Introduction
Patients with non-small cell lung cancer (NSCLC)
possess a high risk of developing brain metastases. The incidence
rate of brain metastases for patients with lung cancer is 23–65%
(1). Once intracerebral metastases
develop, the prognosis is poor. The median survival time for
patients with untreated brain metastases is only ~1 month (2,3). The
principle therapeutic modality for brain metastases is whole-brain
radiation therapy (WBRT), and the median survival time following
this treatment may increase to 4–6 months. Although therapies,
including surgery, WBRT, stereotactic radiotherapy and systematic
chemotherapy, are rapidly improving, the prognosis of patients with
brain metastases from lung cancer remains poor, and the median
survival time for patients remains at ~6.5–10 months (4–7).
In 2004, the genes encoding the read code box of
epidermal growth factor receptor (EGFR) were sequenced (8). In addition, the gene mutation status has
been elucidated and is now widely used in clinical practice. As a
result, the identification of the EGFR mutation and the
introduction of treatment with EGFR-tyrosine kinase inhibitors
(EGFR-TKI) has improved clinical outcomes (9). EGFR-TKI regimens have a good efficacy
against brain metastases in NSCLC. In previous years, increasing
efforts have been made to understand and use prognostic indicators
in patients with brain metastases from NSCLC (9,10).
However, the prognostic factors of EGFR mutation-positive NSCLC
patients with brain metastases following WBRT have not been studied
extensively.
Based on the aforementioned considerations, the
purpose of the present study was to analyze and assess the
prognostic factors in EGFR mutation-positive NSCLC patients with
brain metastases following WBRT and EGFR-TKI treatment, using the
classification tree and Cox proportional hazards regression
models.
Materials and methods
Patients
Between January 2005 and July 2014, 66 EGFR
mutation-positive patients diagnosed with NSCLC were identified
with brain metastases and treated at the Department of Radiation
Oncology and Chemotherapy at The First Affiliated Hospital of
Wenzhou Medical University (Wenzhou, China). The criteria for
inclusion in the present retrospective study were as follows: i)
All patients possessed a histopathological diagnosis of NSCLC,
acquired by bronchial biopsy, fine needle aspiration biopsy or a
surgical excision specimen; ii) all brain metastasis diagnoses were
confirmed by head magnetic resonance imaging (MRI),
contrast-enhanced computed tomography (CT) or positron emission
tomography (PET)/CT scans; iii) the EGFR gene mutation was
detected; iv) all patients were treated with EGFR-TKI until the
disease progressed or the toxicity was intolerable; v) all patients
were treated with WBRT (typically 30 gray units per 10 fractions);
vi) all survival data were up to date on July 31, 2014; and vii)
the clinical data of all patients were complete. In total, 66
patients were available for the present analysis, 34 of which were
males and 32 were females. The median age at the diagnosis of brain
metastasis was 61 years old (range, 38–82 years). A total of 11
(16.7%) patients possessed squamous cell lung carcinoma, while 55
(83.3%) patients possessed adenocarcinoma.
Study design
The first section of the present study was a
retrospective description regarding the recent therapeutic effects
and long-term treatment effects observed in the patients. The
second section of the study was a multivariate analysis that
followed a univariate analysis, including 14 prognostic factors,
using Cox proportional hazards regression. The final section of the
study was a classification tree model.
The following variables were examined to determine
the prognostic value for EGFR mutation-positive NSCLC patients with
brain metastases following WBRT and EGFR-TKI treatment: i) Age at
diagnosis of brain metastasis (for statistical purposes, the
patients were classified into two age groups, <65 vs. ≥65
years); ii) gender (male vs. female); iii) Eastern Cooperative
Oncology Group performance status (ECOG PS; PS ≤2 vs. >2
points); iv) histopathology (adenocarcinoma vs. squamous cell
carcinoma); v) primary tumor node metastasis (TNM) stage (I–III vs.
