Introduction
Cytostatic drug resistance in cancer cells is
defined as multidrug resistance (1).
One of the main mechanisms of drug resistance in cancer cells is
the activation of adenosine triphosphate(ATP)-dependent
transporters, which increase the transmembrane efflux of drugs from
cells, thus decreasing the drug concentration in the cytoplasm.
Consequently, the cell becomes insensitive to the effect of
cytostatic drugs (2). The ATP-binding
cassette (ABC) family is a ubiquitous and important family of
ATP-dependent transporters (3,4). Although
the mechanisms of resistance that affect the ATP-dependent
transporter pathways are not fully elucidated at the molecular
level, P-glycoprotein (P-gp) and multidrug resistance protein 1
(MRP1) are the most important and widely studied members of the ABC
family (5). P-gp is the protein
product of multidrug resistance gene 1 (MDR1), and acts as a drug
efflux pump in cells. P-gp is dependent on two molecules of ATP as
an energy source to export numerous structurally unrelated
chemotherapeutic drugs from the cell to the outside (3). In cells that express increased levels of
P-gp, intracellular drug levels are decreased, which is associated
with a decrease in cytotoxicity (6).
Therefore, novel studies on ATP-dependent transporter pathways may
contribute to preventing drug resistance in cancer.
Cluster of differentiation (CD)38, a 45-kDa antigen
present in the surface of human cells, is a type II transmembrane
glycoprotein that has a short N-terminal cytoplasmic domain and a
long C-terminal extracellular domain (7). The expression of CD38 is used as a
phenotypic marker for the differentiation and activation of T and B
lymphocytes (7–9), in addition to other types of cells such
as erythrocytes (10). CD38 has been
demonstrated to be a bifunctional enzyme with nicotinamide adenine
dinucleotide (NAD)+-glycohydrolase (NADase) and
adenosine diphosphate (ADP)-ribosyl cyclase activities (11,12). The
resulting product of its cyclase activity, cyclic ADP-ribose
(cADPR), has become the focus of several studies due to its role as
an inositol 1,4,5-trisphosphate-independent calcium
(Ca+2) mobilizer (13,14).
Furthermore, CD38 has been revealed to catalyze a base exchange
reaction in which (NAD) phosphate in the presence of nicotinic acid
is converted to nicotinic acid adenine dinucleotide phosphate,
which is a Ca+2 mobilizer (15–17).
Ca2+ acts as a second messenger in the regulation of
signal transduction pathways by modulating kinase activities in
numerous cells (18). Controlled
increases in the intracellular levels of Ca2+ through
the action of Ca+2 pumps, ion channels and
Ca+2 buffering proteins, are important in the regulation
of normal cellular function and cell apoptosis (19). Therefore, the cytotoxic effects of
certain drugs used to treat cancer may occur as a result of an
increase in the intracellular concentration of Ca2+
(20,21).
The present study investigated a
doxorubicin-resistant human chronic myelogenous leukemia (CML) K562
cell line as a model system to evaluate the potential role of CD38
in the development of drug resistance. The results demonstrated
that MRP1 and, in particular, P-gp mediated multidrug resistance in
these cells. K562 cells that were resistant to doxorubicin
expressed high levels of CD38, which was associated with a
transient increase in the levels of cADPR and intracellular
Ca2+ concentration. The effect of specific inhibitors
for P-gp (verapamil) and MRP1 (MK-571) on the cADPR-mediated
release of Ca2+ failed to inhibit the response of cells
to cADPR.
Materials and methods
Cell lines and development of a
resistant subline
Human CML K562 cells were maintained in Dulbecco's
modified Eagle's medium: Nutrient Mixture F-12 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.), L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.) and antibiotics (penicillin/streptomycin; Stemcell
Technologies, Inc., Vancouver, BC, Canada). The cells were
incubated at 37°C in a humidified atmosphere of 5% CO2
in a Heraeus® incubator (Thermo Fisher Scientific, Inc.).
Doxorubicin (Sigma-Aldrich, St. Louis, MO, USA) was applied to the
parental K562 cells in dose increments between 0.1 µM and 1.4 µΜ to
develop the doxorubicin-resistant subline K562/DOX. The adapted
cells were allowed to become confluent in the presence of the
corresponding concentration of drug, and were maintained under
those conditions for 3–4 weeks). Cells that had been selected with
1.4 µΜ doxorubicin were maintained in medium supplemented with 1.4
µΜ doxorubicin for subsequent assays.
Flow cytometry analysis of P-gp, MRP1
and CD38
K562 and K562/DOX cells were centrifuged at 1,500 ×
g and suspended at a cell density of 1×106 cells/ml in
cold phosphate-buffered saline (PBS) prior to be stained for 30 min
at 4°C with the following monoclonal antibodies: Mouse
immunoglobulin (Ig) G anti-human anti-CD38 conjugated to
allophycocyanin (dilution, 1:200 in PBS; catalog no, 340439; BD
Biosciences, San Jose, CA, USA), monoclonal mouse anti-human
anti-P-gp conjugated to phycoerythrin (dilution, 1:100 in PBS;
catalog no. 557003; BD Biosciences) and mouse IgG anti-human
anti-MRP1 conjugated to fluorescein isothiocyanate (dilution, 1:100
in PBS; catalog no., 557593; BD Biosciences). Subsequently, the
cells were washed with PBS (BD Biosciences), centrifuged at 1,500 ×
g for 5 min and analyzed using BD FACSAria II™ flow cytometer and
BD CellQuest™ (BD Biosciences). Gates were set up to exclude
nonviable cells and debris. The negative fraction was determined
using appropriate isotype controls.
