Introduction
Lung cancer is extremely harmful to human health and
has the highest worldwide incidence of all malignant tumors
(1). Overall, ~80% of lung cancers
are classified as non-small cell lung cancer (NSCLC) (2), which is the leading cause of
cancer-associated mortality in the industrialized world (3). Despite the significant progress made in
the treatment of early-stage NSCLC, the survival rate of the
advanced stage NSCLC remains extremely low (4). Although several therapeutic targets have
been developed previously, there continues to be no effective
therapeutic target for the augmentation of the cure rate (5). Therefore, the development of novel and
effective therapeutic targets to successfully treat NSCLC is
urgently required (6,7). Additionally, novel methods to
simultaneously inhibit multiple therapeutic targets, including
well-known targets and innovative therapeutic targets, have been
developed (8,9).
Hypoxia-inducible factor-1α (HIF-1α) is widespread
in mammalian and human cells (10)
under hypoxic conditions (11).
HIF-1α is a transcription factor indicated in extracts of a
hypoxia-induced cell nucleus (12,13).
HIF-1α may promote the transcription of hypoxia adaptation genes,
including vascular endothelial growth factor (VEGF) genes, by
binding with the promoter or enhancer region of these genes
(10,14–16).
Overexpression of the HIF-1α protein has been detected in a variety
of malignant tumors and precancerous lesions (12,17), but
has never been indicated in normal tissues and benign lesions
(18). Certain studies have
demonstrated that the overexpression of HIF-1α is closely
associated with tumor growth (19),
angiogenesis (20), invasion and
metastasis (21–23), but the molecular mechanisms and
signaling pathways involved remain unclear. Numerous studies have
confirmed that HIF-1α is important in hypoxia-mediated apoptosis
and proliferation in testicular tumor cells (14,24).
Hypoxic environments widely exist in NSCLC (25,26), which
indicates the association between HIF-1α and NSCLC (13,27).
However, whether HIF-1α is associated with the proliferation and
apoptosis of NSCLC cells remains unclear.
In the present study, the effects of HIF-1α
knockdown on the proliferation and invasion of NSCLC cells were
investigated, and the specific mechanisms and signaling pathways
were explored. The present study may provide support for the drug
development and clinical treatment of NSCLC.
Materials and methods
Reagents
The rabbit polyclonal anti-human caspase-3 (catalog
no., Ab4051; dilution, 1:400), rabbit polyclonal anti-human
caspase-9 (catalog no., Ab2014; dilution, 1:1,000), rabbit
polyclonal anti-human VEGF (catalog no., Ab46154; dilution,
1:1,000), mouse monoclonal anti-human pigment epithelium-derived
factor (PEDF; catalog no., Ab115489; dilution, 1:1,000) and mouse
monoclonal anti-human phosphoinositide 3-kinase (PI3K; catalog no.,
Ab86714; dilution, 1:1,000) antibodies were obtained from Abcam
(Cambridge, MA, USA). The rabbit polyclonal anti-human protein
kinase B (AKT; catalog no., 9272S; dilution, 1:1,000), rabbit
polyclonal anti-human extracellular signal-regulated kinase (ERK;
catalog no., 9102; dilution, 1:1,000), rabbit monoclonal anti-human
phosphorylated (p-) ERK (catalog no., 4370S; dilution, 1:1,000),
rabbit polyclonal anti-human p-PI3K (catalog no., 4228; dilution,
1:1,000) and rabbit monoclonal anti-human p-AKT (catalog no.,
4058S; dilution, 1:1,000) and rabbit monoclonal anti-human
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; catalog no., 5471;
dilution, 1:1,500) antibodies were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The rabbit polyclonal
anti-human B-cell lymphoma-2 (Bcl-2; catalog no., Sc-492; dilution,
1:100) antibody was obtained from Santa Cruz Biotechnology Inc.
(Dallas, TX, USA). Cell counting kit-8 (CCK-8) was obtained from
Boster Biological Engineering Co., Ltd. (Wuhan, Hubei, China),
Gibco Roswell Park Memorial Institute (RPMI)-1640 medium was
obtained from Thermo Fisher Scientific (Waltham, MA, USA), HyClone
fetal bovine serum (FBS) was obtained from GE Healthcare Life
Sciences (Logan, UT, USA) and glutamine was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The TIANScript reverse
transcription (RT) and RNAsimple Total RNA kits were obtained from
Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China), the Super
Moloney murine leukemia virus (catalog no., PR6502) reverse
transcriptase was obtained from BioTeke Corporation (Beijing,
China), diamminedichloroplatinum (DDP) was obtained from JRDun
Biotechnology Corp. (Shanghai, China), Invitrogen Lipofectamine
2000 (catalog no., 11668027) was purchased from Thermo Fisher
Scientific and dimethyl sulfoxide was obtained from Sigma-Aldrich.
The primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China).
Cell culture
The lung carcinoma NCI-H157 cell line was purchased
from the Cell Culture Center of the Shanghai Aiyan Biological
Technology Co., Ltd. (Shanghai, China). The cells were grown in
RPMI-1640 medium containing 10% FBS and 2 mM glutamine in a
humidified incubator (Mco-15AC; Sanyo Electric Co. Ltd., Moriguchi,
Osaka, Japan) at 37°C in 5% CO2. The cells were split
twice weekly and cells that were in the logarithmic growth phase
were used for experiments.
Small interfering (si)RNA design and
transfection
An irrelevant negative control (MOCK), non-specific
siRNA and siRNA that target human HIF-1α, were designed and
synthesized by Sangon Biotech (Shanghai, China). The cells were
transfected with siRNA using Lipofectamine 2000, and the RNA was
extracted 48 h subsequent to transfection.