IV stage); vi) smoking (heavy vs. no/little); vii) history of
pulmonary lesions radiotherapy (with vs. without); viii) history of
pulmonary lesions surgical resection (with vs. without); ix)
cisplatin-based chemotherapy (with vs. without); x) number of brain
metastases (single vs. multiple); xi) extracranial metastases (with
vs. without); xii) carcinoembryonic antigen (CEA) levels at brain
metastases diagnosis (for statistical purposes, the patients were
classified into two groups, ≤10 µg/ml vs. >10 µg/ml); xiii) the
status of the primary tumor (controlled vs. uncontrolled); and xiv)
supportive chemotherapy (yes vs. no). No/little smoking was defined
as a smoking index (SI) of <200 (11). SI was defined as the number of
cigarettes smoked per day multiplied by the number of years
smoked.
Evaluation
The therapeutic effects were evaluated using the
RECIST 1.1 criteria (12). The
therapeutic effects may be divided into complete response (CR),
partial response (PR), stable disease (SD) and progressive disease
(PD). The objective response rate (ORR) refers to the percentage of
CR+PR patients out of the total number of patients, and the disease
control rate (DCR) refers to the percentage of CR+PR+SD patients
out of the total number of patients. The long-term treatment
effects were evaluated by recording the progression free survival
(PFS) and overall survival (OS) rates. PFS was defined as the
interval between the diagnosis of brain metastasis and the initial
observation of PD or mortality from any cause. The OS was measured
between the date of the diagnosis of brain metastasis and the time
of the mortality of the patient or the deadline for the study (July
31, 2014).
Statistical analysis
The clinical data were described by median,
frequency and percentage. Survival analyses for each prognostic
factor were performed using the Kaplan-Meier method, using SPSS
software version 19.0 (IBM SPSS; Armonk, NY, USA). The log rank
test was used in statistical comparisons. The multivariate analysis
was conducted using the Cox proportional hazard regression model.
The classification tree model was subsequently applied. A P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
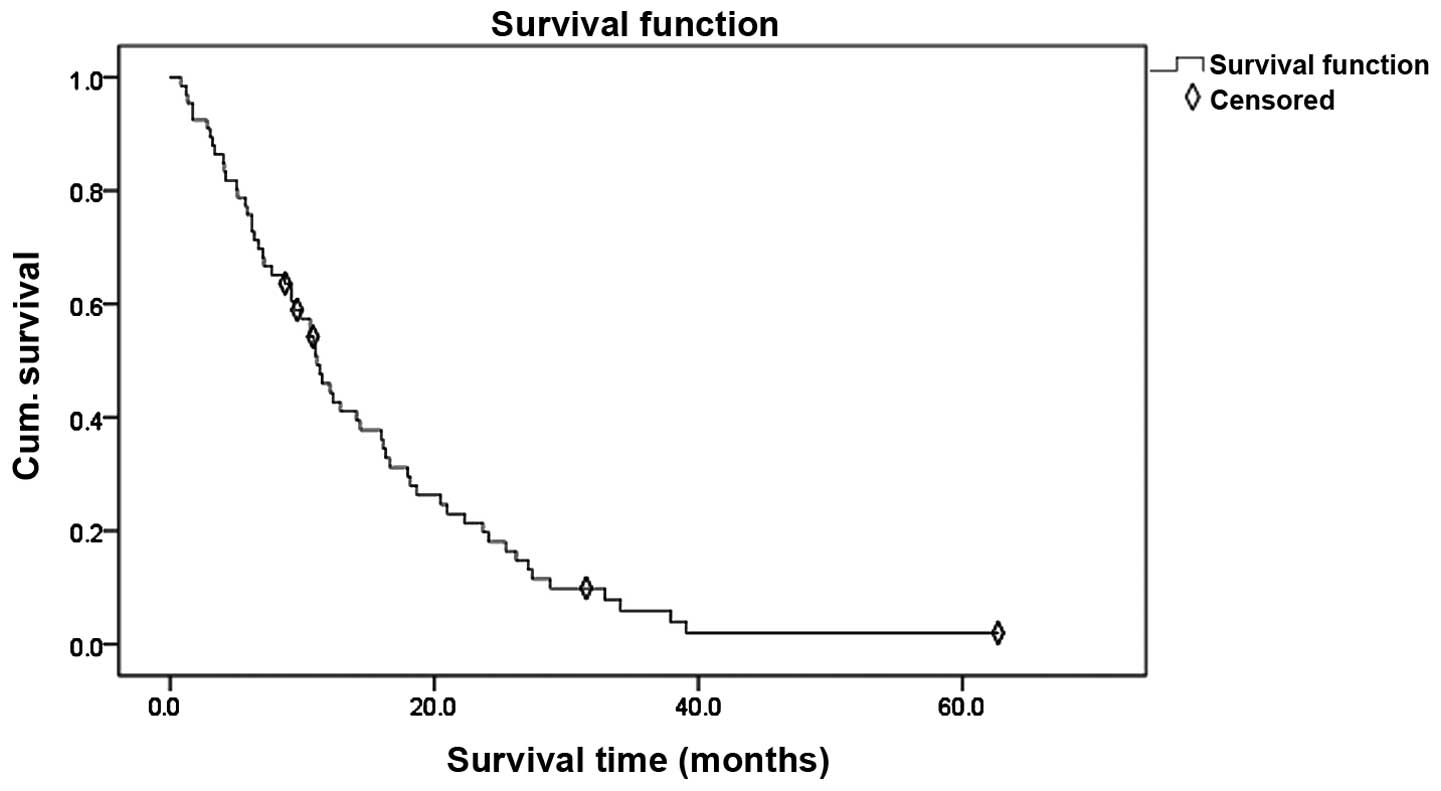
Statistical description
A total of 66 patients were analyzed. Of these, 3
