauthey JN and Abdalla EK: Treatment sequencing
strategy for hepatic epithelioid haemangioendothelioma. HPB
(Oxford). 16:677–685. 2014. View Article : Google Scholar : PubMed/NCBI
|