RNA detection
RNA molecules were extracted using the RNAsimple
Total RNA kit. Complementary DNA was synthesized using the
TIANScript RT kit. Quantitative polymerase chain reaction (PCR) was
performed to detect the RNA levels of HIF-1α. GAPDH was used an
internal control. The primers used were as follows: HIF-1α forward,
5′-TCGGCGAAGTAAAGAATC-3′ and reverse, 5′-TTCCTCACACGCAAATAG-3′; and
GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′. The results were analyzed using
Exicycler™ 96 (Bioneer Corporation, Daejeon, South Korea). Each
experiment was repeated 3 times.
CCK-8 assay
NCI-H157 cell viability was measured using a CCK-8
assay with
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
salt. The NCI-H157 cells were seeded at a density of
5×103 cells per well into a 96-well microplate and
incubated for 24 h at 37°C. The peripheral wells of the microplate
were filled with 200 µl sterile phosphate-buffered saline (PBS).
Subsequent to the addition of MOCK, 4 pmol HIF-1α siRNA, 5 µg/ml
DDP, 4 pmol HIF-1α siRNA + 5 µg/ml DDP or MOCK+ 5 µg/ml DDP, the
cells were incubated for 0, 24 and 48 h. Subsequently, 10 µl CCK-8
solution was added to each well and the wells were incubated for 1
h at 37°C. Optical density (OD) was measured at a wavelength of 450
nm using a Multiskan Spectrum microplate reader (Thermo Fisher
Scientific).
Flow cytometry assay
The NCI-H157 cells were inoculated into 6-well
plates (5×105 cells/well). Subsequent to 24 h
incubation, siRNA or 5 µg/ml DDP were added to the cells and the
plates were incubated for another 24 h. The flow cytometry assay
was conducted according to the manufacturer's protocol for Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Abcam,
Cambridge, UK). Briefly, NCI-H157 cells were routinely digested,
washed with 500 µl PBS and centrifuged at 200 × g for 5 min. The
supernatant was aspirated and 100 µl of Annexin V-FITC, at 1 g/ml
in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) buffer with 1.8 mM CaC12 (Beijing Solarbio
Science & Technology Co., Ltd.), was added to the NCI-H157
cells and incubated for 5–10 min at room temperature in the dark.
Finally, 1 ml of HEPES that contained 10 g/ml propidium iodide was
added. Samples were analyzed immediately using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Detection of the cell invasive
capacity using a Transwell assay
The Transwell chambers (Corning Inc., Corning, NY,
USA) were first coated with Matrigel (dilution, 1:3; BD
Biosciences) in Gibco serum-free RPMI-1640 for 2 h at 37°C. The
NCI-H157 cells were then digested with 0.25% trypsin (Beijing
Solarbio Science & Technology Co., Ltd.) to produce a single
cell suspension. The suspension was diluted to a density of
1×105 cells/ml for use. A total of 800 µl culture medium
containing 20% FBS was added into the coated lower Transwell
chambers, and 200 µl of cell suspension was added into the upper
