Introduction
A pharyngeal fistula may occur due to saliva stored
in an incision of the skin or tissue, which may result in the
rupture of an abscess to form skin at the incision edge. Therefore,
incisions in the hypopharynx and esophagus may grow towards the
sinus cavity, with skin communicating through this channel.
Eventually, the saliva or food may overflow from the incision, and
the fistula between the pharynx and the sinus cavity is formed
(1). A pharyngeal fistula is a
frequent complication that often occurs following the performance
of a total laryngectomy. The incidence of a fistula occurring due
to a primary total laryngectomy is 14.3% (1). Infection, the location of the primary
tumor, for example on the glottis and transglottic region (2), preoperative radiotherapy (2), a preoperative tracheotomy (3), tumor cells at the surgical margin
(4), chronic systemic disease
(2,5–11),
including diabetes, severe hypertension, anaemia, hypoproteinemia,
renal insufficiency and thyroid dysfunction, and locally advanced
tumors (T3 and T4 stages) (12) are
all risk factors following a total laryngectomy for pharyngeal
fistulas. Salvage surgery is associated with more complications
compared with primary surgery; and the incidence of salvage
laryngectomy fistula is 27.6% while the incidence of salvage
surgery with flap-reinforced closure is 10.3% (1). Pharyngeal fistulas not only inflict
increasing physical pain and psychological distress, but may also
prolong hospitalization, negatively affect patient recovery (in
terms of local infections, swallowing and vocal capabilities) and
increase mortality (1,13,14). The
treatment of pharyngeal fistulas is complicated; although smaller
fistulas occasional heal well with a local dressing, fistulas >1
cm in diameter, and those occurring subsequent to radiotherapy,
typically require surgical repair (14–17).
Case report
In June 2012, due to the hypopharynx discomfort, a
56-year-old man was admitted to the Ganzhou Tumor Hospital
(Ganzhou, China). Laryngoscopy examination identified a hypopharynx
tumor on the right side of the hypopharynx, and subsequent
pathological analysis of a biopsy (employing hematoxylin and eosin
staining techniques) revealed disordered carcinoma nests and tumor
cells with large nuclei, in addition to a number of prominent
nucleoli, abundant cytoplasm and keratoses. Such observations lead
to the diagnosis of highly-differentiated pharyngeal squamous cell
carcinoma, and the clinical stage of the tumor was reported as
cT2N1M0, stage III (18). Following 2
cycles of docetaxel (75 mg/m2 on day 1), cisplatin (75
mg/m2 on day 1) and 5-fluorouracil (0.5 mg/m2
on days 2–5) induction chemotherapy, the patient underwent a total
laryngectomy with a right functional carotid dissection and
tracheostomy. Following the surgery, the incision appeared to heal
well. A regimen of radiotherapy to the neck was then performed,
with a total dose of 66 Gy in 33 fractions for a duration of 45
days. Following 20 doses of radiation, a small amount of milk
leaked from the edge of the tracheal stoma subsequent to eating and
resulted in the patient choking; the possibility of a pharyngeal
fistula was then considered. Relevant therapeutic processes,
including fasting and nutritional support, were adopted and
radiotherapy was continued. Following completion of the
radiotherapy regimen and regular follow-up, no symptoms of a
pharyngeal fistula were evident.
However, on May 25, 2013, a fistula was detected at
the top of the tracheal stoma, with secretions being excreted
continuously from the passage; aspiration and coughing symptoms
also progressively worsened. The pharyngeal fistula was concurrent
with the initiation of post-operative radiotherapy. Subsequently,
the patient underwent pharyngeal fistula excision and local repair
surgery under general anesthesia on June 28, 2013. However, it was
observed that the negative pressure drainage tube was leaking 3
days later, and the neck incision was dehiscent after 6 days. The
skin of the neck gradually became necrotic, and the hypopharynx was
exposed, with foul-smelling secretions. A local anti-inflammatory
treatment was applied [1.0 g ceftazidine three times a day (tid)
and ciprofloxacin hydrochloride 0.2 g two times a day (bid) for 5
days], and the fistula was then washed and a dressing applied;
however, the local infection persisted.