Rhodamine (Rho)-123 efflux assay
P-gp activity was determined using Rho-123
(Sigma-Aldrich) as a marker of P-gp efflux, since Rho-123 is a
fluorescent dye and a substrate for P-gp (22). K562 and K562/DOX cells
(1×106 cells/ml) were incubated with 2 µg/ml Rho-123 in
the presence or absence of the P-gp inhibitor verapamil (20 µg/ml;
Sigma-Aldrich) for 30 min at 37°C in a humidified atmosphere of 5%
CO2. Upon washing with PBS, the cells were incubated for
1 h in Rho-123-free medium supplemented with 10% FCS, in the
presence or absence of verapamil. Finally, the cells were analyzed
using BD FACSAria II flow cytometer.
cADPR activity assay
cADPR activity was evaluated using nicotinamide
guanine dinucleotide (NGD+; Sigma-Aldrich) as a
substrate, and measuring the production of cyclic guanosine
diphosphate-ribose (cGDPR) as an increase in fluorescence. cGDPR,
the guanine nucleotide equivalent to cADPR, is resistant to
hydrolysis, in contrast to cADPR (23) and is also fluorescent, which allows
continuous monitoring of the reaction fluorometrically. Cells
(5×106 cells) were incubated for 1 h at 37°C in 1.5 ml
PBS containing 50 µM NGD+ and 20 mM Tris-hydrochloride
(pH, 7.4; Sigma-Aldrich). The excitation wavelength was set at 300
nm, and the emission was measured at 410 nm using LS 45
Fluorescence Spectrometer (PerkinElmer, Inc., Waltham, MA, USA).
The quantity of cGDPR produced was determined by comparing the
fluorescence intensity of the sample with that of the cGDPR
standards (24).
Western blotting of CD38
K562 and K562/DOX cells were harvested, and equal
amounts of cell lysate proteins were analyzed using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE;
acrylamide, Sigma-Aldrich; bisacrylamide, Amresco, Solon, OH, USA;
Tris, Sigma-Aldrich; TEMED, Sigma-Aldrich; ammonium persulfate,
Sigma-Aldrich), as previously described (25). Briefly, equal amounts (10 µg) of cell
lysate were loaded onto SDS-PAGE gels using the Mini-PROTEAN®
Electrophoresis System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Proteins were next transferred electrophoretically onto
nitrocellulose membranes. The membranes were blocked for 1 h at
room temperature or overnight at 4°C with Tris-buffered saline and
Tween 20 (TBST; 50 mM Tris, ph 7.6; 150 mM NcCl, Honeywell
Specialty Chemicals, Seelze, Germany; 0.1% Tween-20, Honeywell
Specialty Chemicals) containing 3% bovine serum albumin, followed
by incubation with anti-CD38 monoclonal antibody (catalog no,
OKT10; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
anti-P-gp antibody (catalogue number D11; Santa Cruz Biotechnology,
Inc.) in 3% bovine serum albumin overnight at 4°C or for 2 h at
room temperature. Following incubation, the membranes were washed 3
times with TBST. Detection of the primary antibodies was achieved
by incubation with alkaline phosphatase-conjugated bovine anti-goat
antibody for 1 h at room temperature (dilution 1:1,000 in 3% TBST;
catalog no., Sc-2381; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), followed by 3 washes with TBST. Alkaline phosphatase activity
was detected using 5-bromo-4-chloro-3′-indolyl phosphate/nitro-blue
tetrazolium as a substrate (Promega Corp., Madison, WI, USA) for
colorimetric detection.
Ca2+ release
To determine Ca2+ release,
3×106 cells were rinsed with Hank's Balanced Salt
Solution (HBSS; Gibco; Thermo Fisher Scientific, Inc.).
Subsequently, 0.5 ml of 5 µM Fura-2 acetoxymethyl ester (AM;
Sigma-Aldrich) dissolved in HBSS [stock solution, 1.5 mM in
dimethyl sulfoxide (AppliChem GmbH; Darmstadt, Germany) containing
20% Pluronic® F-127 (Sigma-Aldrich)] was added to the cells for 30
min at room temperature. Upon rinsing the cells twice with HBSS, LS
45 Fluorescence Spectrometer was used for the fluorometric
measurement of Ca2+ (excitation wavelengths, 340 and 380
nm; emission wavelength, 510 nm). Maximum and minimum fluorescence
ratios (Rmax and Rmin, respectively) were obtained by the addition
of 10 µM ionomycin (EMD Millipore, Billerica, MA, USA) and 4 mM
ethylene glycol tetraacetic acid (Sigma-Aldrich). 8-bromo-cADPR is
a specific antagonist of cADPR. It was used at a 100 µM
concentration (Sigma-Aldrich). Thapsigargin is potent endomembrane
Ca2+-ATPase inhibitor, which can release Ca2+
from intacellular stores. It was used at 1 µΜ concentration
(Sigma-Aldrich). The fluorescence signal was calibrated using the
Grynkiewicz equation (26).
