Introduction
Ovarian cancer is one of the most prevalent
gynecological neoplasms and major cause of cancer-associated
mortality in women. The five-year survival rate remains <40% for
stage IIB to IV ovarian cancers, despite advancements in surgical
techniques and treatments (1). Thus,
novel and more effective agents for the treatment of ovarian cancer
are required.
Panax notoginseng, a common Chinese herb, is
known to contain ginsenosides, a class of compounds with a number
of biological activities, including antioxidation (2), immunomodulatory effects (3) and anticancer properties (4,5). The
ability of ginsenosides to inhibit metastasis has been demonstrated
by strong pleiotropic anti-cancer effects observed previously in
several cancer lines (6,7). The aglycone compounds of ginsenosides
have been seldom studied, despite intensive research into the
physiological activities of ginsenosides.
Osteopontin (OPN) is a protein that is overexpressed
in ovarian cancer, its expression is frequently identified in
ovarian carcinoma effusions, and it has an involvement in
tumorigenesis and metastasis (8,9). OPN
silencing by small interfering RNA suppresses the proliferation of
human ovarian cancer cell line HO-8910PM in vitro (10), suggesting that increased OPN
expression may be key in the carcinogenesis, progression, and
differentiation of epithelial ovarian carcinoma. Regulation of OPN
activity may therefore be useful in ovarian cancer therapy.
However, to date there are no reports on the regulation of OPN
expression using ginseng extracts, such as 20(S)-protopanaxadiol
saponins (PDS).
The aim of the present study was to investigate the
anti-tumour effects of PDS and conduct a preliminary study of its
mechanisms of action. This included studying the effects of PDS on
the migration of SKOV3 cells, and the effects of PDS on the
expression of OPN.
Materials and Methods
Materials and chemicals
20(S)-protopanaxadiol saponins from Panax
notoginseng (Burkill) F. H. Chen, with ginsenosides Rb1, Rb3
and Rd (56.42, 22.99 and 13.40%, respectively) were obtained from
Kunming Pharmaceutical Corp. (Kunming, China). An
immunohistochemistry analysis kit was purchased from Zhongshan
Golden Bridge Biotechnology Co., Ltd (Beijing, China). Gibco®
RPMI-1640 medium was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Fetal bovine serum (FBS), penicillin and
streptomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA)
and Matrigel™ was obtained from BD Biosciences (Franklin Lakes, NJ,
USA). Crystal violet and polylysine were purchased from Beyotime
Institute of Biotechnology (Beijing, China). SP9001 rabbit
streptavidin peroxidase (SP) test kit was purchased from Zhongshan
Jinqiao Biotech Company (Beijing, China). The following antibodies
were used: Mouse anti-human monoclonal pro-caspase-3 (catalog no.,
sc-56053; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse
anti-human monoclonal B-cell lymphoma (Bcl)-2 (catalog no.,
sc-7382; Santa Cruz Biotechnology, Inc.), mouse anti-human
monoclonal Bcl-2-like protein 4 (Bax) (catalog no., sc-70405; Santa
Cruz Biotechnology, Inc.), rabbit anti-human polyclonal OPN
(catalog no., SAB2700986; Sigma-Aldrich, St. Louis, MO, USA), mouse
monoclonal anti-human β-actin (catalog no., sc-47778; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) and HRP-labeled goat
anti-rabbit and anti-mouse immunoglobulin (Ig)G (catalog nos.,
A0208 and A0216, respectively; dilution, 1:1,000; Beyotime
Institute of Biotechnology). All chemicals used were of reagent or
analytical grade.
Cancer cell lines and cell
culture
The SKOV3 ovarian cancer cell line, obtained from
the American Type Culture Collection (Manassas, VA, USA) was
cultured in RPMI-1640 containing 10% FBS and 1%
penicillin/streptomycin in a humidified atmosphere with 5%
CO2 at 37°C.
