Introduction
In basic and applied research, related to lung
cancer pathogenesis and antitumor drugs, animal models have a
central role (1,2). Mouse, rat, hamster and rabbit and other
rodents have been used to establish a variety of animal models in
the study of lung cancer (1–3). However, these animals are not similar to
human and are different with regard to living environment, and
social psychological status (3).
Tupaia belangeri, the tree shrew from the Yunnan province in
China, is a fast breeding, small animal that is easy to domesticate
and simple to breed. The metabolic and anatomic structure of the
tree shrew is closer to human than the dog, rat and other animal
models, and has a unique advantage as an animal model for human
diseases (4–9).
Previous studies showed that the rat lung cancer
model established using 3-methylcholanthrene (3-MC) and
diethylnitrosamine (DEN) provided investigators with a better
description of gene modification in precancerous and invasive
lesions of human lung cancer (10,11).
In the present study, the tree shrew was selected as
the animal model due to its resemblance with regard to human
structure, function and metabolism. Lung cancer was induced in tree
shrews with one-time instillation of the iodized oil suspension of
3-MC and DEN. Through observation of the tree shrews survival and
tumor formation rates, we selected the best experimental conditions
for developing the best tree shrew lung cancer model, to provide a
new type of experimental animal model for the study of lung
cancer.
Materials and methods
Experimental materials
Tree shrews from the Yunnan region were selected for
the present study. A total of 80 healthy specific pathogen-free
sub-generation tree shrews were provided by Kunming Institute of
Zoology of the Chinese Academy of Sciences (Kunming, China). There
were 40 female and 40 male animals, with an average age of 6 months
± 7 days, and a weight of 120–160 g. In addition, 3-MC (Sigma, St.
Louis, MO, USA); DEN (Sigma); and iodized oil injection (Shanhai
Xudong Haipu Pharmaceutical Co. Ltd., Shanghai, China) were
purchased for the present study.
Experimental method
Experiment and animal grouping
Experimental groups were set-up and exposed to four
different concentrations of iodized oil suspension of 3-MC and DEN
(Table I). The blank control group
received an equal volume of normal saline, and the solvent control
group received lipiodol solvent. The experiments were divided into
six groups. Eighty tree shrews were randomly divided into six
groups. Each of the four experimental groups had 15 animals, and
the two control groups had 10 animals each. During the experiment
the tree shrews were kept in a laboratory animal environment of
specific pathogen-free at room temperature (20–25°C) and received
adequate amounts of food and water.
 | Table I.Components dosage form of 0.1 ml
lipiodol suspension in each experimental group. |
Table I.
Components dosage form of 0.1 ml
lipiodol suspension in each experimental group.
| Experimental
group | 3-Methylcholanthrene,
mg | Diethylnitrosamine,
ml | Iodized oil, ml |
|---|
| 1 | 10 | 0.01 | 0.09 |
| 2 | 20 | 0.01 | 0.09 |
| 3 | 10 | 0.02 | 0.08 |
| 4 | 20 | 0.02 | 0.08 |
Replicate animal model
After the injection of ketamine and atropine, the
skin in the neck region was cut, and thyroid cartilage was exposed.
In the upper part of the thyroid cartilage, a special needle
(comprised of 1 ml syringe needle, designed to be 30°) was
punctured into the left inferior pulmonary lobe. Tree shrews in
each group received a one-time infusion of 0.1 ml iodized oil
suspension, saline or iodized oil solvent containing different
doses of 3-MC and DEN. Doses received by animals in each
experimental group are shown in Table
I.
Experimental animal exclusion criteria
Animals did not become ill and had no infectious or
non-infectious diseases during the course of the experiment.
General observation of tree shrews
Animal weights in the experimental groups were
measured in the 1st, 3rd, 5th, 7th, 9th and 11th weeks. The
activity and survival of tree shrews were monitored closely.
Lung imaging of tree shrews
Tree shrews in the experimental groups underwent
X-ray examinations in the 3rd, 5th, 7th, 9th and 11th weeks.
Radiographic changes within the chest area were recorded.
Tumor detection of tree shrews
Tree shrews in the experimental groups were
sacrificed by cervical dislocation and lung tissue pathology
studies were performed in the 3rd, 5th, 7th, 9th and 11th weeks.
Light microscope (ECLIPSE E800, Nikon Corporation, Japan)
hematoxylin and eosin (H&E) staining was used to visualize
bronchial epithelial pathological changes and tumors.
The present study was approved by the institutional
research ethics committee of The Tumor Hospital of Yunnan Province
(no. ky201110).