Statistical analysis
Statistical significance between control conditions
and each of the exposure samples was calculated with Student's
t test, using SPSS version 21 software (IBM SPSS, Armonk,
NY, USA). Data are presented as the mean ± standard deviation of ≥3
replicate experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
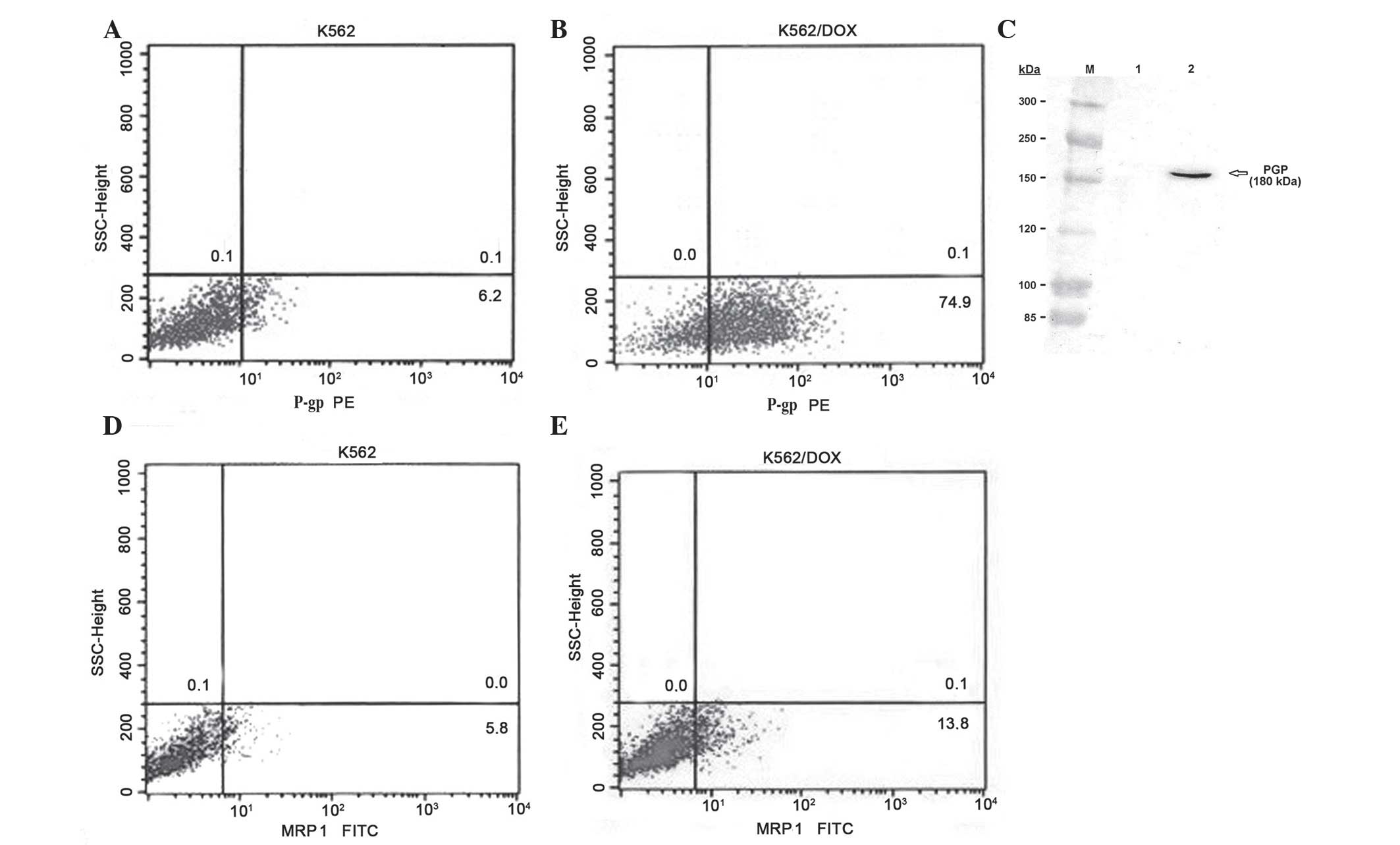
P-gp and MRP1 expression analysis
The present study analyzed the expression of
multidrug resistance proteins P-gp and MRP1 in human CML K562 cells
and in K562/DOX cells, a K562 subline resistant to doxorubicin.
Fluorocytometric analysis confirmed that K562/DOX cells exhibited
significant expression of P-gp, compared with the parental K562
cell line (74.9 vs. 6.2%; Fig. 1A and
B). The expression of P-gp in K562/DOX cells was confirmed
using western blot analysis, whereby a 180-kDa band in the K562/DOX
cells was demonstrated with anti-P-gp antibody (Fig. 1C). The percentage of cells expressing
MRP1 was 5.8% in parental K562 cells, and 13.8% in K562/DOX cells
(Fig. 1D and E).