Determination of cytotoxicity of SKOV3
cells
The in vitro anti-proliferative effect of PDS
on SKOV3 cells was examined using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Thermo Fisher Scientific, Inc.) assay. Cells were placed in 96-well
plates (6×103 cells/well) and subsequently were treated
with 0.013, 0.025, 0.05, 0.1, 0.2 and 0.4 mg/ml of PDS. Following a
treatment time of 12, 24 or 48 h, 25 µl MTT solution (5 mg/ml) was
added to each well and incubated at 37°C in 5% CO2 for 4
h. Dimethyl sulfoxide (150 µl; Thermo Fisher Scientific, Inc.) was
added to dissolve any crystal formation. The cytotoxicity against
cancer cells was determined by measuring the absorbance of the MTT
at 490 nm using a Spectra Max 190 microplate spectrophotometer
(Molecular Devices Corporation, Sunnyvale, CA, USA). Cytotoxicity
of each sample was expressed as an IC50 value. The
IC50 value is the concentration of test sample that
causes 50% inhibition of cell growth, averaged from 3–5 replicate
experiments, and was obtained by plotting the percentage inhibition
vs. concentration of a test sample. Oxaliplatin (OXA; Jiangsu
Aosaikang Pharmaceutical Co., Ltd., Nanjing, China) was used as a
positive control, which is an effective anticancer drug. The
results are expressed as the mean ± standard deviation (SD) of
three independent experiments.
Wound-healing migration assay
A confluent single-layer of cells was seeded onto
polylysine-coated (100 µg/ml) coverslips and the cells were left to
adhere overnight in the incubator. Scraping a sterile P200 pipette
tip across the cells produced a wound in the monolayer.
Subsequently, the cells were treated with 0.2 mg/ml PDS for 0, 6,
12 or 24 h. Cell debris was removed by washing the coverslips once
with sterile phosphate-buffered saline (PBS), and cells were fixed
by incubation with 80% ethanol for 10 min at room temperature. The
cells were stained with crystal violet and migrating cells were
counted in 5 regions. Images were captured using an IX71 Inverted
Microscope with a DP73 digital camera (Olympus Corp., Tokyo, Japan;
magnification, ×40). Cell migration was expressed as the mean ± SD
of 5 independent experiments.
Tube structure formation assay
An in vitro Matrigel™ substratum assay was
used to measure tube structure formation. A 96-well plate (Thermo
Fisher Scientific, Inc.) coated with a total of 50 µL of Matrigel™
basement membrane matrix was incubated for 1 h at 37°C. A total of
2×104 SKOV3 cells in 100 µl of medium were seeded into
the wells, and 0.2 mg/ml or 0.4 mg/ml PDS was then added. Tube-like
SKOV3 cell structures formed after 6 h of incubation at 37°C in 5%
CO2 were recorded using an IX71 Inverted Microscope
(magnification, ×40).
Immunohistochemical analysis of OPN
expression
Immunohistochemistry analysis was performed using
the SP9001 rabbit SP test kit, according to manufacturer's
protocol. The immunoreaction was visualized using 3,
3′-diaminobenzidine (DAB; Zhongshan Jinqiao Biotech Company)
staining (yellow or brown visualization). The cells were
counterstained with hematoxylin (blue visualization; Beyotime
Institute of Biotechnology). Briefly, cells were plated onto
coverslips and treated with 0.2 mg/ml PDS in serum-free RPMI-1640
for 24 h and subsequently fixed with 80% ethanol for 10 min. The
cells were incubated with polyclonal anti-OPN antibodies (dilution,
1:400) for 1 h at room temperature, followed by incubation with
HRP-labeled goat anti-rabbit IgG for 15 min, washed with PBS three
times, and subsequently incubated with streptavidin-horseradish
peroxidase (Zhongshan Jinqiao Biotech Company) for 15 min. The
cells were washed with PBS three times, were stained with DAB for 8
min and washed with distilled water for 2 min. The cells were
stained with hematoxylin and washed with PBS three times, then
examined and photographed with an IX71 Inverted Microscope with a
DP73 digital camera.
Western blot analysis
The expression levels of four proteins, Bcl-2, Bax,
pro-caspase-3 and OPN, were evaluated using western blot analysis.
SKOV3 cells (1×105 cells/well) were seeded in 6-well
plates (Thermo Fisher Scientific, Inc.) and were treated with
various concentrations of PDS for 6 or 12 h. The medium was removed
and the cells were washed with PBS. The cells were lysed in 100 µl
lysis buffer (Beyotime Institute of Biotechnology) for 30 min on
ice. The lysates were centrifuged (Eppendorf® Multipurpose
centrifuge 580R; Eppendorf, Hambur, Germany) at 15,000 × g for 15
min. A protein quantification assay kit was obtained from Bio-Rad
Laboratories (catalog no., 5000001; Bio-Rad Laboratories, Hercules,
CA, USA). Absorbance was determined using a Spectra Max 190
microplate spectrophotometer. Proteins (30 µg) were resolved using
electrophoresis on a 4–12% sodium dodecyl sulfate-polyacrylamide
gels and were transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). Transferred blots were incubated
with blocking agents [5% non-fat milk in PBS-Tween 20 (Thermo
Fisher Scientific, Inc.)]. The membranes were incubated with
antibodies against pro-caspase-3 (dilution, 1:200), Bcl-2
(dilution, 1:200), Bax (dilution, 1:200), β-actin (dilution, 1:500)
and OPN (dilution, 1:500) overnight. The membranes were
subsequently washed 3 times with PBS and incubated with goat
anti-rabbit or anti-mouse IgG. The proteins were then detected
using Pierce™ enhanced chemiluminescence Western Blotting Substrate
(Thermo Fisher Scientific, Inc.). β-actin was used as a
protein-loading control.