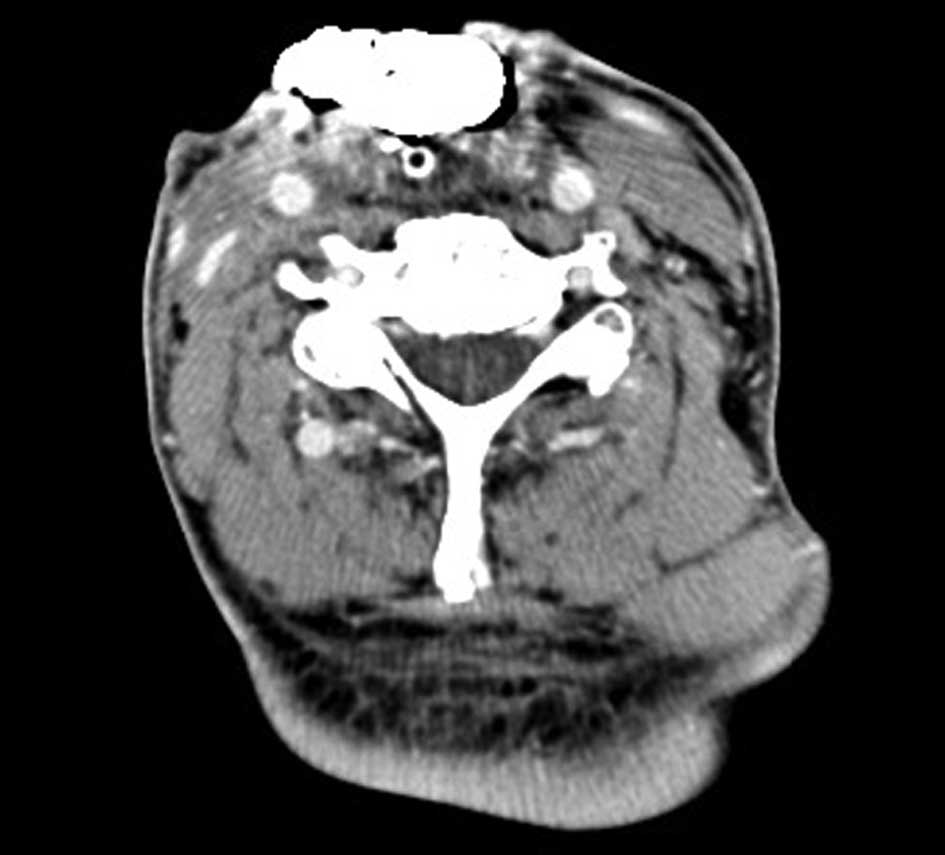
Computed tomography examination revealed no
recurrence of the tumor, however, an irregular fistula (~7×5 cm)
was identified in the neck on July 18, 2013 (Fig. 1), and was associated with oral
secretions. A pharyngeal mucosal defect (5×3 cm) was present in the
hypopharynx, and the entrance to the esophagus and stomach were
directly visible. The tracheal stoma was 2.0×1.5 cm in size and was
located below the neck fistula, with no signs of local swelling or
abnormal secretions. The airway was smooth and the neck lymph nodes
were not palpable. Pseudomonas aeruginosa was cultured from
the fistula secretions. Despite regular treatment with hydrogen
peroxide, saline and iodoform strips, no significant change was
observed in the size of the fistula after 2 weeks, and the
foul-smelling secretions persisted. In light of the emotional
instability and suicidal ideation of the patient, the available
treatment options were discussed with the patient and their family.
In the Ganzhou Tumor Hospital, on August 6, 2013, a pharyngeal
fistula resection was conducted, with a right pectoralis major flap
prosthesis, under general anesthesia, as approved by the hospital
ethics committee. The hypopharyngeal mucosa and skin defects were
repaired with a bi-folded pectoralis major muscle flap.