Transwell chambers at a density of 5×104 cells/well. The
plate was cultured for 24 h. The Transwell chambers were removed
and gently washed with PBS. The cells on the upper layer of the
microporous membrane were removed using a cotton swab, fixed with
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) for 20 min at room temperature, and stained with hematoxylin
staining solution (Beijing Solarbio Science & Technology Co.,
Ltd.) for 3 min. The cells that migrated to the lower layer of the
microporous membrane were counted in 5 fields under an inverted
microscope (Olympus IX71/IX81; Olympus Corporation, Tokyo, Japan;
magnification, ×200) for each sample. The experiment was repeated
three times.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Data were evaluated using the SPSS 16.0 statistical
software package (SPSS, Inc., Chicago, IL, USA). Data were analyzed
using one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
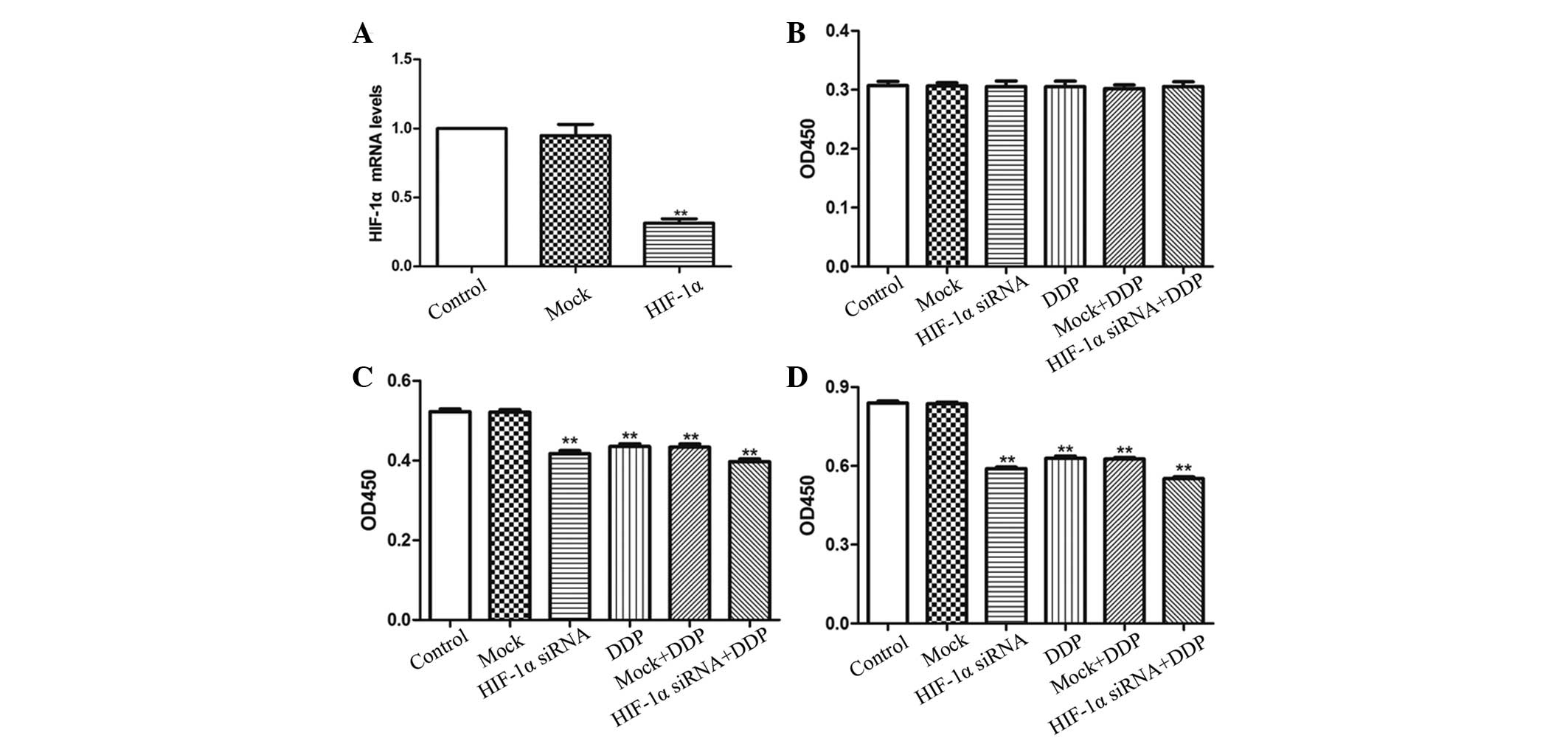
Knockdown of HIF-1α by RNA
interference decreased the proliferation of NCI-H157 cells
siRNA that targets human HIF-1α was designed and
transfected into NCI-H157 cells. HIF-1α expression was inhibited by
~70% by HIF-1α siRNA (P<0.01; Fig.
1A), whereas HIF-1α expression remained unaffected in the MOCK
group. The type of HIF-1α siRNA that inhibited HIF-1α expression
was selected for additional study.
The well-known anti-tumor drug DDP was used in the
present study. A CCK-8 assay was performed in order to determine
the effects of HIF-1α siRNA administered with or without DDP on the
cell proliferation capability of NCI-H157 cells. All groups
possessed the same number of cells at 0 h (Fig. 1B). The growth capacity of NCI-H157
cells transfected with HIF-1α siRNA or treated with DDP was
significantly decreased at 24 h (Fig.
1C) and 48 h (Fig. 1D) compared
with the Control and MOCK groups (P<0.01). More notably, the
HIF-1α siRNA+DPP group demonstrated a stronger inhibition of
NCI-H157 cell proliferation compared with the NCI-H157 cells that
were treated with DPP or HIF-1α siRNA separately (P<0.05). The
present results indicate that the downregulation of HIF-1α may
decrease the growth capacity of NCI-H157 cells, and that this
effect may be augmented by DDP treatment.
HIF-1α knockdown increased the
apoptosis of NCI-H157 cells
To explore the involvement of HIF-1α in cell
apoptosis, Annexin V/propidium iodide (PI) staining was performed.
As exhibited in Fig. 2, cell
apoptosis was markedly increased in the HIF-1α siRNA, DDP, MOCK+DDP
and HIF-1α siRNA+DDP groups, when compared with the expression in
the control group (P<0.01). In addition, the cell apoptosis of
the HIF-1α siRNA+DDP group was greater than that of the HIF-1α
siRNA, DDP and MOCK+DDP groups (P<0.01). The present results
indicate that the downregulation of HIF-1α may increase the
apoptosis of NCI-H157 cells, which may be strengthened by DDP
treatment.