patients achieved CR, 27 patients demonstrated a PR, 25 patients
remained to possess SD and 11 patients developed a PD; therefore,
the patients demonstrated an ORR of 45.5% (30/66) and a DCR of
83.3% (55/66). At the time of analysis, 5 patients (7.58%) were
alive, while 61 patients (92.42%) had succumbed. The median PFS was
5.9 months (95% CI, 4.2–8.8 months) and the median survival time of
the entire cohort was 10.9 months (95% CI, 8.7–14.1 months). The
survival curve for EGFR mutation-positive NSCLC patients with brain
metastases is shown in Fig. 1.
Survival analysis
In the univariate analysis, the following variables
at the diagnosis of brain metastasis were significantly associated
with an improved PFS and OS (P-values, respectively): Age (0.008,
0.028); CEA (0.035, 0.031); and the status of primary tumor (0.015,
0.026). The prognostic factors for PFS and OS in the univariate
analysis are presented in Table
I.
 | Table I.Prognostic factors for PFS and OS in
univariate analysis. |
Table I.
Prognostic factors for PFS and OS in
univariate analysis.
| Prognostic
factors | No. of patients | PFSa | PFS P-value | OSa | OS P-value |
|---|
| Age, years |
|
|
|
|
|
| <65 | 40 | 8.7 | 0.008 | 13.2 | 0.028 |
| ≥65 | 26 | 3.4 |
| 9.0 |
|
| Gender |
|
|
|
|
|
| Male | 34 | 6.9 | 0.481 | 10.1 | 0.929 |
|
Female | 32 | 4.6 |
| 11.0 |
|
| ECOG PS |
|
|
|
|
|
| ≤2 | 48 | 6.7 | 0.424 | 11.4 | 0.348 |
|
>2 | 18 | 3.2 |
| 4.1 |
|
| Primary TNM
stage |
|
|
|
|
|
|
I–III | 15 | 3.3 | 0.314 | 6.2 | 0.125 |
| IV | 51 | 7.1 |
| 11.3 |
|
| Histopathology |
|
|
|
|
|
|
Adenocarcinoma | 55 | 6.2 | 0.341 | 10.9 | 0.455 |
| Squamous
cell carcinoma | 11 | 5.7 |
| 7.7 |
|
| Smoking |
|
|
|
|
|
|
Heavy | 24 | 8.7 | 0.478 | 11.1 | 0.427 |
|
No/little | 42 | 4.6 |
| 10.7 |
|
| Pulmonary lesions
radiotherapy |
|
|
|
|
|
| With | 12 | 4.6 | 0.464 | 9.0 | 0.514 |
|
Without | 54 | 6.1 |
| 10.9 |
|
| Pulmonary lesions
surgical resection |
|
|
|
|
|
| With | 6 | 7.5 | 0.189 | 11.7 | 0.512 |
|
Without | 60 | 5.6 |
| 10.9 |
|
| Cisplatin-based
chemotherapy |
|
|
|
|
|
|
With | 37 | 6.3 | 0.340 | 11.3 | 0.823 |
|
Without | 29 | 3.6 |
|
9.9 |
|
| Brain
metastases |
|
|
|
|
|
|
Single | 22 | 6.0 | 0.538 | 11.2 | 0.715 |
|
Multiple | 44 | 5.4 |
| 10.7 |
|
| Extracranial