On day 3 post-surgery, a small amount of
non-foul-smelling turbid liquid spilled from the drainage tube,
however, this gradually decreased. Pseudomonas aeruginosa
was cultured from the drainage tube secretions on day 7
post-surgery, and antibiotic treatment (1.5 g
cefoperazone-sulbactam bid combined with 0.2 g ciprofloxacin
hydrochloride bid for 3 days) was selected to treat the infection.
On day 10 post-surgery, Escherichia coli was cultured from
the tracheal stoma secretions, and this infection was also treated
with 1.5 g cefoperazone-sulbactam bid combined with 0.2 g
ciprofloxacin hydrochloride bid for 2 days. By day 12 post-surgery,
the drainage from the right upper neck tube had decreased; however,
extensive secretions continued to be excreted from the area
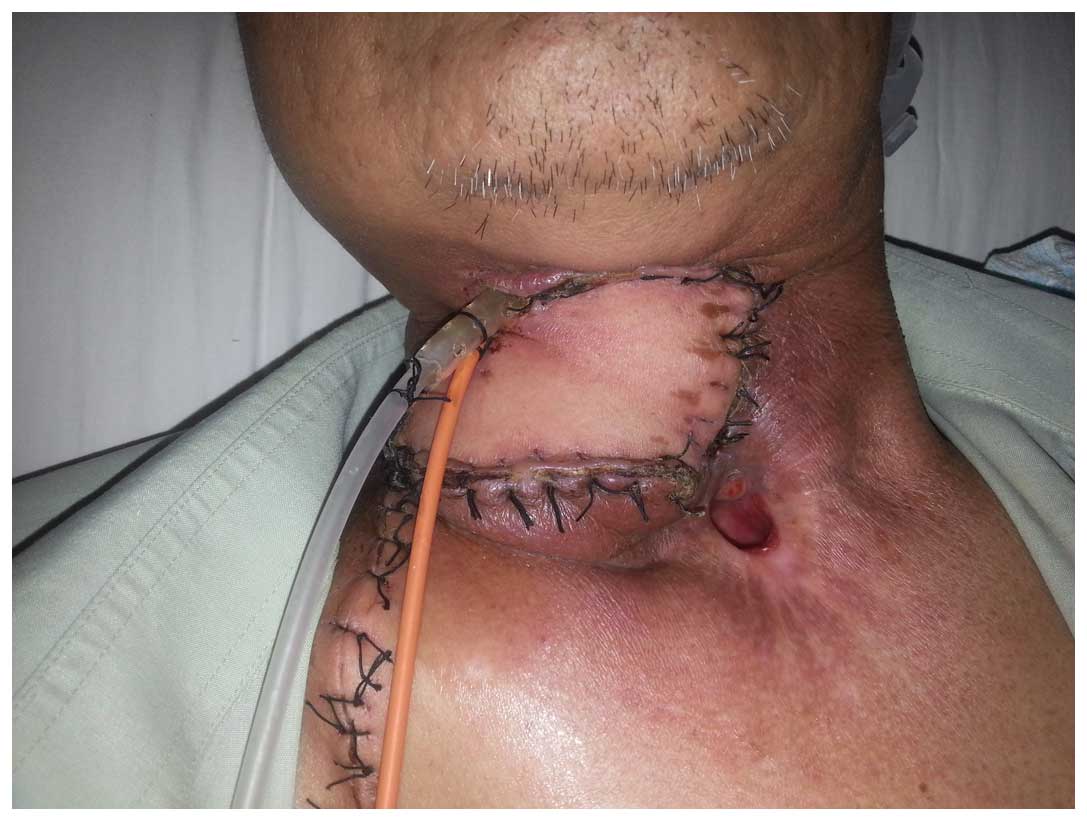
surrounding the tube. During a laryngoscopy, a gap of ~2 cm was
detected at the upper left section of the hypopharyngeal mucosa,
which had been repaired with the pectoralis major muscle flap. The
right upper neck drainage tube was removed, and a dual tube was
inserted to a depth of ~5 cm with continuous negative
pressure-flush (Fig. 2). All
antibiotics were stopped and nutritional therapy was strengthened.