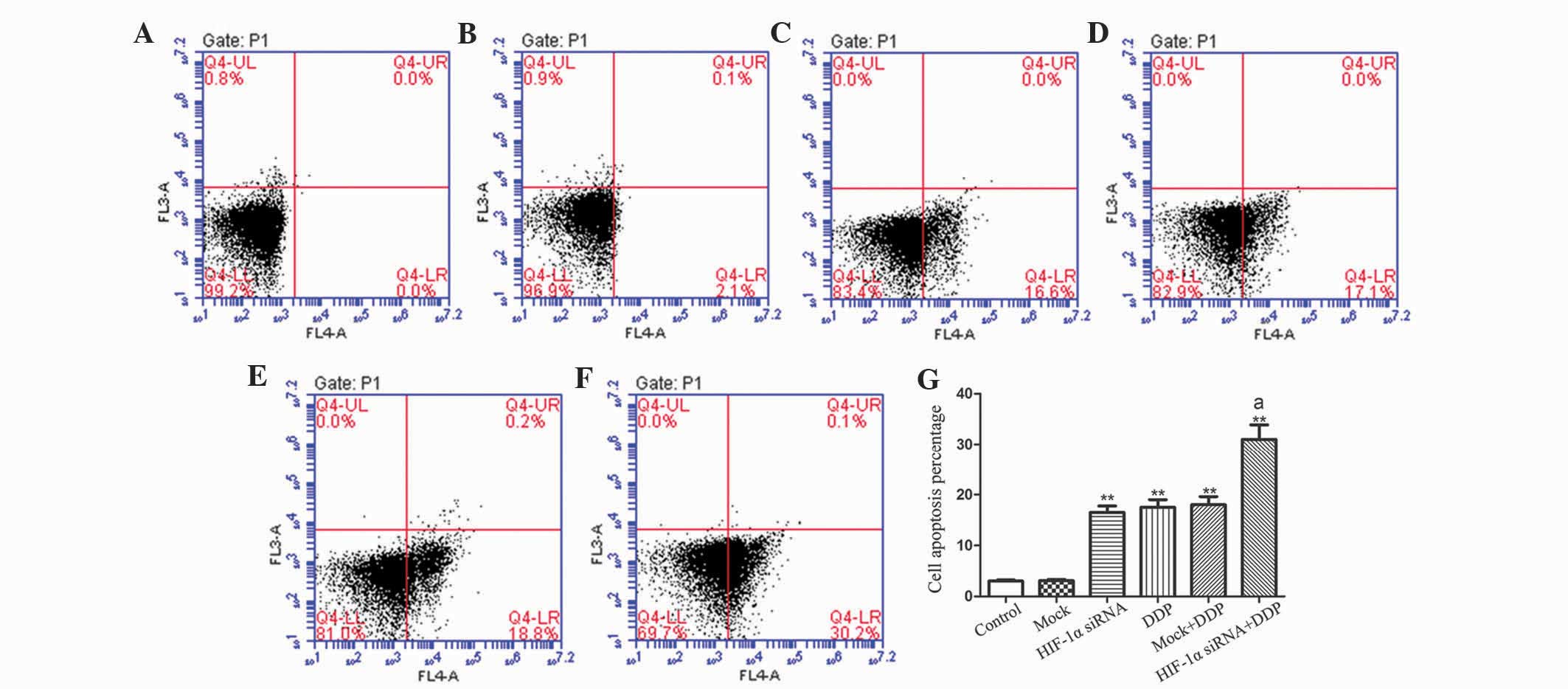 | Figure 2.Apoptosis of NCI-H157 cells that were
detected using Annexin V/propidium iodide staining in the (A)
control (no treatment), (B) MOCK (controls transfected with one
irrelevant siRNA), (C) HIF-1α siRNA, (D) DDP, (E) MOCK with DDP and
(F) HIF-1α siRNA with DDP groups. (G) Statistical results of 3
experiments. The data are expressed as the mean ± standard
deviation (n=6). Data were analyzed using one-way analysis of
variance. *P<0.05, **P<0.01 vs. control.
aP<0.01 vs. HIF-1α siRNA, DDP and MOCK+DDP. HIF-1α,
hypoxia-inducing factor-1α; siRNA, small interfering RNA; MOCK,
irrelevant; DDP, diamminedichloroplatinum. |
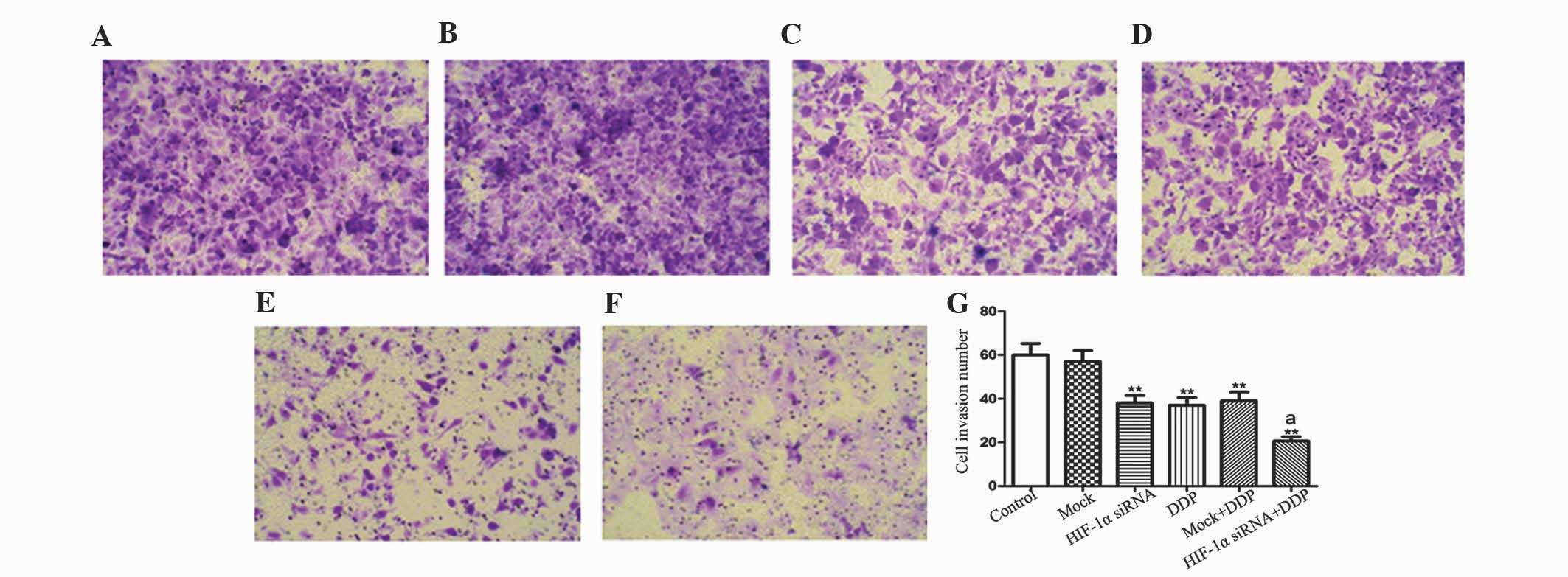
HIF-1α knockdown decreased the
invasion ability of NCI-H157 cells
Invasive ability is essential for the malignant
biological behaviors of tumors. The present study also investigated
whether HIF-1α affected the invasive ability of NCI-H157 cells. An
in vitro invasion assay was performed in chambers that had
the upper wells coated with Matrigel in order to mimic the
extracellular matrix. In sharp contrast to the control cells, the
HIF-1α siRNA group, DDP group, Mock+DDP group and HIF-1α siRNA+DDP
groups demonstrated a dramatically reduced invasive ability
(P<0.01; Fig. 3). The number of
invasive cells in the HIF-1α siRNA+DDP group was significantly
increased compared to the number in the HIF-1α siRNA or DDP groups
(P<0.05, P<0.01). The present results indicate that the
downregulation of HIF-1α may decrease the invasive ability of
NCI-H157 cells, which may be potentiated by DDP treatment.