Statistical analysis
In total, 3–5 independent replicates were conducted
for all experiments. The results are expressed as the mean ± SD.
The Student's t test with a significance level of P<0.05
was used to calculate statistically significant differences.
OriginLab version 8.5 software (OriginLab, Northampton, MA, USA)
was used to analyze the experimental data.
Results
PDS inhibits the viability of SKOV3
cells
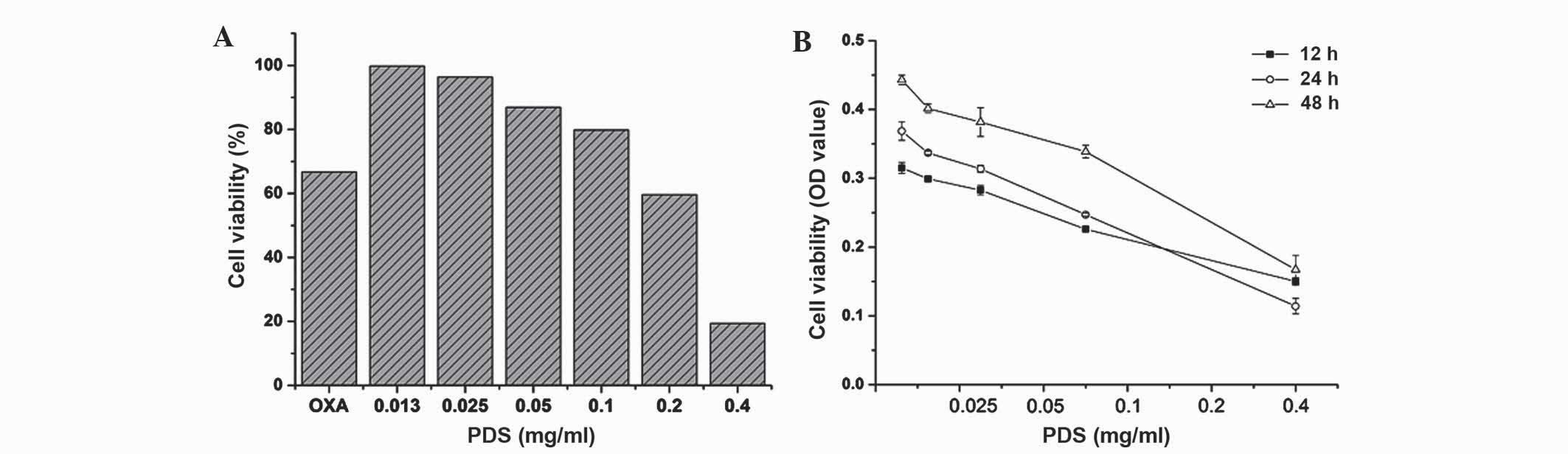
The MTT assay demonstrated that PDS significantly
inhibited SKOV3 cell proliferation. As shown in Fig. 1, PDS significantly inhibited the
viability of SKOV3 cells when treated with various concentrations
of SDS (0–0.4 mg/ml) during various incubation times (12–48 h).
Notably, the optimum treatment time was achieved at 24 h
(IC50=0.30 mg/ml). OXA was used as a positive control.
The inhibition rate of the cells by PDS was increased compared with
OXA during the early stage of drug treatment, including at a 24 h
treatment time at 0.2 mg/ml PDS decreased SKOV3 cell viability by
~40%, whereas SKOV3 cell viability decreased by ~27% following
treatment with 0.2 mg/ml OXA. In addition, cell viability decreased
by ~81% when the cells were treated with 0.4 mg/ml of PDS,
suggesting that PDS exhibits toxic effects at high concentrations.
However, following 24 h treatment, the cytotoxicity of PDS
decreased as the treatment time increased. This suggests that PDS
has a short-duration cytotoxicity on SKOV3 cells.