Statistical analysis
The SPSS 17.0 software package (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. The results were
presented as mean ± standard deviation. The paired t-test was used
to pair the mean number of the design data. Skewed distribution
data were assessed by the rank sum test. P<0.05 was considered
to indicate a statistically significant difference.
Results
General
During the experiment, the body weights of the tree
shrews in the control group did not change significantly, whereas
in the experimental group the body weights decreased gradually.
Weight measurement comparisons between the two groups in the 5th,
7th, 9th and 11th weeks demonstrated statistically significant
differences (P<0.05) (Table
II).
 | Table II.Comparison of weight changes in tree
shrews at different time points in the experimental and control
groups (g, mean ± standard deviation). |
Table II.
Comparison of weight changes in tree
shrews at different time points in the experimental and control
groups (g, mean ± standard deviation).
| Experimental
grouping | Control group | Experimental
group | P-value |
|---|
| 1 week | 146.00±5.78 | 147.55±12.56 | P>0.05 |
| 3 week | 148.00±4.70 | 135.45±11.34 | P<0.05 |
| 5 week | 147.00±8.04 | 134.23±12.26 | P<0.05 |
| 7 week | 149.00±7.67 | 133.45±13.17 | P<0.05 |
| 9 week | 152.00±8.72 | 132.67±12.78 | P<0.05 |
| 11 week | 148.00±6.58 | 130.34±11.78 | P<0.05 |
The survival rate of the tree shrews in the blank
control group until the end of the experiment was 100%. In the
solvent control group, 8 of 10 animals (80% survival rate). The
death of the two tree shrews (20%) was confirmed to be associated
with puncture-induced hemopneumothorax (Tables III and IV).
 | Table III.Comparison of the survival rates for
tree shrews in the experimental and control groups. |
Table III.
Comparison of the survival rates for
tree shrews in the experimental and control groups.
| Group | Quantity for
experiment, no. | Surviving quantity,
no. | Survival rate, % |
|---|
| Blank control | 10 | 10 | 100.0 |
| Solvent control | 10 | 8 | 80.0 |
| Experimental | 60 | 23 | 38.3 |
 | Table IV.Comparison of the survival rates for
tree shrews with different doses of drug perfusions in each
experimental group. |
Table IV.
Comparison of the survival rates for
tree shrews with different doses of drug perfusions in each
experimental group.
| Experimental
group | Quantity for