Associated proteins were regulated by
the downregulation of HIF-1α
The results of the invasion assay indicated the
involvement of HIF-1α in the proliferation, apoptosis and invasion
of NCI-H157 cells. To investigate the possible mechanisms, the
expression levels of associated proteins were determined using
western blotting. There were no evident differences observed in the
levels of detected proteins between the control and MOCK groups
(Fig 4). The expression levels of
caspases 3 and 9 were significantly increased in the HIF-1α siRNA,
DDP, MOCK+DDP and HIF-1α siRNA+DDP groups (P<0.01), whereas the
expression levels of Bcl-2, VEGF, p-PI3K and p-AKT were decreased.
Additionally, the effect of HIF-1α knockdown on the expression of
these proteins was strengthened by DDP treatment.
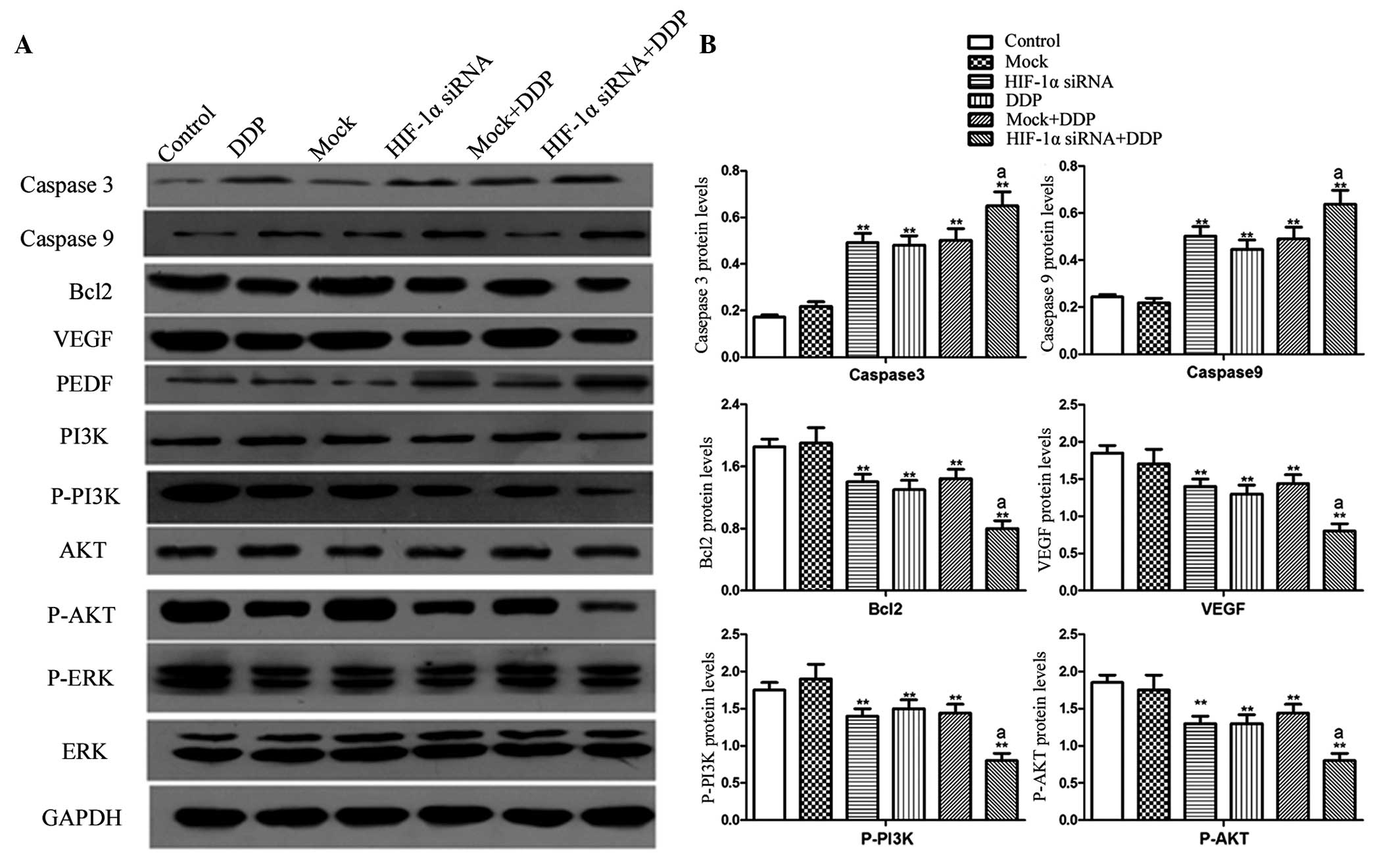 | Figure 4.(A) The expression of caspase 3,
caspase 9, Bcl-2, VEGF, p-PI3K and p-AKT was detected using western
blotting. (B) Quantification of the western blotting membrane
signal intensity was performed, and the statistical results of the
3 experiments were presented. The data are expressed as the mean ±
standard deviation (n=6). *P<0.05, **P<0.01 vs. control.