The effect of PDS on SKOV3 cell
migration
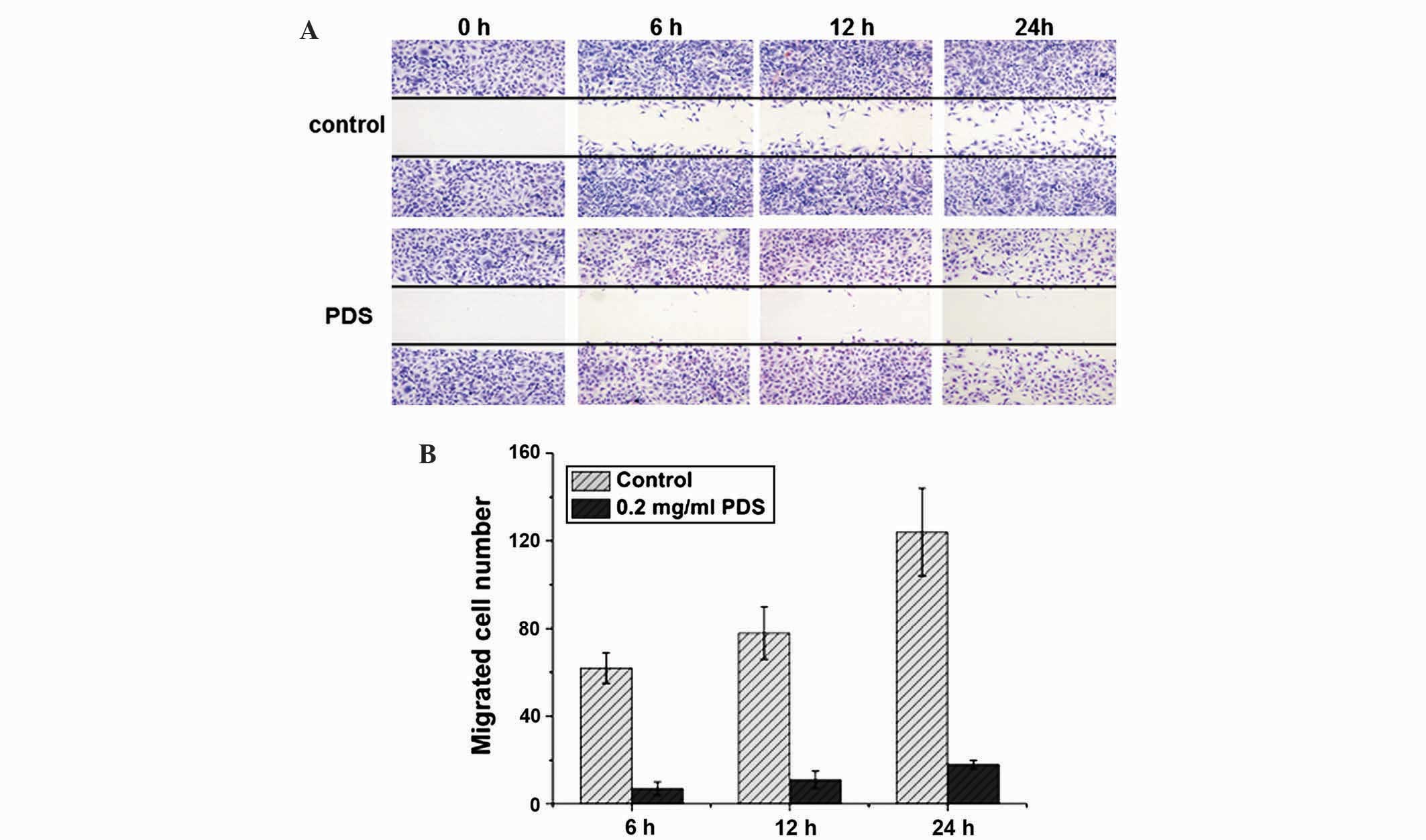
Following treatment with either 0.2 or 0.4 mg/ml of
PDS, wound-healing experiments were conducted and the number of
SKOV3 cells migrating to the wound area every 6 h for 1 day was
quantified to determine if PDS inhibits cell migration (Fig. 2A). The migrating cells were counted in
5 regions and the results were calculated as the means from 5
replicates of each experiment. As shown in Fig. 2B, PDS inhibited SKOV3 migration in a
time-dependent manner. Quantitatively, SKOV3 cell viability was
decreased by ~40% following 24 h treatment by 0.2 mg/ml of PDS
(Fig. 1B), suggesting the effect of
inhibitory SKOV3 migration was not due to cell death.