metastases |
|
|
|
|
|
|
With | 35 | 6.2 | 0.747 | 11.0 | 0.441 |
|
Without | 31 | 4.4 |
|
9.4 |
|
| CEA, µg/ml |
|
|
|
|
|
|
≤10 | 27 | 9.3 | 0.035 | 16.0 | 0.031 |
|
>10 | 39 | 4.1 |
|
8.7 |
|
| Primary tumor
status |
|
|
|
|
|
|
Uncontrolled | 15 | 2.7 | 0.015 |
6.7 | 0.026 |
|
Controlled | 51 | 7.1 |
| 11.3 |
|
| Supportive
chemotherapy |
|
|
|
|
|
|
Yes | 24 | 5.4 | 0.450 | 11.6 | 0.731 |
| No | 42 | 7.5 |
| 10.6 |
|
In the multivariate analysis, the prognostic
predictors for PFS for EGFR mutation-positive NSCLC patients with
brain metastases were age, CEA and status of the primary tumor
(P=0.006, 0.014 and 0.005, respectively). Age, CEA and status of
the primary tumor were also predictive factors for OS (P=0.009,
0.013 and 0.009, respectively). The results of the prognostic
factors for PFS and OS in the multivariate analysis are summarized
in Tables II and III.
 | Table II.Multivariate analysis of prognostic
factors for progression free survival. |
Table II.
Multivariate analysis of prognostic
factors for progression free survival.
| Variable | B | SE | Wald | Exp (B) | P-value |
|---|
| Age, years | −0.721 | 0.261 | 7.664 | 0.486 | 0.006 |
| CEA, µg/ml | −0.652 | 0.266 | 6.003 | 0.521 | 0.014 |
| Primary tumor
status |
0.872 | 0.312 | 7.807 | 2.391 | 0.005 |
 | Table III.Multivariate analysis of prognostic
factors for overall survival. |
Table III.
Multivariate analysis of prognostic
factors for overall survival.
| Variable | B | SE | Wald | Exp (B) | P-value |
|---|
| Age, years | −0.706 | 0.272 | 6.738 | 0.494 | 0.009 |
| CEA, µg/ml | −0.674 | 0.271 | 6.178 | 0.510 | 0.013 |
| Primary tumor
status |
0.818 | 0.313 | 6.855 | 2.267 | 0.009 |
Classification tree model. Based on the clinical
data, a classification and regression tree method (13,14), a
non-parametric regression method, was used. In addition, the method
selected the automatic depth, which was length of the
classification tree model. The terminal parent and child nodes were
defined as 20 and 10, respectively. The adjusted significance level
was defined as P<0.05 in the splitting and merging of a tree
branch. The nodes were combined into the same group where the
significance level of the statistical difference between the
survival distributions of two terminal nodes was >0.05.