aP<0.01 vs. HIF-1α siRNA, DDP and MOCK+DDP. Bcl-2,
B-cell lymphoma-2; VEGF, vascular endothelial growth factor; PEDF,
pigment epithelium-derived factor; PI3K, phosphoinositide 3-kinase;
P-PI3K, phosphorylated-PI3K; AKT, protein kinase B; P-AKT,
phosphorylated-AKT; ERK, extracellular signal-regulated kinase;
P-ERK, phosphorylated-ERK; HIF-1α, hypoxia-inducing factor-1α;
siRNA, small interfering RNA; DDP, diamminedichloroplatinum; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
HIF-1α knockdown in NCI-H157 cells may inhibit cell
proliferation and promote cell apoptosis. Previously, an increase
in HIF-1α expression was indicated to be associated with the
progression of gastric cancer (28).
Wang et al indicated an association between the breast
cancer diffused optical tomography-synthesis diagnostic index and
the expression of HIF-1α (29). Zhou
et al indicated that HIF-1α may promote breast cancer growth
(30). Similar to previous studies,
the results of the CCK-8 assay in the present study indicated that
HIF-1α knockdown may inhibit NCI-H157 cell proliferation (Fig. 1), which was similar to the effect of
the anticancer drug DDP.
Hepatic cholesterol has been previously reported to
activate HIF-1α, which may in turn damage the liver cells (31). Jo et al indicated that
glucosamine hydrochloride may be used to treat oral tumors that
demonstrate a high expression of HIF-1α, through reducing HIF-1α
expression (32). Similar to the
study by Jo et al (32), the
Annexin V/PI staining data from the present study indicates that
HIF-1α knockdown may promote the apoptosis of NCI-H157 cells
(Fig. 2), which was the same effect
that was caused by DDP. Additionally, the effects of HIF-1α
knockdown on cell proliferation and apoptosis were strengthened by
DDP, which indicated that HIF-1α siRNA may be combined with DPP for
tumor therapy.
HIF-1α knockdown significantly reduced the invasive
ability of NCI-H157 cells. It has been previously reported that
HIF-1α overexpression may be a useful independent prognostic
biomarker in gastric cancer (33).
Hypoxia is considered to be an important stimulus for tumor
angiogenesis, as hypoxia may induce HIF-1α overexpression and
therefore activate platelet-derived growth factor (PDGF) (34). Preventing the microenvironmental
adaptations of tumors by reducing the expression of HIF-1α may be a
good method to inhibit craniopharyngioma (35). However, the role of HIF-1α on the cell
invasion of NSCLC has not yet been studied. In the present study,
the invasion ability of NCI-H157 was significantly decreased by
HIF-1α knockdown (Fig. 3). Notably,
HIF-1α siRNA and DDP demonstrate combined effects. Therefore HIF-1α
siRNA may be combined with anti-tumor drugs for tumor
treatment.
There are certain signaling pathways that are
affected by HIF-1α knockdown. Mitochondria-mediated apoptosis is
regarded as the major apoptotic pathway (36), and caspases 3 and 9 may be used as
markers of cell apoptosis. Bcl-2 is an anti-apoptotic protein
(37). As revealed in Fig. 4, HIF-1α knockdown significantly
increased the expression of caspases 3 and 9, but notably decreased
Bcl-2 expression. The present data indicated that HIF-1α knockdown
may induce mitochondria-mediated apoptosis.
The PI3K/Akt and Raf/MEK/ERK signaling pathways are
important in the process of tumor development (38). In a previous study, the PI3K/AKT
pathway and the transcription of HIF-1α were activated in a
cellular model of oral-esophageal carcinogenesis (39). Numerous studies have reported that
HIF-1α expression may lead to organ damage through the MEK/ERK
signaling pathway (40–42). Exposure of the neonatal rat brain to
hypoxia-ischemia was demonstrated to cause a neonatal brain injury
due to the increase of HIF-1α protein levels and the activation of
ERK and AKT (43). In this study,
HIF-1α knockdown significantly decreased the phosphorylation of
PI3K and Akt.
Angiogenesis is required for the growth and
metastasis of invasive tumors. VEGF is reported to induce
endothelial cell proliferation and promote cell migration, and is
therefore important in angiogenesis (34,44). PEDF
is considered to be an effective inhibitor of angiogenesis by
reducing the expression of VEGF (45). In the present study, HIF-1α knockdown
was demonstrated to downregulate VEGF and upregulate PEDF, which
indicated that there were inhibitory effects of HIF-1α knockdown on
the VEGF/PEDF pathway.
In addition, the effects of HIF-1α siRNA on the
aforementioned signaling pathways may be strengthened by DDP, which
provided additional confirmation in the present study that HIF-1α
siRNA may be combined with DDP for tumor treatment.
In the present study, the knockdown of HIF-1α was
suggested to inhibit the cell proliferation and invasion ability of
NCI-H157 cells, which may be associated with the regulation of the
PI3K/AKT, Raf/MEK/ERK and VEGF/PEDF pathways. The knockdown of
HIF-1α may also increase mitochondria-mediated cell apoptosis.
Furthermore, HIF-1α siRNA may inhibit tumor growth more effectively
when treated with DDP simultaneously.