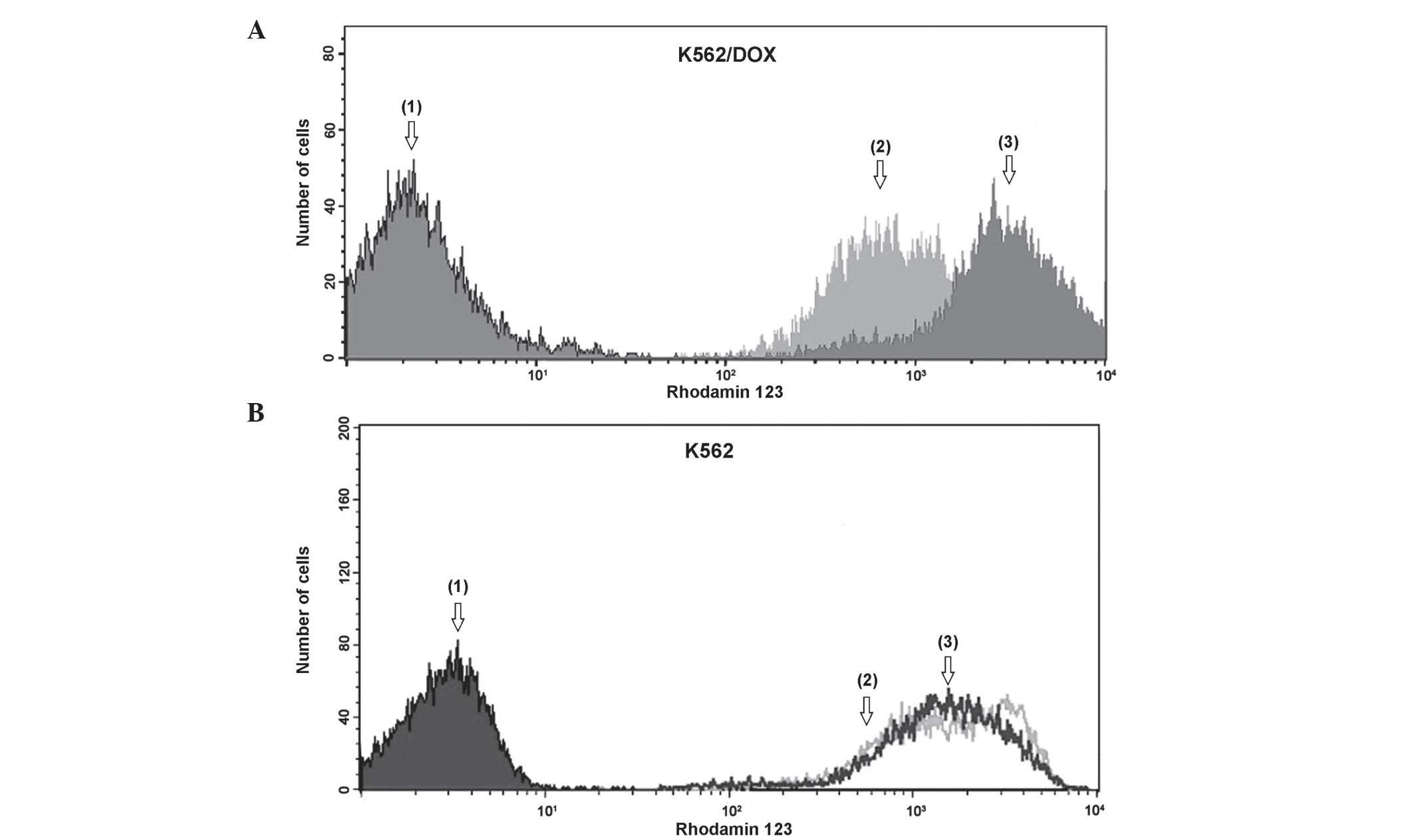
Rho-123 efflux assay
Rho-123 assay was used to investigate P-gp active
efflux in parental K562 and K562/DOX cells. The results of the
Rho-123 accumulation and efflux assay, used to determine the number
of cells with low levels of Rho-123, were based on the extent of
efflux that was blocked by the P-gp inhibitor verapamil. As
revealed by Fig. 2A, drug-resistant
K562/DOX cells demonstrated a significantly reduced accumulation of
Rho-123, compared with parental K562 cells (Fig. 2B). Verapamil clearly attenuated the
activity of P-gp, which lead to a clear increase in the
accumulation of Rho-123 in K562/DOX cells. K562 cells exhibited no
significant efflux differences with verapamil.