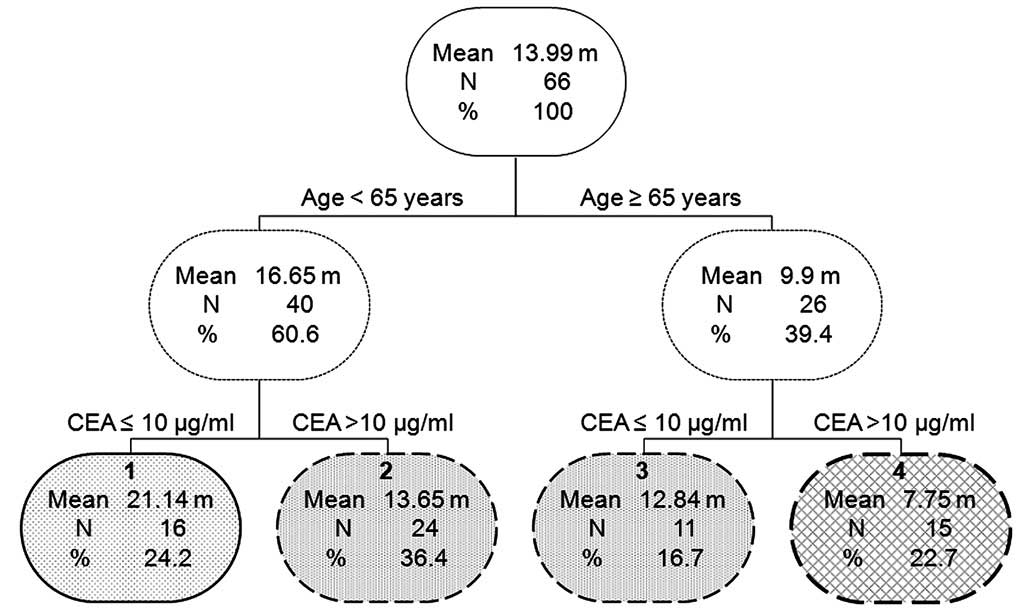
Following the application of the classification tree
model, a survival tree was generated (Fig. 2), in which the first prognostic split
occurred between patients aged <65 years vs. ≥65 years. Within
patients aged <65 years or ≥65 years, the CEA level at the
diagnosis of brain metastasis (≤10 µg/ml vs. >10 µg/ml) became a
dividing factor, finally resulting in four groups. No significant
difference in the survival time between groups 2 and 3 was
identified (P=0.962; Fig. 2).
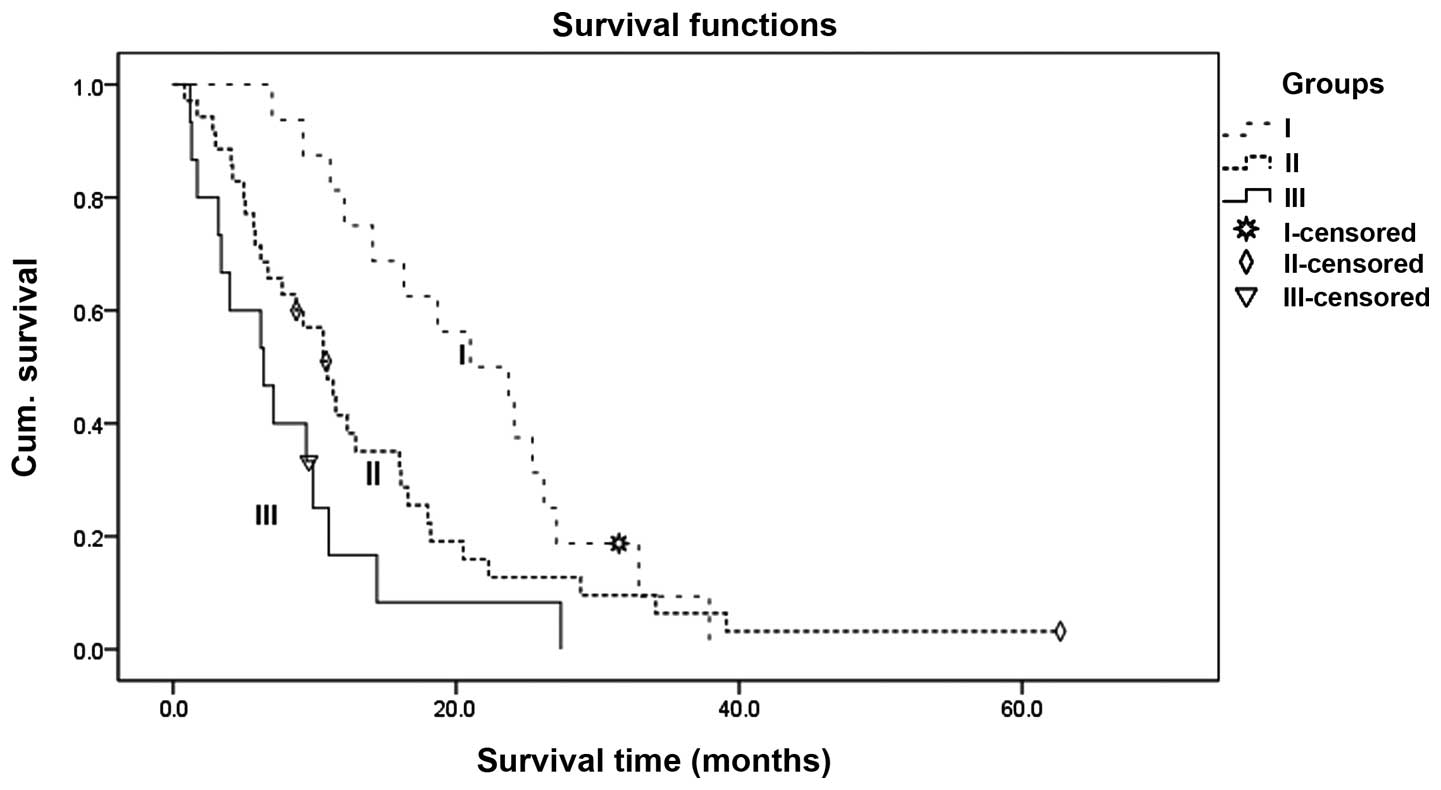
Therefore, groups 2 and 3 were combined. Finally, the patients were
divided into three groups: Group I, age <65 years and CEA ≤0
µg/ml; Group II, age <65 years and CEA >10 µg/ml or age ≥65
years and CEA ≤10 µg/ml; and Group III, age ≥65 years and CEA
>10 µg/ml. The survival curves for the three groups that were
determined by classification tree analysis are shown in Fig. 3 (P=0.004).
Discussion
The brain is one of the most common sites of
metastasis in patients with lung cancer, and lung cancer is the
most common intracranial metastatic tumor (15). The incidence of brain metastases in
lung cancer is 20% at diagnosis and 40% at autopsy (16). In addition, brain metastases
contribute to increased morbidity and mortality and herald a poor
prognosis in patients with metastatic lung cancer (16). WBRT continues to be an important
palliative treatment option for patients with brain metastases from
NSCLC. In previous studies, WBRT combined with EGFR-TKI treatment
was demonstrated to be a safe and effective treatment for EGFR
mutation-positive NSCLC patients with brain metastases, with a
median survival time of 7.7–13.0 months (9,17,18). The median survival time in the present
analysis was 10.9 months, which confirms these expectations.
Predicting the survival time of patients is important, and the fact
that a significant percentage of patients have a limited survival
time suggests that accurate survival prediction models may assist
in avoiding overtreatment (19,20).
In 1997, the Radiation Therapy Oncology Group
established the prognostic scores recursive partitioning analysis