experiment, no. | Surviving quantity,
no. | Survival rate, % |
|---|
| 1 | 15 | 14 | 93.3 |
| 2 | 15 | 5 | 33.3 |
| 3 | 15 | 4 | 26.7 |
| 4 | 15 | 0 | 0.0 |
In each experimental group, mortalities started from
the second day following perfusion, and deaths occurred mostly
within 3–7 days after the drug perfusion. The survival rate in each
experimental group was negatively correlated with the infusion
doses (Table IV). In experimental
group 4, the 15 tree shrews died within 2–7 days after drug
infusion and the survival rate was 0%. Obvious lung congestion was
observed during the autopsies and deaths were confirmed to be due
to the lethal drug toxicity.
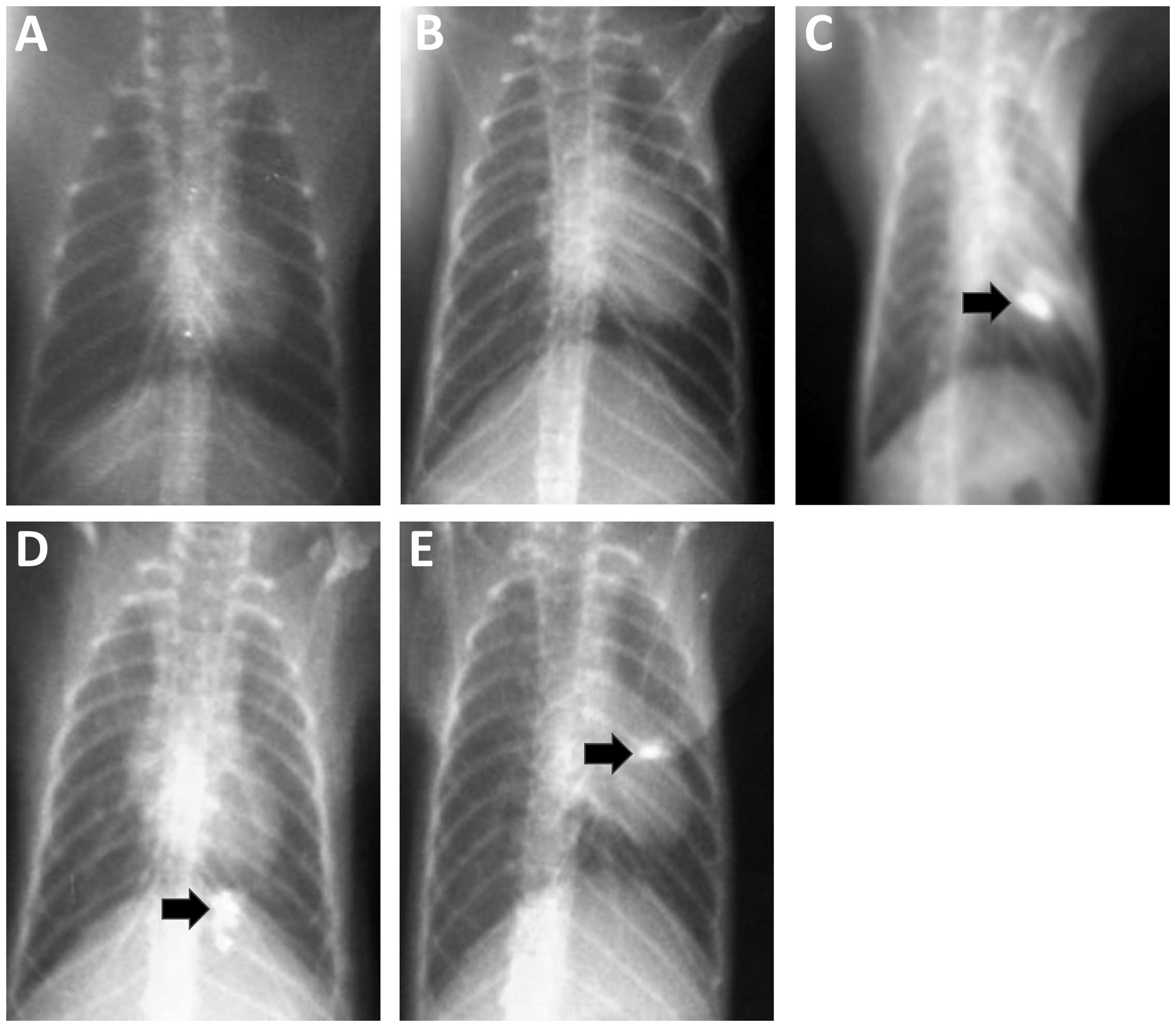
Lung imagings of tree shrews
The results from the chest X-ray examinations
conducted on animals in the blank control and solvent control
groups at each time point revealed no obvious abnormalities
(Fig. 1A and B). In the experimental
group, the chest X-ray examination results for tree shrews in the
3rd, 7th and 11th weeks after perfusion showed focals of
high-density shadow spots within the perfusion sites (Fig. 1C and E). We considered these
high-density shadows to be the product of lipiodol incomplete
metabolism. Pathological studies, performed on the high-density
areas, confirmed changes in the bronchial epithelium.
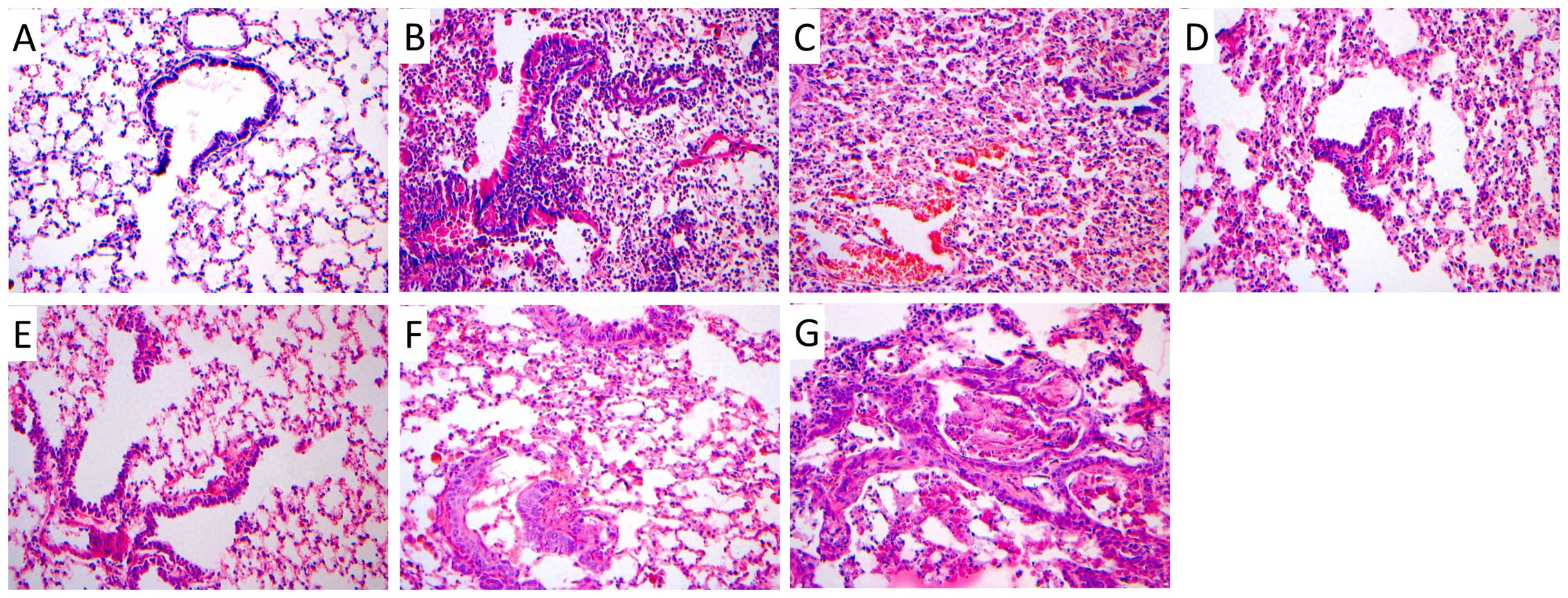
Tumor results of the tree shrews
H&E staining was conducted on lung tissues
collected from tree shrews in each group. We observed no obvious
abnormal pathological changes in the bronchial epithelial structure
in lung tissues from tree shrews in the blank control and solvent
control groups at different time points (Fig. 2A). The results from the H&E
staining conducted on tissues from the experimental groups in the
3rd week revealed lung tissue congestion. We observed inflammatory
changes, hemorrhage and bronchial epithelial exfoliation
pathological changes (Fig. 2B and C).
In the 5th, 7th, 9th and 11th weeks, we detected mild to severe
atypical hyperplasia in bronchial epithelium (Fig. 2D and F), and even some visible early
invasive carcinoma (Fig. 2G).
Bronchial epithelial changes were accompanied by inflammation and
haemorrhagic manifestations. In the same lung tissue sections, we
detected alterations in all the stages. The occurrence of the
pathological alterations observed in lung tissues collected from