Tube structure formation is inhibited
by PDS in SKOV3 cells
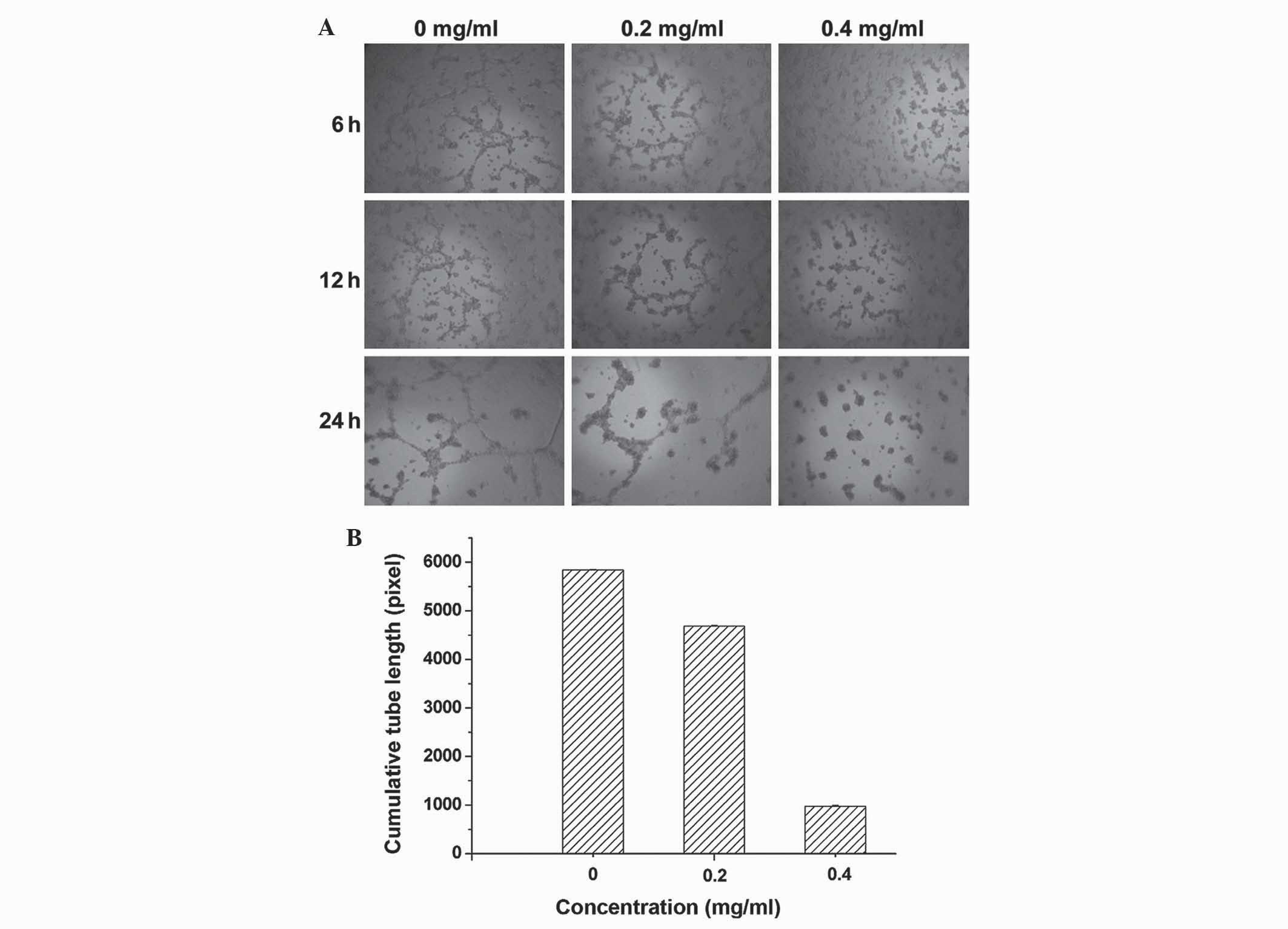
Following incubation of SKOV3 cells in
Matrigel™-coated 96-well plates for 6 h with medium, the results
demonstrate that untreated SKOV3 cells formed well-organized
tube-like structures, while PDS incubated cells formed
significantly fewer and shorter tubes (Fig. 3A). When the concentration of PDS was
increased from 0.2 to 0.4 mg/ml, PDS incubation significantly
reduced the average summated length of capillary tubes
(P<0.005). Therefore, SKOV3 cell tube structure formation was
inhibited by PDS (Fig. 3B).