(RPA) classification, which was the first prognostic scoring system
for assessing the prognosis of patients with brain metastases
(21–23). The detailed parameters of the model
contained age, Karnofsky performance status (KPS), with or without
extracranial metastases and the status of the primary tumor. Later,
other established prognostic scores, including the basic score for
brain metastases, systemic inflammatory response and graded
prognostic assessment, were developed for the general population of
patients with brain metastases.
Previous studies have demonstrated several
prognostic factors in NSCLC patients with brain metastases. Gerosa
et al (23) concluded that the
performance status, age, extracranial metastases and primary tumor
control caused a potential effect on survival. Zindler et al
(24) revealed the prognostic value
of performance status, age, absence of extracranial metastases,
primary tumor site, gender and steroid response for OS. Rotin et
al (25) indicated that the
factors influencing survival were the number of brain metastases
and KPS. Rades et al (26)
revealed that the prior performance status, a younger age and the
absence of extracranial metastases were associated with increased
survival time. Therefore, in numerous published studies, age was
commonly manifested as a prognostic factor. Additionally, patients
having no PD in in the lung tumor was commonly mentioned as a
prognostic factor.
The present Cox proportional hazards regression
analysis of an EGFR mutation-positive NSCLC patient population with
brain metastases confirmed that the prognostic implications of age
(<65 years), CEA (≤10 µg/ml) and primary tumor control were
favorable factors for survival time. Unlike previous studies, the
clinical value of CEA in the prognosis of EGFR mutation-positive
NSCLC patients with brain metastases was indicated to be extremely
important. Recently, Fiala et al (27) showed that an increased level of CEA
and CYFRA21-1 may be associated with a poor outcome for patients
with NSCLC that are treated with erlotinib. These findings indicate
that tumor biomarkers may be used for predicting the effect of
therapy and the prognosis of patients.