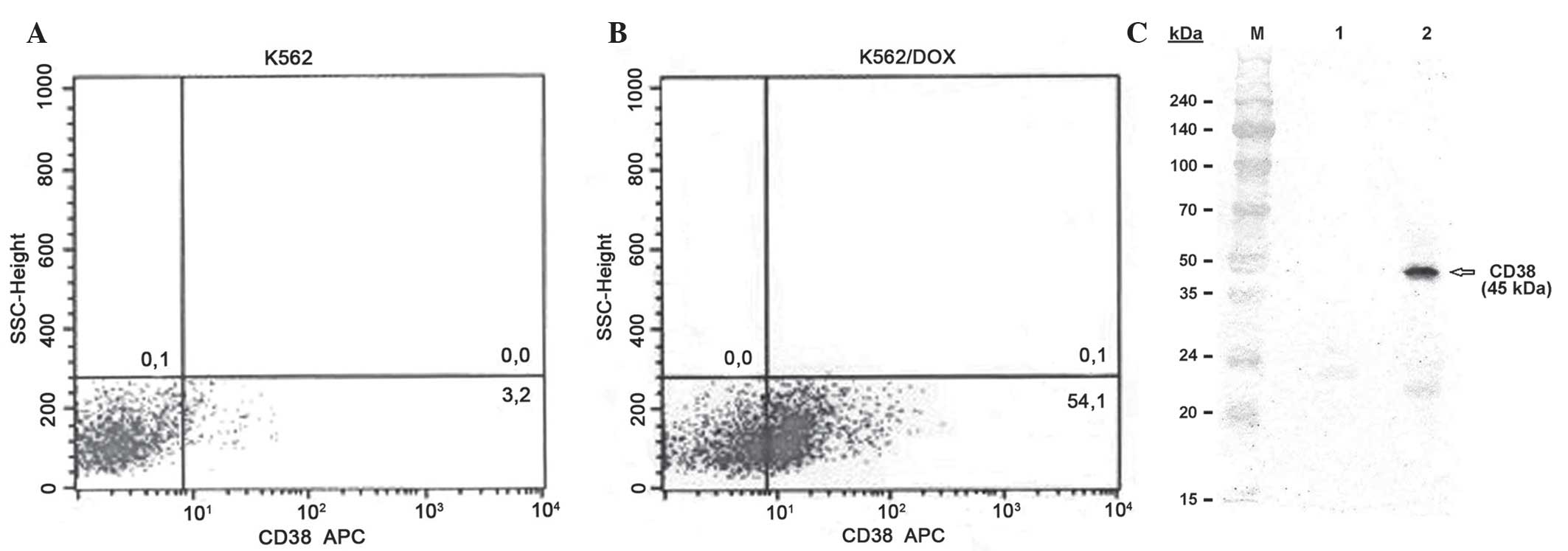
CD38 expression
A phenotypic analysis of K562 and K562/DOX cells to
evaluate the expression of CD38 was performed using flow cytometry.
As revealed by Fig. 3A and B, the
expression levels of CD38 were 3.2 and 54.1% in the K562 and
K562/DOX cells, respectively. Western blotting analysis using the
anti-CD38 antibody demonstrated the presence of a 45-kDa protein in
the K562/DOX cells (Fig. 3C).
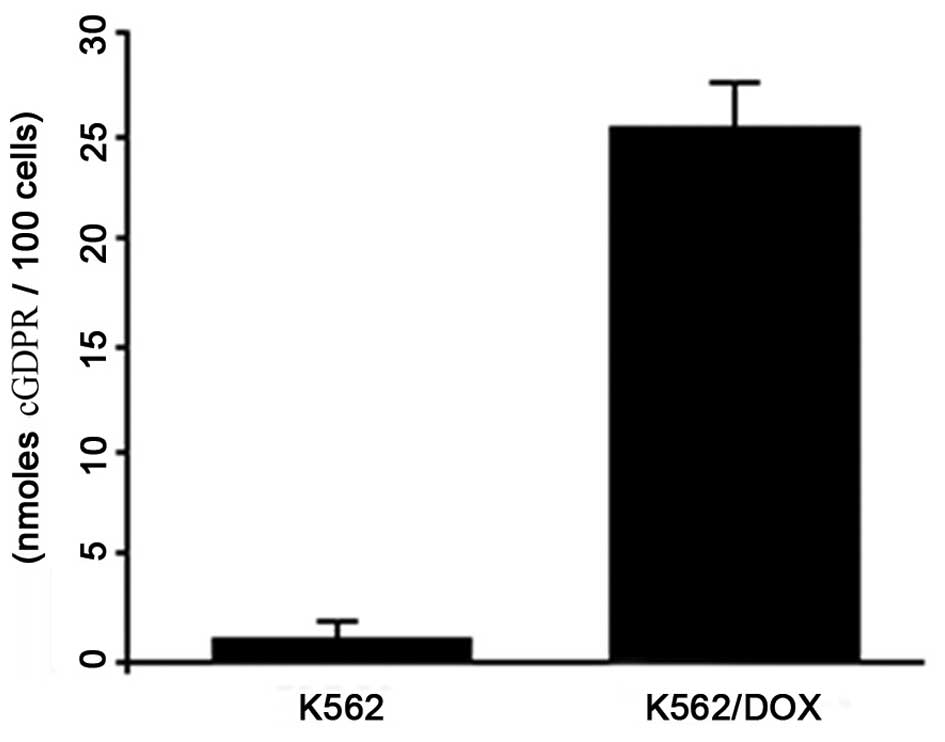
ADP-ribosyl cyclase activity
assays
To investigate endogenous cADPR formation, a
spectrophotometric assay of GDPR-cyclase activity in K562 and
K562/DOX cells was performed (Fig.
4). This method is based on the conversion of NGD+
by ADPR cyclases into a cyclic derivative termed cGDPR, which is
resistant to hydrolysis. cGDPR production was ~25.48
nM/1×106 in K562/DOX cells, which was significantly
higher compared with control cells (P<0.01). No cGDPR activity
was observed in the parental K562 cells.