By day 35, the flush drainage was clear and the drain fluid intake
and output were similar, with the skin incision surrounding the
tracheal stoma fistula healing well. The dual tube, ~2 cm from the
fistula, was removed with continuous flushing, and the depth of the
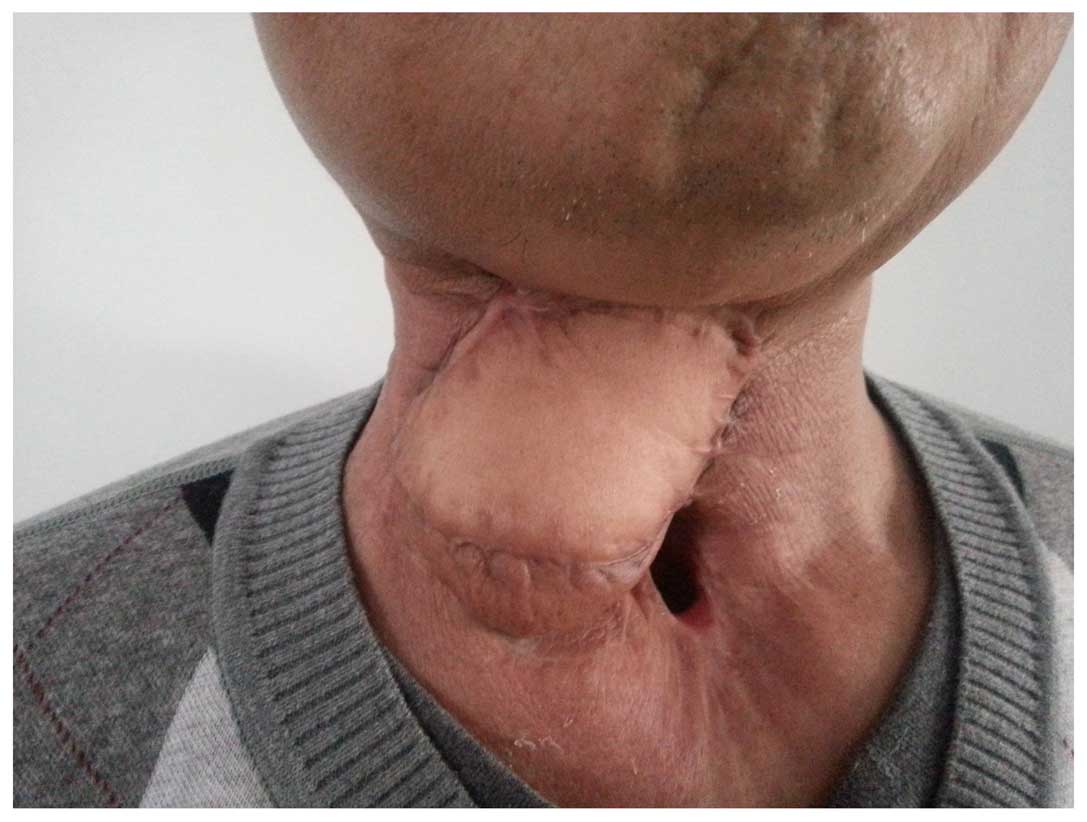
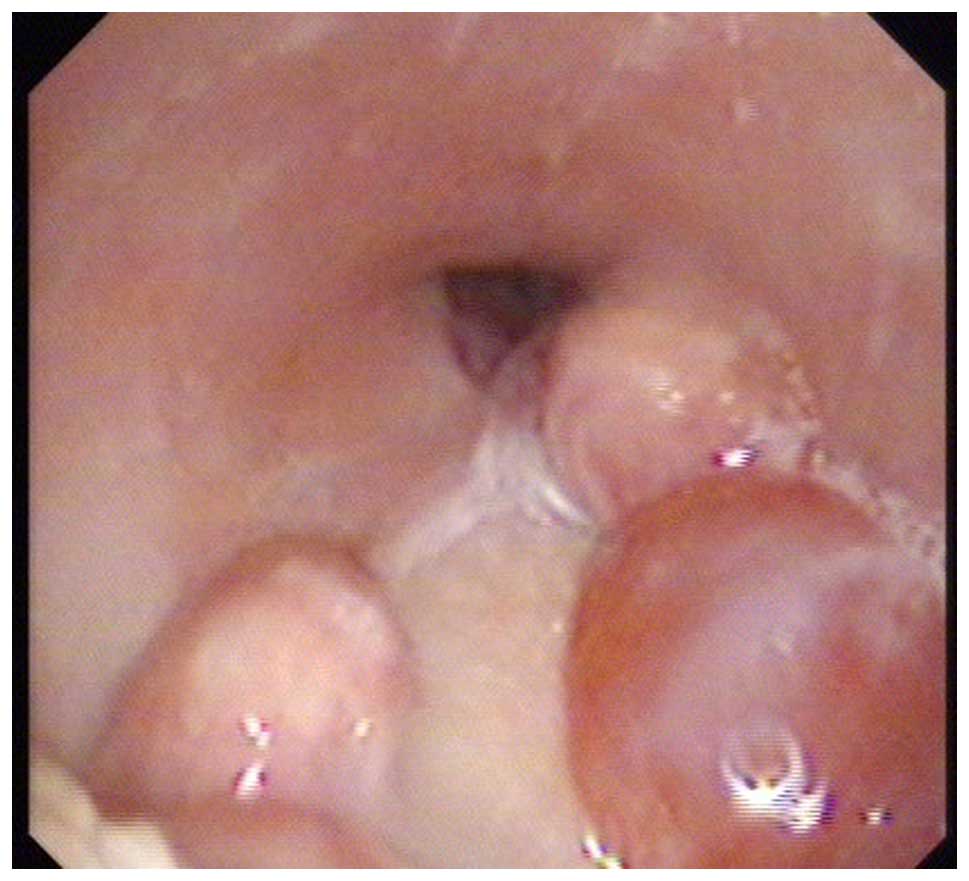
double tube was gradually adjusted. At day 70 post-surgery, the
dual tube was withdrawn from the fistula with no local exudate, and
the fistula was packed with iodoform gauze. The patient underwent a
laryngoscopic examination 5 days later, demonstrating that the
fistula had closed, with the flap healing well, and the patient had
returned to normal oral eating. The patient remained free from
recurrence and metastasis throughout 15 months of follow-up
examinations (Figs. 3 and 4).
Discussion
In the present case, severe symptoms of a pharyngeal
fistula reappeared ~6 months after the initiation of symptomatic
treatment and radiotherapy to treat a highly-differentiated
pharyngeal squamous cell carcinoma. Despite suturing the fistula
directly, a larger pharyngeal fistula was observed with a skin
defect of ~8×6 cm and a mucosal defect of ~6×4 cm. The patient
exhibited suicidal tendencies with unstable emotions during
hospitalization. Following careful consideration, the fistula was
repaired with a bi-fold pectoralis major muscle flap; this has the
advantages of a rich blood supply, strong resistance to infection
and a rapid healing time. However, the pharyngeal fistula recurred
3 days after reconstructive surgery. Despite anti-infection
treatment, nutritional therapy and drainage, the fistula worsened,
with another fistula developing just above the tracheal stoma; this
was now considered to be a complicated pharyngeal fistula. The
treatment options available for this complicated pharyngeal fistula
were limited, and prolonged dressing of the surgical incision was
likely to lead to renewed suicidal ideation.