Previous studies that investigated the prognostic
factors of patients with brain metastases from NSCLC have indicated
that traditional models focus on assessing the relative prognostic
factors using the Cox proportional hazards regression model. A
previous study indicated that, in combination with Cox proportional
hazards regression, the survival tree method may aid prognostic
analysis (28). To the best of our
knowledge, none of the published prognostic classification models
have involved EGFR mutation-positive NSCLC patients with brain
metastases.
The present study aimed to use the Cox proportional
hazards regression and classification tree models to analyze and
explore prognostic factors in EGFR mutation-positive NSCLC
patients, following WBRT and EGFR-EKI treatment. Age and CEA were
the dominant prognostic factors identified in the classification
tree model. Combining the aforementioned results of the Cox
proportional hazard regression model, age (≥65 years) and CEA
(>10 µg/ml) were considered to be adverse prognostic factors. In
particular, the present analysis succeeded in splitting patients
with brain metastases from EGFR mutation-positive NSCLC into three
groups. The identification of prognostic groups between patients
may provide prognostic information and serve as a basis of
classification for future trials. In addition, the primary tumor
status was indicated to be a prognostic factor in the Cox
proportional hazards regression analysis. However, as the third
dividing factor of the classification tree model, primary tumor
control may be used a good predictor of prognosis, but may not be
as reliable as age and CEA.
Regarding the present study, additional prospective
studies are recommended to be performed in order to increase the
accuracy of the results. Ideally, the sample size would have been
larger. In conclusion, the major prognostic predictors of EGFR
mutation-positive NSCLC patients with brain metastases following
WBRT and EGFR-TKI were age and CEA. Age (≥65 years) and CEA (>10
µg/ml) were considered to be the adverse prognostic factors. In
addition, primary tumor control may be important for predicting
survival.
Acknowledgements
The authors would like to acknowledge the following
people, who have made the completion of the present study possible:
Professor Changlin Zou, for vital guidance and support, and Mr.
Lucheng Zhu, for encouragement and assistance.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
NSCLC
|
non-small cell lung cancer
|
|
WBRT
|
whole-brain radiation therapy
|
|
EGFR-TKI
|
epidermal growth factor
receptor-tyrosine kinase inhibitors
|
References
|
1
|
Kong DS, Lee JI, Nam DH and Park K, Kim
JH, Kim JG, Park JO and Park K: Prognosis of non-small cell lung
cancer with synchronous brain metastases treated with gamma knife
radiosurgery. J Korean Med Sci. 21:527–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimm S, Wampler GL, Stablein D, Hazra T
and Young HF: Intracerebral metastases in solid-tumor patients:
Natural history and results of treatment. Cancer. 48:384–394. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
4
|
Gaspar L, Scott C, RotmaIl M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lagerwaard FJ, Levendag PC, Nowak PJ,
Eijkenboom WM, Hanssens PE and Schmitz PI: Prognostic factors in
patients with brain metastases: A review of 1292 patients. Int J
Radiat Oncol Biol Phys. 43:795–803. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng H, Ma M and Han B: Survival analysis
of 1,742 patients with stage IV non-small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 14:362–366. 2011.(In Chinese). PubMed/NCBI
|
|
7
|
Mekhail T, Sombeck M and Sollaccio R:
Adjuvant whole-brain radiotherapy versus observation after
radiosurgery or surgical resection of one to three cerebral
metastases: Results of the EORTC 22952–26001 study. Curr Oncol Rep.
13:255–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieder N, Bremnes RM and Andratschke NH:
Prognostic scores in patients with brain metastases from non-small
cell lung cancer. Thorac Oncol. 4:1337–1341. 2009. View Article : Google Scholar
|
|
10
|
Abacioglu U, Caglar H, Atasoy BM,
Abdulloev T, Akgun Z and Kilic T: Gamma knife radiosurgery in non
small cell lung cancer patients with brain metastases: Treatment
results and prognostic factors. J BUON. 15:274–280. 2010.PubMed/NCBI
|
|
11
|
Yu PJ, Chen WG, Feng QL, Chen W, Jiang MJ
and Li ZQ: Association between CYP1B1 gene polymorphisms and risk
factors and susceptibility to laryngeal cancer. Med Sci Monit.
21:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gass K, Klein M, Sarnat SE, Winquist A,
Darrow LA, Flanders WD, Chang HH, Mulholland JA, Tolbert PE and
Strickland MJ: Associations between ambient air pollutant mixtures
and pediatric asthma emergency department visits in three cities: A
classification and regression tree approach. Environ Health.
14:582015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rovlias A, Theodoropoulos S and
Papoutsakis D: Chronic subdural hematoma: Surgical management and
outcome in 986 cases: A classification and regression tree
approach. Surg Neurol Int. 6:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong DS, Lee JI, Nam DH and Park K, Kim
JH, Kim JG, Park JO and Park K: Prognosis of non-small cell lung
cancer with synchronous brain metastases treated with gamma knife
radiosurgery. J Korean Med Sci. 21:527–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ebert BL, Niemierko E, Shaffe K and Salgia
R: Use of temozolomide with other cytotoxic chemotherapy in the
treatment of patients with recurrent brain metastases from lung
cancer. Oncologist. 8:69–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arrieta O, Villarreal-Garza C, Zamora J,
Blake-Cerda M, de la Mata MD, Zavala DG, Muñiz-Hernández S and de
la Garza J: Long-term survival in patients with non-small cell lung
cancer and synchronous brain metastasis treated with whole-brain
radiotherapy and thoracic chemoradiation. Radiat Oncol. 6:1662011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma S, Xu Y, Deng Q and Yu X: Treatment of
brain metastasis from non-small cell lung with whole brain
radiotherapy and gefitinib in a Chinese population. Lung Cancer.
65:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lock M, Chow E, Pond GR, Do V, Danjoux C,
Dinniwell R, Lea J and Bezjak A: Prognostic factors in brain
metastases: Can we determine patients who do not benefit from
whole-brain radiotherapy. Clin Oncol (R Coll Radiol). 16:332–338.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang Z, Chen J, Zhang H, Shen L and Wei
Q: Whole brain radiotherapy-based combined modality treatment of
brain metastases from non-small cell lung cancer: A retrospective
analysis of prognostic factors. Oncol Res Treat. 38:35–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaspar LE, Scott C, Murray K and Curran W:
Validation of the RTOG recursive partitioning analysis (RPA)
classification for brain metastases. Int J Radiation Oncol Biol
Phys. 47:1001–1006. 2000. View Article : Google Scholar
|
|
23
|
Gerosa M, Nicolato A, Foroni R, Tomazzoli
L and Bricolo A: Analysis of long-term outcomes and prognostic
factors in patients with non-small cell lung cancer brain
metastases treated by gamma knife radiosurgery. J Neurosurg.
102(Suppl): 75–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zindler JD, Rodrigues G, Haasbeek CJ, De
Haan PF, Meijer OW, Slotman BJ and Lagerwaard FJ: The clinical
utility of prognostic scoring systems in patients with brain
metastases treated with radiosurgery. Radiother Oncol. 106:370–374.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rotin DL, Paklina OV, Kobiakov GL,
Shishkina LV, Kravchenko ÉV and Stepanian MA: Lung cancer
metastases to the brain: Clinical and morphological prognostic
factors. Zh Vopr Neirokhir Im N N Burdenko. 77:24–28. 2013.(In
Russian), discussion 29. PubMed/NCBI
|
|
26
|
Rades D, Schild SE, Lohynska R, Veninga T,
Stalpers LJ and Dunst J: Two radiation regimens and prognostic
factors for brain metastases in non-small cell lung cancer
patients. Cancer. 110:1077–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fiala O, Pessek M, Finek J, Benesova L,
MinariK M, Bortlicek Z and Topolcan O: Predictive role of CEA and
CYFRA21-1 in patients with advanced-stage NSCLC treated with
erlotinib. Anticancer Res. 34:3205–3210. 2014.PubMed/NCBI
|
|
28
|
Schilling C, Mortimer D, Dalziel K, Heeley
E, Chalmers J and Clarke P: Using Classification and Regression
Trees (CART) to identify prescribing thresholds for cardiovascular
disease. Pharmacoeconomics. Nov 17–2015.(Epub ahead of print).
|