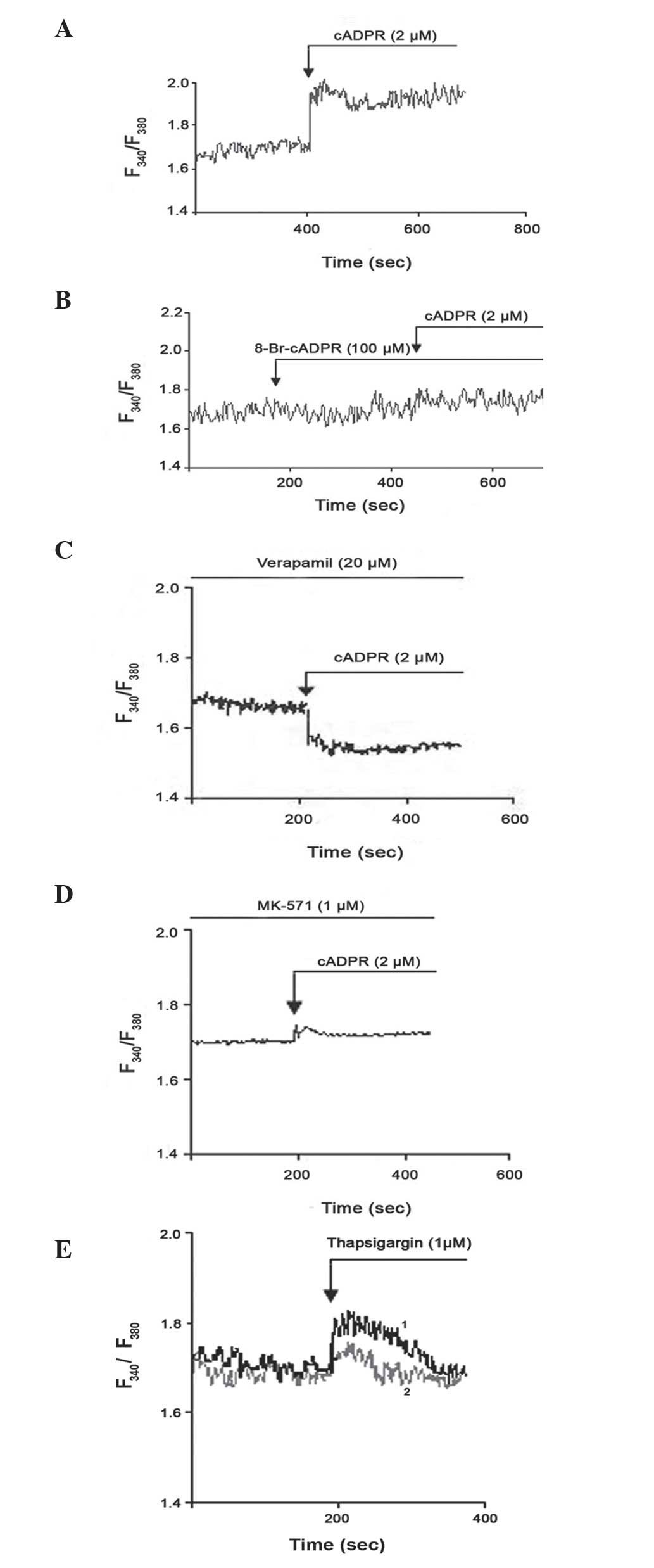
Ca+2 release
Several studies have demonstrated that altered
intracellular Ca+2 homeostasis contributes to anticancer
drug resistance (18,19). CD38 generates and degrades the second
messenger cADPR, and is key in the regulation of transient changes
in the intracellular concentration of Ca2+ (27). To investigate the possible involvement
of CD38 on Ca2+ metabolism in K562/DOX cells, Fura-2 AM
was added to the cells. K562/DOX cells responded rapidly to cADPR
(Fig. 5A), and 8-bromo-cADPR (an
antagonist of cADPR) blocked the cADPR-mediated release of
Ca2+ (Fig. 5B). The effect
of verapamil and MK-571 on the cADPR-mediated release of
Ca2+ was also investigated in the present study. It was
demonstrated that verapamil (Fig. 5C)
and MK-571 (Fig. 5D) failed to
inhibit the response to cADPR. Thapsigargin is an inhibitor of the
sarco/endoplasmic reticulum Ca2+ ATPase (28). Thapsigargin induces the mobilization
of intracellular Ca2+ and the depletion of
Ca2+ from the sarco/endoplasmic reticulum, which is an
important intracellular Ca2+ store (29). Thapsigargin has been demonstrated to
be a P-gp substrate; therefore, thapsigargin may be eliminated from
the cytoplasm in P-gp+ cells (30). To confirm this, the present study
compared the effect of thapsigargin on K562/DOX and K562 cells. The
results revealed that K562/DOX cells were more resistant to
thapsigargin than K562 cells (Fig.
5E). Therefore, the expression of P-gp in cells may cause
resistance to thapsigargin.
Discussion
Multidrug resistance to anticancer drugs remains one
of the most serious challenges for anticancer therapy, and is
mainly responsible for the failure of cancer chemotherapy (3). Multidrug ABC transporters, including
P-gp and MRP1, are important in the efflux of drugs from tumor
cells. Thus, overexpression of ABC transporters may result in the
failure of anticancer chemotherapy (2–4).