Continuous negative pressure-flush through a dual
tube has been utilized successfully for the treatment of
anastomotic fistulas (19). This
approach involves using an outer tube, comprising a medical
silicone pipe with a scale, with two to four pairs of holes punched
in the sides (conducted using a leather hole puncher). The inner
tube is a suction pipe, with its tip 3–5 cm from the outer pipe tip
and fixed to the outer tube by a thread. Bacterial secretions and
exudates in the body cavities may be aspirated by a dual-tube
continuous negative pressure-flush, thus reducing the risk of
infection and local inflammation. This may also accelerate the
development of novel blood vessels and granulation tissue,
promoting healing of the fistula. Although spontaneous fistula
closure occurs in the majority of cases undergoing conservative
management, Wiseman et al (20) described a case of a post-laryngectomy
pharyngocutaneous fistula that had developed in a previously
irradiated patient; this case was successfully managed through the
incorporation of fibrin glue into the surgical closure. However,
fibrin glue-reinforced closure is only suitable for smaller
pharyngeal fistulas, and was not applicable to the present case. A
small number of pharyngeal fistulas may require surgical flap
repair, whereby the inner mucosa and the outer skin are
reconstructed (21). However,
surgical repair of fistulas is challenging, with the likelihood of
bleeding, infection and necrosis, and the fistula being
substantially more difficult to treat post-surgery.
Following careful consideration, with the consent of
the patient and his family, and with authorization from the
hospital ethics committee, it was decided that continuous negative
pressure-flush would be applied through a dual tube in the
complicated pharyngeal fistula. Continuous negative pressure-flush
was applied through a dual tube on day 12 post-surgery, without
antibiotics. The depth of the dual tube was gradually adjusted, and
the nutritional support of the patient was strengthened. The
fistula had healed well by day 35 and the dual tube was removed at
day 70 post-surgery. By day 75, the patient was considered to be
cured. The dual tube was retained for a total of 58 days,
suggesting that the condition of the patient may have worsened due
to the administration of radiotherapy and also the possibility that
the fistula infection was not fully controlled prior to surgery.
The treatment strategies for pharyngeal fistula with
flap-reinforced closure include re-operation repair, dressing and
other nonsurgical treatments. The fistula increases the patient's
pain and reduces the quality of life (15–17).
In conclusion, continuous negative pressure-flush
through a dual tube to treat a complicated pharyngeal fistula has
numerous advantages, including no requirements for an incision,
daily dressing or antibiotics, whilst shortening the healing time
of the fistula. Additionally, the lack of an incision is associated
with positive cosmetic results. This technique may aid in reducing
the psychological burden associated with pharyngeal fistulas,
whilst also improving patient quality of life.
References
|
1
|
Sayles M and Grant DG: Preventing
pharyngo-cutaneous fistula in total laryngectomy: A systematic
review and meta-analysis. Laryngoscope. 124:1150–1163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galli J, De Corso E, Volante M, Almadori G
and Paludetti G: Postlaryngectomy pharyngocutaneous fistula:
Incidence, predisposing factors, and therapy. Otolaryngol Head Neck
Surg. 133:689–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cecatto SB, Soares MM, Henriques T,
Monteiro E and Moura CI: Predictive factors for the
postlaryngectomy pharyngocutaneous fistula development: Systematic
review. Braz J Otorhinolaryngol. 80:167–177. 2014.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markou KD, Vlachtsis KC, Nikolaou AC,
Petridis DG, Kouloulas AI and Daniilidis IC: Incidence and
predisposing factors of pharyngocutaneous fistula formation after
total laryngectomy. Is there a relationship with tumor recurrence?