animals in the experimental groups is shown in Table V. In experimental group 1, 12 tree
shrews were identified with bronchial epithelial atypical
hyperplasia and 2 with apparent changes in carcinoma in
situ.
 | Table V.Pathological changes detected in lung
tissues collected from the animals in the experimental group. |
Table V.
Pathological changes detected in lung
tissues collected from the animals in the experimental group.
|
| Dysplasia |
|
|---|
|
|
|
|
|---|
| Group | Mild | Moderate | Severe | Carcinoma in
situ |
|---|
| Experimental group
1 | 1 | 1 | 10 | 2 |
| Experimental group
2 | 0 | 1 | 3 | 1 |
| Experimental group
3 | 0 | 1 | 2 | 1 |
Discussion
Mouse, rat, hamster, rabbit and other rodent animal
models have been employed for lung cancer studies (1–3). Rodents,
to some extent, can simulate human lung cancer occurrence and the
development process of lung tumors. However, due to species
variation, there is a big difference in living environment, cause
of disease and social psychological status between rodent and human
(2,12). Currently, there is no ideal animal
model for lung cancer and the lack of an ideal animal model is one
of the most important obstacles for lung cancer researchers. Thus,
it is imperative to identify an effective and accurate model for
lung cancer studies.
More evolved animals have more complex organ
structures and functions. More complex organs in animals render
them closer to human organs (13,14). To
develop animal models with full simulation, those animals with
closest structure, function and metabolism to human beings should
be selected. Tupaia belangeri or tree shrew with some
metabolic and anatomic similarities to human has been widely used
in biomedical research (4–6). Tree shrews are phylogenetically close to
primates and have some primitive primate characteristics. The tree
shrew may therefore be regarded as a primate model. In addition,
the tree shrew has been considered the intermediary ring connecting
the insectivorous and primate taxa (4). Physiological functions of tree shrews
are close to those of human, and may therefore be suitable to be
used as animal models for human diseases (7–9).
Currently, the tree shrew is mainly used for the construction of
hepatitis B virus, hepatocellular carcinoma, myopia and the social
psychological stress model (6,14–15). To the best of our knowledge, no study
has been conducted on the tree shrew as a model for the study of
lung cancer.