Overexpression of P-gp, which is an integral membrane protein,
represents one of the major mechanisms contributing to the
development of the multidrug resistance phenotype, and leads to
increased drug efflux (2). However,
drug resistance in various cancer cells overexpressing P-gp is not
completely reversed by specific inhibitors or gene-specific small
interfering RNA, which suggests that there may be additional
mechanisms that lead to the development of the multidrug resistance
phenotype (31).
In the present study, human CML K562 cells were
rendered resistant to the cytotoxicity of doxorubicin by
progressive adaptation of the sensitive parental K562 cells to
doxorubicin. The resulting subline was termed K652/DOX. The present
study investigated the expression of P-gp in K562 and K562/DOX
cells. K562/DOX cells exhibited clear P-gp protein levels in
western blot analysis, and clear P-gp activity was observed using
flow cytometry. By contrast, in the parental K562 cells P-gp was
not observed. P-gp activity is measured by the efflux of Rho-123,
and is inhibited by verapamil. The results of the Rho-123 assay,
based on the extent of Rho-123 efflux that verapamil was capable of
inhibiting, indicated that the differences observed in the
fluorescence intensity of Rho-123 were due to P-gp activity in K562
and K562/DOX cells.
The present study revealed that K562/DOX cells
expressed CD38 and exhibited ADP-ribosyl cyclase enzymatic
activity. The expression levels of CD38 in K562/DOX cells was ~54%,
and western blot analysis clearly demonstrated the presence of a
45-kDa band in the K562/DOX cell lysate, but not in the parental
K562 cell lysate. To the best of our knowledge, the present study
is the first to report direct CD38 involvement in tumor cell drug
resistance. CD38 is a single-chain type II transmembrane
glycoprotein that is expressed by a variety of hematological cells,
and its expression is dependent on cell activation and
differentiation (32). In addition to
its role as a cell surface marker, CD38 is a multifunctional enzyme
that synthesizes cADPR, which is a second messenger (14–17). cADPR
is an endogenous Ca2+ mobilizing cyclic nucleoside that
targets the stores of Ca2+ located in the endoplasmic
reticulum of numerous types of cells and species, including
protozoa, plants, animals and humans (17,33). To
investigate the role of CD38 in Ca2+ homeostasis and
drug resistance, the present study observed the effect of cADPR on
Fura-2 AM-loaded cells. The results demonstrated that cADPR led to
an increase in intracellular Ca2+, and this effect was
inhibited by pre-incubation of the cells with the cADPR antagonist
8-bromo-cADPR. In addition, the present study demonstrated that
verapamil and MK-571 did not inhibit the effect of cADPR.
CD38 has been identified as a major cellular NADase
that appears to regulate the activity of silent mating type
information regulation 2 homolog 1 (SIRT1) and the cellular levels
of NAD, which is a key molecule in energy production and signal
transduction, two processes that undergo crucial alterations in
tumor cells (34). Furthermore,
previous evidence indicates that the mammalian stress response gene
SIRT1 regulates multiple aspects of cancer drug resistance
(35). Chu et al (36) and Oh et al (37) reported that SIRT1 is activated in
multiple drug-resistant cancer cell lines and tumor biopsies. The
activation of SIRT1 increases the expression of P-gp (35). Therefore, the increase in the
expression of ABC transporters induced by SIRT1 may increase drug
efflux from cells, which may lead to a decrease in drug
concentration in tumor cells and may result in drug resistance.
There is no direct evidence to indicate that an induction of CD38
expression in K562/DOX cells and the presence of ADP-ribosyl
cyclase enzymatic activity are regulated through SIRT1 (38). Additional studies are required to
establish the mechanisms involved.
In conclusion, the present study demonstrated that
CD38 participates in the mechanism of drug resistance to
chemotherapy, and the present findings may contribute to future
studies aimed to identify a molecular basis for the reversal of
chemotherapeutic drug resistance in cancer cells.
Acknowledgements
The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University
(Istanbul, Turkey; project no. 3124).
References
|
1
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug Resistance in Cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kovalev AA, Tsvetaeva DA and Grudinskaja
TV: Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the
development of primary and acquired multiple drug resistance in
patients with early and metastatic breast cancer. Exp Oncol.
35:287–290. 2013.PubMed/NCBI
|
|
3
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higgins CF: Multiple molecular mechanisms
for multidrug resistance transporters. Nature. 446:749–757. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nooter K, de la Riviere Brutel G, Look MP,
van Wingerden KE, Henzen-Logmans SC, Scheper RJ, Flens MJ, Klijn
JG, Stoter G and Foekens JA: The prognostic significance of
expression of the multidrug resistance-associated protein (MRP) in
primary breast cancer. Br J Cancer. 76:486–493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Juranka PF, Zastawny RL and Ling V:
P-glycoprotein: Multidrug-resistance and a superfamily of
membrane-associated transport proteins. FASEB J. 3:2583–2592.