Eur Arch Otorhinolaryngol. 261:61–67. 2004. View Article : Google Scholar
|
|
5
|
Akduman D, Naiboğlu B, Uslu C, Oysu C, Tek
A, Sürmeli M, Kiliçarslan Y and Yanilmaz M: Pharyngocutaneous
fistula after total laryngectomy: Incidence, predisposing factors,
and treatment. Kulak Burun Bogaz Ihtis Derg. 18:349–354. 2008.(In
Turkish). PubMed/NCBI
|
|
6
|
Paydarfar JA and Birkmeyer NJ:
Complications in head and neck surgery: A meta-analysis of
postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head
Neck Surg. 132:67–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boscolo-Rizzo P, De Cillis G, Marchiori C,
Carpene S and Da Mosto MC: Multivariate analysis of risk factors
for pharyngocutaneous fistula after total laryngectomy. Eur Arch
Otorhinolaryngol. 265:929–936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White HN, Golden B, Sweeny L, Carroll WR,
Magnuson JS and Rosenthal EL: Assessment and incidence of salivary
leak following laryngectomy. Laryngoscope. 122:1796–1799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cavalot AL, Gervasio CF, Nazionale G,
Albera R, Bussi M, Staffieri A, Ferrero V and Cortesina G:
Pharyngocutaneous fistula as a complication of total laryngectomy:
Review of the literature and analysis of case records. Otolaryngol
Head Neck Surg. 123:587–592. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erdag MA, Arslanoglu S, Onal K, Songu M
and Tuylu AO: Pharyngocutaneous fistula following total
laryngectomy: Multivariate analysis of risk factors. Eur Arch
Otorhinolaryngol. 270:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hier M, Black MJ and Lafond G:
Pharyngo-cutaneous fistulas after total laryngectomy: Incidence,
etiology and outcome analysis. J Otolaryngol. 22:164–166.
1993.PubMed/NCBI
|
|
12
|
Saki N, Nikakhlagh S and Kazemi M:
Pharyngocutaneous fistula after laryngectomy: Incidence,
predisposing factors, and outcome. Arch Iran Med. 11:314–317.
2008.PubMed/NCBI
|
|
13
|
Sayles M, Koonce SL, Harrison L, Beasley
N, McRae AR and Grant DG: Pharyngo-cutaneous fistula complicating
laryngectomy in the chemo-radiotherapy organ-preservation epoch.
Eur Arch Otorhinolaryngol. 271:1765–1769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basheeth N, O'Leary G and Sheahan P:
Pharyngocutaneous fistula after salvage laryngectomy: Impact of
interval between radiotherapy and surgery, and performance of
bilateral neck dissection. Head Neck. 36:580–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Dong J, Lu M, Yang BH, Li HP and
He XJ: Diagnosis of pharyngeal fistula by video laryngoscopy and
its nonsurgical treatment: A case report. Orthop Surg. 6:158–161.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan NA, Medina JE, Sanclement JA and
Krempl GA: Fistula rates after salvage laryngectomy: Comparing
pectoralis myofascial and myocutaneous flaps. Laryngoscope.
124:1615–1617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mirghani H, Leymarie N, Amen F, Qassemyar
Q, Leclère FM and Kolb F: Pharyngotracheal fistula closure using
the internal mammary artery perforator island flap. Laryngoscope.
124:1106–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology: Head and Neck
Cancers. Version 1. 2010.https://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed.
August 27–2012
|
|
19
|
Lin C, Zhang Z, Wang Y, Huang S, Wang L
and Wang B: Continuous negative pressure-flush through
extraperitoneal dual tube in the treatment and prevention for
rectal cancer patients with anastomotic leakage after low anterior
resection. Zhonghua Wei Chang Wai Ke Za Zhi. 17:469–472. 2014.(In
Chinese). PubMed/NCBI
|
|
20
|
Wiseman S, Hicks W Jr, Loree T,
Al-kasspooles M and Rigual N: Fibrin glue-reinforced closure of
postlaryngectomy pharyngocutaneous fistula. Am J Otolaryngol.
23:368–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guha G, Saha S and Kundu I: Surgical
repair of postlaryngectomy pharyngocutaneous fistulas. Indian J
Otolaryngol Head Neck Surg. 59:103–107. 2007. View Article : Google Scholar : PubMed/NCBI
|