Effects of PDS on protein localization
of OPN
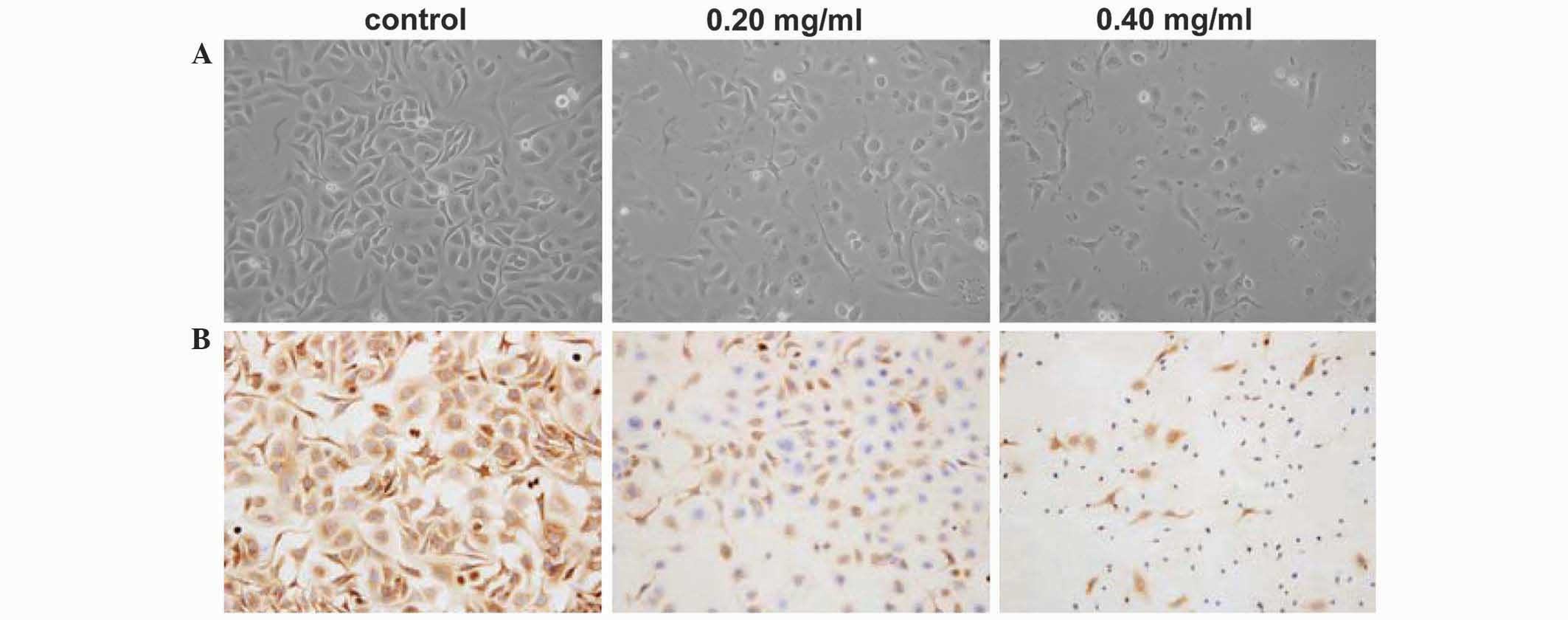
Immunohistochemical analysis was used to evaluate
the expression of OPN in SKOV3 cells. Compared with control cells,
PDS-treated cells exhibited a number of morphological changes
(Fig. 4A). In particular, the cells
shrunk, lost cytoplasm volume and had ‘naked nuclei’ following 24 h
of 0.4 mg/ml PDS treatment. OPN was weakly-expressed in PDS-treated
cell groups, and the expression was PDS dose-dependent, indicating
that the expression of OPN protein was inhibited by PDS (Fig. 4B). The expression of OPN protein was
mostly in the cytoplasm of control cells with yellow or brown
yellow staining.