The present study provides an experimental basis for
additional studies that investigate the role of HIF-1α in tumor
progression, although the detailed mechanisms remain to be
explored. The present study expands the current understanding of
tumor growth and provides an important combined approach for the
treatment of tumors.
Acknowledgements
The present study was supported by the Science Fund
of Shanghai Chest Hospital (grant no., YZ14-09).
References
|
1
|
Fenton-Ambrose L and Kazerooni EA:
Preventative care: Lung-cancer screens now worth the cost. Nature.
514:352014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellis P: Minimally invasive thoracic
surgery for early stage non-small cell lung cancer. BMJ.
349:g58492014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lococo F, Cesario A, Leuzzi G and Apolone
G: Second primary on-small-cell lung cancer: Implications of the
new adenocarcinoma classification in the challenging decision of
the best surgical strategy. Eur J Cardiothorac Surg. 45:1115–1116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farías RO, Bortolussi S, Menéndez PR and
González SJ: Exploring Boron Neutron Capture Therapy for non-small
cell lung cancer. Phys Med. 30:888–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Ma M and Han B: GOLPH3 high
expression predicts poor prognosis in patients with resected
non-small cell lung cancer: An immunohistochemical analysis. Tumour
Biol. 35:10833–10839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W,
Zhang Z, Liu B and Ma S: MicroRNA-10b indicates a poor prognosis of
non-small cell lung cancer and targets E-cadherin. Clin Transl
Oncol. 17:209–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Qian J, Qiang Y, Huang H, Wang C,
Li D and Xu B: Down-regulation of miR-4500 promoted non-small cell
lung cancer growth. Cell Physiol Biochem. 34:1166–1174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeuner A, Francescangeli F, Contavalli P,
Zapparelli G, Apuzzo T, Eramo A, Baiocchi M, De Angelis ML, Biffoni
M, Sette G, et al: Elimination of quiescent/slow-proliferating
cancer stem cells by Bcl-XL inhibition in non-small cell lung
cancer. Cell Death Differ. 21:1877–1888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Lan T, Hou J, Li J, Fang R, Yang
Z, Zhang M, Liu J and Liu B: NOX4 promotes non-small cell lung
cancer cell proliferation and metastasis through positive feedback
regulation of PI3K/Akt signaling. Oncotarget. 5:4392–4405. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu S, Cheng Z, Yu L, Song W and Tao Y:
Expression of CD82/KAI1 and HIF-1α in non-small cell lung cancer
and their relationship to vasculogenic mimicry. Zhongguo Fei Ai Za
Zhi. 14:918–925. 2011.(In Chinese). PubMed/NCBI
|
|
11
|
Wang Q, Hu DF, Rui Y, Jiang AB, Liu ZL and
Huang LN: Prognosis value of HIF-1α expression in patients with
non-small cell lung cancer. Gene. 541:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Qiu X, Zhang S, Zhang Q and Wang E:
Hypoxia induced CCR7 expression via HIF-1α and HIF-2α correlates
with migration and invasion in lung cancer cells. Cancer Biol Ther.
8:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv Y and Zhang P: Advances of HIF-1α and
its role in lung cancer. Zhongguo Fei Ai Za Zhi. 12:147–151.
2009.(In Chinese). PubMed/NCBI
|
|
14
|
Jang BC: The fruit juice of Morinda
citrifolia (noni) downregulates HIF-1α protein expression through
inhibition of PKB, ERK-1/2, JNK-1 and S6 in manganese-stimulated
A549 human lung cancer cells. Int J Mol Med. 29:499–504.
2012.PubMed/NCBI
|
|
15
|
Liang J, Qian Y, Xu D, Yin Q and Pan HJ:
Serum tumor markers, hypoxia-inducible factor-1α HIF-1α and
vascular endothelial growth factor, in patients with non- small
cell lung cancer before and after intervention. Asian Pac J Cancer
Prev. 14:3851–3854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Li LH, Gao GD, Wang G, Qu L, Li JG
and Wang CM: HIF-1α up-regulates NDRG1 expression through binding
to NDRG1 promoter, leading to proliferation of lung cancer A549
cells. Mol Biol Rep. 40:3723–3729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Hu M, Yu JM, Yang GR, Guo HB and Gao
Y: Correlations of 99 Tc m-HL91 SPECT hypoxia imaging with HIF-1α
and VEGF expression in non-small cell lung cancer. Zhonghua Zhong
Liu Za Zhi31. 669–673. 2009.(In Chinese).
|
|
18
|
Choi YJ, Rho JK, Lee SJ, Jang WS, Lee SS,
Kim CH and Lee JC: HIF-1α modulation by topoisomerase inhibitors in
non-small cell lung cancer cell lines. J Cancer Res Clin Oncol.
135:1047–1053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuo S, Ji Y, Wang J and Guo J: Expression
and clinical implication of HIF-1α and VEGF-C in non-small cell
lung cancer. J Huazhong Univ Sci Technolog Med Sci. 28:674–676.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Zhang Q, Jiang L, Qiu X and Wang E:
Upregulation of the chemokine receptor CCR7 expression by HIF-1α
and HIF-2α in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi.
11:724–728. 2008.(In Chinese). PubMed/NCBI
|
|
21
|
Ai B, Pan T, Zheng Z and Chen T: The
relationship of expression of GLUT1, HIF-1α and the uptake of FDG
in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 10:508–512.