The rat lung cancer model established by 3-MC+DEN is
the model of choice for investigators working in this field
(9,10). Induction of the disease by perfusing
rats with ipiodol suspension liquid of 3-MC+DEN leads to
morphological changes in the airway epithelium and the development
of invasive squamous cell carcinoma. The development of squamous
metaplasia, atypical hyperplasia, early invasive carcinoma, and
extensive infiltration of highly differentiated squamous cell
carcinoma may be observed (9,10). In addition, induction of lung cancers
may contain highly and poorly differentiated squamous cell
carcinoma at the same time. This improved animal model of lung
cancer had a high success rate, was cost-effective and had a short
induction time. As a single factor induced the experimental animal
model, it provided a more suitable method for lung cancer
mechanisms, tumor immunity and anticancer treatment studies
(9,10).
In the present study, we aimed to develop a suitable
animal model for lung cancer research using tree shrews from the
Yunnan region in China. Several physiological and structural
similarities between this animal and human led to investigation of
the possibility of using the tree shrew as our animal model. We
successfully induced lung cancer in tree shrews by one-time
endotracheal introduction of iodized oil suspension of 3-MC and
DEN.
The results of the chest X-ray H&E staining
confirmed those findings. As a result, it is plausible to consider
the tree shrew as a new animal model in future lung cancer
studies.
Acknowledgements
The present study was funded by the National Natural
Science Fund (nos. U1202224 and 81460278) and the Natural Science
Foundation of Yunnan Province (nos. 2013FZ276 and 2014FA048).
References
|
1
|
Westcott PM, Halliwill KD, To MD, Rashid
M, Rust AG, Keane TM, Delrosario R, Jen KY, Gurley KE, Kemp CJ, et
al: The mutational landscapes of genetic and chemical models of
Kras-driven lung cancer. Nature. 517:489–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwon MC and Berns A: Mouse models for lung
cancer. Mol Oncol. 7:165–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bach PB: Is our natural-history model of
lung cancer wrong? Lancet Oncol. 9:693–697. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XH, Dai ZX, Zhang GH, Han JB and
Zheng YT: Molecular characterization, balancing selection, and
genomic organization of the tree shrew (Tupaia belangeri)
MHC class I gene. Gene. 522:147–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baldwin MK, Wei H, Reed JL, et al:
Cortical projections to the superior colliculus in tree shrews
(Tupaia belangeri). J Comp Neurol. 521:1614–1632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petruzziello F, Fouillen L, Wadensten H,
Kretz R, Andren PE, Rainer G and Zhang X: Extensive
characterization of Tupaia belangeri neuropeptidome using an
integrated mass spectrometric approach. J Proteome Res. 11:886–896.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amako Y, Tsukiyama-Kohara K, Katsume A,
Hirata Y, Sekiguchi S, Tobita Y, Hayashi Y, Hishima T, Funata N,
Yonekawa H, et al: Pathogenesis of hepatitis C virus infection in
Tupaia belangeri. J Virol. 84:303–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Chang Q, Zhang Y, Zou X, Chen L,
Zhang L, Lv L and Liang B: Relationships between body weight,
fasting blood glucose concentration, sex and age in tree shrews
(Tupaia belangeri chinensis). J Anim Physiol Anim Nutr
(Berl). 97:1179–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhattacharya S and Haldar PK:
Chemopreventive property of Trichosanthes dioica root
against 3-methylcholanthrene-induced carcinogenesis in albino mice.
J Environ Pathol Toxicol Oncol. 31:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta C, Vikram A, Tripathi DN, Ramarao P
and Jena GB: Antioxidant and antimutagenic effect of quercetin
against DEN induced hepatotoxicity in rat. Phytother Res.
24:119–128. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thuy TT, Morita T, Yoshida K, Wakasa K,
Iizuka M, Ogawa T, Mori M, Sekiya Y, Momen S, Motoyama H, et al:
Promotion of liver and lung tumorigenesis in DEN-treated
cytoglobin-deficient mice. Am J Pathol. 179:1050–1060. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudyanto RD, Bastarrika G, de Biurrun G,
Agorreta J, Montuenga LM, Ortiz-de-Solorzano C and Muñoz-Barrutia
A: Individual nodule tracking in micro-CT images of a longitudinal
lung cancer mouse model. Med Image Anal. 17:1095–1105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coppola A and Moshé SL: Animal models.
Handb Clin Neurol. 107:63–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miziara ID, Magalhães AT, Santos Md, Gomes
EF and Oliveira RA: Research ethics in animal models. Braz J
Otorhinolaryngol. 78:128–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Zhou QX, Lv LB, Xu L and Yang YX:
A depression model of social defeat etiology using tree shrews.
Dongwuxue Yanjiu. 33:92–98. 2012.(In Chinese). PubMed/NCBI
|