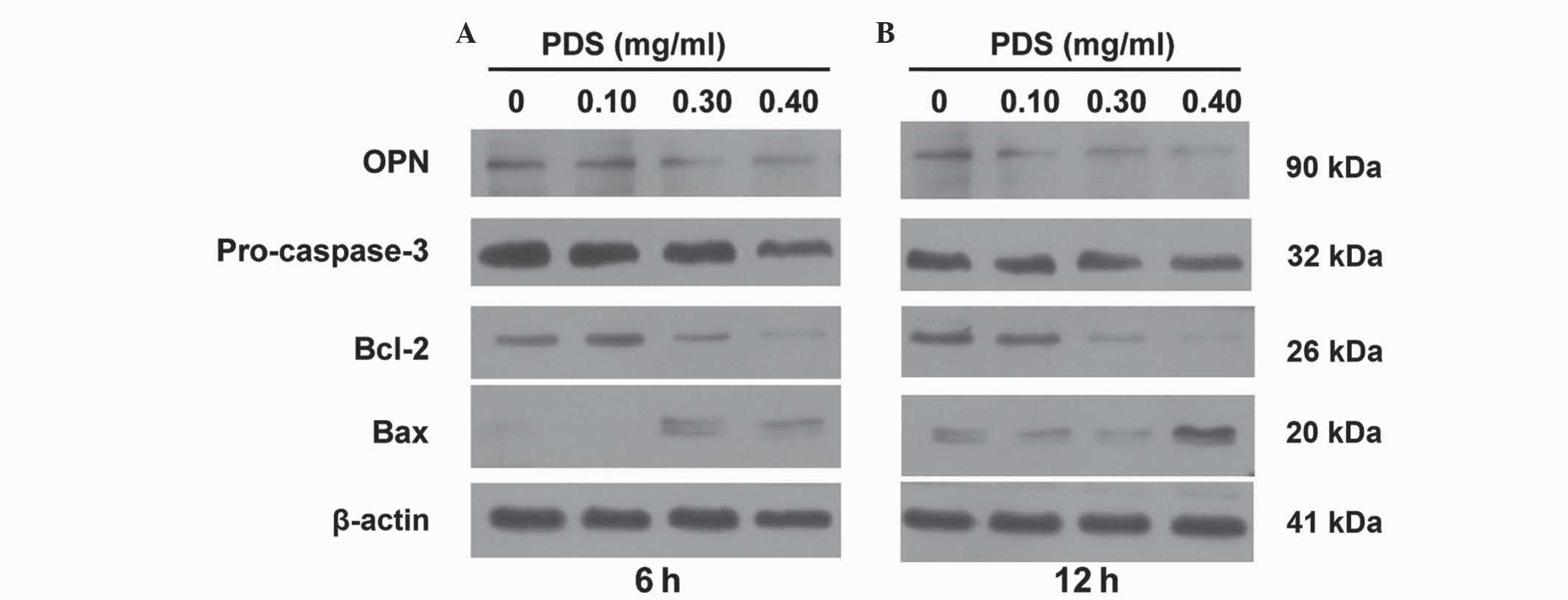
Regulation of the expression of
several proteins
The changes in protein expression levels following
PDS treatment was assessed. The levels of OPN protein were
visualized using western blot analysis with protein extracts from
PDS-treated cells. The results demonstrate that PDS treatment led
SKOV3 cells to downregulate OPN protein expression (Fig. 5). The migration of SKOV3 ovarian
cancer cells was significantly inhibited by PDS, and this may be
associated with the observed reduction in OPN expression.
Furthermore, the protein expression levels of pro-caspase-3, Bcl-2
and Bax were investigated, which are important in regulating and
initiating apoptosis in ovarian cancer (11,12). As
shown in Fig. 5, the pro-caspase-3
and Bcl-2 protein expression levels were downregulated by PDS and
Bax was upregulated. This has also been reported by Park et
al (5). Therefore, PDS is a
potent antitumor molecule that may be developed as a cancer
therapeutic agent.
Discussion
The present study demonstrates that the migration of
SKOV3 ovarian cancer cells was inhibited by dammarane glycoside
20(S)-protopanaxadiol, extracted from Panax notoginseng, and
this was accompanied by a reduction in the expression of OPN
protein in these cells.
Cell migration and invasion are fundamental aspects
of cancer cell growth (13),
requiring that neighbouring tumor cells loosen their intercellular
junctions and that the ECM is proteolytically degraded (14). Cell migration and invasion are
particularly important processes in ovarian cancer progression and
have gained widespread attention (15). One such marker of tumor invasion and
metastasis, OPN, may inhibit malignant tumor invasion and
metastasis when its expression is suppressed (16). It has been reported previously that
OPN downregulation significantly inhibits OPN protein expression in
MDA-MB-231 breast cancer cells, reduces cell proliferation, and
increases cell sensitivity to doxorubicin (17). The present study reveals that PDS
decreased OPN protein in a dose-dependent manner. It is reasonable
to postulate that the downregulation of OPN protein by PDS may also
decrease cell-matrix adhesion. Therefore, if the factors that bind
to these regulatory elements were blocked, the migration of SKOV3
cells could possibly be inhibited. Furthermore, PDS may not only be
a potential agent for ovarian cancer treatment, but may also be
applicable in preventing primary or metastatic tumors. Furthermore,
pro-caspase-3 and Bcl-2 were observed to be downregulated by PDS
and Bax was upregulated in the present study, which was also
reported by Park et al (5).