2007.(In Chinese). PubMed/NCBI
|
|
22
|
Song X, Liu X, Chi W, Liu Y, Wei L, Wang X
and Yu J: Hypoxia-induced resistance to cisplatin and doxorubicin
in non-small cell lung cancer is inhibited by silencing of HIF-1α
gene. Cancer Chemother Pharmacol. 58:776–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu C, Wang X, Zheng H, Liu T, Li Y, Sun
C, Wang A and Zhao F: Expressions of EphB4 and HIF-1α in human lung
cancer and their significances. Zhongguo Fei Ai Za Zhi. 8:99–102.
2005.PubMed/NCBI
|
|
24
|
Chang H, Shyu KG, Lee CC, Tsai SC, Wang
BW, Lee Hsien Y and Lin S: GL331 inhibits HIF-1α expression in a
lung cancer model. Biochem Biophys Res Commun. 302:95–100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jacoby JJ, Erez B, Korshunova MV, Williams
RR, Furutani K, Takahashi O, Kirkpatrick L, Lippman SM, Powis G,
O'Reilly MS, et al: Treatment with HIF-1α antagonist PX-478
inhibits progression and spread of orthotopic human small cell lung
cancer and lung adenocarcinoma in mice. J Thorac Oncol. 5:940–949.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan J, Ma J, Mei J and Shan G: The effects
of HIF-1α on gene expression profiles of NCI-H446 human small cell
lung cancer cells. J Exp Clin Cancer Res. 28:1502009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng Z, Shan C and Wang H: Expression of
VHL and HIF-1α and its clinical significance in the lung cancer
tissue. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 34:331–334. 2009.(In
Chinese). PubMed/NCBI
|
|
28
|
Naruke A, Azuma M, Takeuchi A, Ishido K,
Katada C, Sasaki T, Higuchi K, Tanabe S, Saegusa M and Koizumi W:
Comparison of site-specific gene expression levels in primary
tumors and synchronous lymph node metastases in advanced gastric
cancer. Gastric Cancer. 18:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang HL and Zhang ZL: Analysis of the
relationship between ultrasound of breast cancer DOT-SDI and the
expression of MVD, VEGF and HIF-1α. Cell Biochem Biophys.
70:205–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Z, Liu F, Zhang ZS, Shu F, Zheng Y,
Fu L and Li LY: Human rhomboid family-1 suppresses
oxygen-independent degradation of hypoxia-inducible factor-1α in
breast cancer. Cancer Res. 74:2719–2730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anavi S, Hahn-Obercyger M, Madar Z and
Tirosh O: Mechanism for HIF-1 activation by cholesterol under
normoxia: A redox signaling pathway for liver damage. Free Radic
Biol Med. 71:61–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jo JR, Park YK and Jang BC: Short-term
treatment with glucosamine hydrochloride specifically downregulates
hypoxia-inducible factor-1α at the protein level in YD-8 human
tongue cancer cells. Int J Oncol. 44:1699–1706. 2014.PubMed/NCBI
|
|
33
|
Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q,
Jia B, Wei B, Wei L, Dai G, et al: HIF-1 α overexpression
correlates with poor overall survival and disease-free survival in
gastric cancer patients post-gastrectomy. PLoS One. 9:e906782014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clara CA, Marie SK, de Almeida JR,
Wakamatsu A, Oba-Shinjo SM, Uno M, Neville M and Rosemberg S:
Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1α in
human glioblastoma. Neuropathology. 34:343–352. 2014.PubMed/NCBI
|
|
35
|
Liu H, Liu Z, Li J, Li Q, You C and Xu J:
Relative quantitative expression of hypoxia-inducible factor 1α
messenger ribonucleic acid in recurrent craniopharyngiomas. Neurol
India. 62:53–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heeg S, Hirt N, Queisser A, Schmieg H,
Thaler M, Kunert H, Quante M, Goessel G, von Werder A, Harder J, et
al: EGFR overexpression induces activation of telomerase via
PI3K/AKT-mediated phosphorylation and transcriptional regulation
through Hif1-α in a cellular model of oral-esophageal
carcinogenesis. Cancer Sci. 102:351–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Richard DE, Berra E, Gothié E, Roux D and
Pouysségur J: p42/p44 mitogen-activated protein kinases
phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and
enhance the transcriptional activity of HIF-1. J Biol Chem.
274:32631–32637. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Minet E, Arnould T, Michel G, Roland I,
Mottet D, Raes M, Remacle J and Michiels C: ERK activation upon
hypoxia: Involvement in HIF-1 activation. FEBS Lett. 468:53–58.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hur E, Chang KY, Lee E, Lee SK and Park H:
Mitogen-activated protein kinase kinase inhibitor PD98059 blocks
the trans-activation but not the stabilization or DNA binding
ability of hypoxia-inducible factor-1α. Mol Pharmacol.
59:1216–1224. 2001.PubMed/NCBI
|
|
43
|
van den Tweel ER, Kavelaars A, Lombardi
MS, Nijboer CH, Groenendaal F, van Bel F and Heijnen CJ: Bilateral
molecular changes in a neonatal rat model of unilateral
hypoxic-ischemic brain damage. Pediatr Res. 59:434–439. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang E, Feng X, Liu F, Zhang P, Liang J
and Tang X: Roles of PI3K/Akt and c-Jun signaling pathways in human
papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8
expression and in vitro angiogenesis in non-small cell lung
cancer cells. PLoS One. 9:e1034402014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamagishi S, Matsui T, Nakamura K, Yoshida
T, Shimizu K, Takegami Y, Shimizu T, Inoue H, Imaizumi T, et al:
Pigment-epithelium-derived factor (PEDF) inhibits
angiotensin-II-induced vascular endothelial growth factor (VEGF)
expression in MOLT-3 T cells through anti-oxidative properties.
Microvasc Res. 71:222–226. 2006. View Article : Google Scholar : PubMed/NCBI
|