In summary, the metastasis of ovarian cancer was
significantly inhibited by PDS, partially due to the inhibition of
tumor-induced cell invasion and migration. This may be associated
with a decrease of OPN expression in the ovarian cancer cells.
Acknowledgements
The present study was financially supported by
Yunnan Educational Committee Grant (grant no. 09ZZ113) and Key
Project of the Applied Basic Research Programs of the Science and
Technology Department of Yunnan Province (grant no. 2013FC006). The
authors would like to thank Mr. Lloyd Zhao (School of Medicine,
Duke University, Durham, NC, USA) for revising the English language
of the manuscript.
References
|
1
|
Banerjee S and Gore M: The future of
targeted therapies in ovarian cancer. Oncologist. 14:706–716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang B, Wang C, Han Y, Hu X, Zheng L and
Zhao Y: Isolation and identification of minor bioactive saponins
from the leaves of Panax notoginseng. Zhong Yao Cai.
27:489–491. 2004.(In Chinese). PubMed/NCBI
|
|
3
|
Sun H, Yang Z and Ye Y: Structure and
biological activity of protopanaxatriol-type saponins from the
roots of Panax notoginseng. Int Immunopharmacol. 6:14–25.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Popovich DG and Kitts DD:
Structure-function relationship exists for ginsenosides in reducing
cell proliferation and inducing apoptosis in the human leukemia
(THP-1) cell line. Arch Biochem Biophys. 406:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SC, Yoo HS, Park C, Cho CK, Kim GY,
Kim WJ, Lee YW and Choi YH: Induction of apoptosis in human lung
carcinoma cells by the water extract of Panax notoginseng is
associated with the activation of caspase-3 through downregulation
of Akt. Int J Oncol. 35:121–127. 2009.PubMed/NCBI
|
|
6
|
Popovich DG and Kitts DD: Ginsenosides
20(S)-protopanaxadiol and Rh2 reduce cell proliferation and
increase sub-G1 cells in two cultured intestinal cell lines,
Int-407 and Caco-2. Can J Physiol Pharmacol. 82:183–190. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen G, Yang M, Nong S, Yang X, Ling Y,
Wang D, Wang X and Zhang W: Microbial transformation of
20(S)-protopanaxadiol by Absidia corymbifera. Cytotoxic
activity of the metabolites against human prostate cancer cells.
Fitoterapia. 84:6–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tilli TM, Franco VF, Robbs BK, Wanderley
JL, da Silva FR, de Mello KD, Viola JP, Weber GF and Gimba ER:
Osteopontin-c splicing isoform contributes to ovarian cancer
progression. Mol Cancer Res. 9:280–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei S and Lu XY: Effects of RNA
interference targeting OPN on the in vitro proliferation of human
ovarian cancer cell line HO-8910PM. Acta Acad Med Sinicae XuZhou.
30:504–510. 2010.(In Chinese).
|
|
11
|
Zhang X, Samadi AK, Roby KF, Timmermann B
and Cohen MS: Inhibition of cell growth and induction of apoptosis
in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural
withanolide Withaferin A. Gynecol Oncol. 124:606–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao
L, Liu J, Liu S, Xiao J, Chen X, Zhang X and Wang H: Paris saponin
II of Rhizoma raridis- a novel inducer of apoptosis in human
ovarian cancer cells. Biosci Trends. 6:201–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Comoglio PM and Trusolino L: Invasive
growth: From development to metastasis. J Clin Invest. 109:857–862.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang L and Li Y: Inhibitory effects of
natural food dyes genistein on invasion of SKOV3 human ovarian
carcinoma cells in vivo and in vitro. Adv Mat Res 781–784.
1152–1155. 2013. View Article : Google Scholar
|
|
16
|
Kim JH, Skates SJ, Uede T, Wong KK,
Schorge JO, Feltmate CM, Berkowitz RS, Cramer DW and Mok SC:
Osteopontin as a potential diagnostic biomarker for ovarian cancer.
JAMA. 287:1671–1679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Wei L, Zhao W, Wang X, Zheng G,
Zheng M, Song X and Zuo W: Down-regulation of osteopontin
expression by RNA interference affects cell proliferation and
chemotherapy sensitivity of breast cancer MDA-MB-231 cells. Mol Med
Rep. 5:373–376. 2012.PubMed/NCBI
|