1989.PubMed/NCBI
|
|
7
|
Malavasi F, Funaro A, Roggero S,
Horenstein A, Calosso L and Mehta K: Human CD38: A glycoprotein in
search of a function. Immunol Today. 15:95–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson DG and Bell JI: Isolation of a
cDNA encoding the human CD38 (T10) molecule, a cell surface
glycoprotein with an unusual discontinuous pattern of expression
during lymphocyte differentiation. J Immunol. 144:2811–2815.
1990.PubMed/NCBI
|
|
9
|
Summerhill RJ, Jackson DG and Galione A:
Human lymphocyte antigen CD38 catalyzes the production of cyclic
ADP-ribose. FEBS Lett. 335:231–233. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zocchi E, Franco L, Guida L, Benatti U,
Bargellesi A, Malavasi F, Lee HC and De Flora A: A single protein
immunologically identified as CD38 displays NAD+
glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase
activities at the outer surface of human erythrocytes. Biochem
Biophys Res Commun. 196:1459–1465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howard M, Grimaldi JC, Bazan JF, Lund FE,
Santos-Argumedo L, Parkhouse RM, Walseth TF and Lee HC: Formation
and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen
CD38. Science. 262:1056–1059. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takasawa S, Tohgo A, Noguchi N, Koguma T,
Nata K, Sugimoto T, Yonekura H and Okamoto H: Synthesis and
hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and
inhibition of the hydrolysis by ATP. J Biol Chem. 268:26052–26054.
1993.PubMed/NCBI
|
|
13
|
Inageda K, Takahashi K, Tokita K, Nishina
H, Kanaho Y, Kukimoto I, Kontani K, Hoshino S and Katada T: Enzyme
properties of Aplysia ADP-ribosyl cyclase: Comparison with NAD
glycohydrolase of CD38 antigen. J Biochem. 117:125–131.
1995.PubMed/NCBI
|
|
14
|
Galione A: Ca(2+)-induced
Ca2+ release and its modulation by cyclic ADP-ribose.
Trends Pharmacol Sci. 13:304–306. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HC: Cyclic ADP-ribose: A new member of
a super family of signalling cyclic nucleotides. Cell Signal.
6:591–600. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aarhus R, Graeff RM, Dickey DM, Walseth TF
and Lee HC: ADP-ribosyl cyclase and CD38 catalyze the synthesis of
a calcium-mobilizing metabolite from NADP. J Biol Chem.
270:30327–30333. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HC: Physiological functions of cyclic
ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol
Toxicol. 41:317–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smaili SS, Pereira GJ, Costa MM, Rocha KK,
Rodrigues L, do Carmo LG, Hirata H and Hsu YT: The role of calcium
stores in apoptosis and autophagy. Curr Mol Med. 13:252–265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sulová Z, Seres M, Barancík M, Gibalová L,
Uhrík B, Poleková L and Breier A: Does any relationship exist
between P-glycoprotein-mediated multidrug resistance and
intracellular calcium homeostasis. Gen Physiol Biophys. 28:F89–F95.
2009.PubMed/NCBI
|
|
21
|
Florea AM and Büsselberg D: Anti-cancer
drugs interfere with intracellular calcium signaling.
Neurotoxicology. 30:803–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pétriz J and García-López J: Flow
cytometric analysis of P-glycoprotein function using rhodamine 123.
Leukemia. 11:1124–1130. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graeff RM, Walseth TF, Fryxell K, Branton
WD and Lee HC: Enzymatic synthesis and characterizations of cyclic
GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl
cyclase activity. J Biol Chem. 269:30260–30267. 1994.PubMed/NCBI
|
|
24
|
Yalcintepe L, Albeniz I, Adin-Cinar S,
Tiryaki D, Bermek E, Graeff RM and Lee HC: Nuclear CD38 in retinoic
acid-induced HL-60 cells. Exp Cell Res. 303:14–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grynkiewicz G, Poenie M and Tsien RY: A
new generation of Ca2+ indicators with greatly improved
fluorescence properties. J Biol Chem. 260:3440–3450.
1985.PubMed/NCBI
|
|
27
|
Zocchi E, Usai C, Guida L, Franco L,
Bruzzone S, Passalacqua M and De Flora A: Ligand-induced
internalization of CD38 results in intracellular Ca2+ mobilization:
Role of NAD+ transport across cell membranes. FASEB J. 13:273–283.
1999.PubMed/NCBI
|
|
28
|
Watson WD, Facchina SL, Grimaldi M and
Verma A: Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors
identify a novel calcium pool in the central nervous system. J
Neurochem. 87:30–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JS, Agarwal N and Mehta K:
Multidrug-resistant MCF-7 breast cancer cells contain deficient
intracellular calcium pools. Breast Cancer Res Treat. 71:237–247.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gutheil JC, Hart SR, Belani CP, Melera PW
and Hussain A: Alterations in Ca2+ transport ATPase and
P-glycoprotein expression can mediate resistance to thapsigargin. J
Biol Chem. 269:7976–7981. 1994.PubMed/NCBI
|
|
31
|
Kellner U, Hutchinson L, Seidel A, Lage H,
Danks MK, Dietel M and Kaufmann SH: Decreased drug accumulation in
a mitoxantrone-resistant gastric carcinoma cell line in the absence
of P-glycoprotein. Int J Cancer. 71:817–824. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dürig J, Naschar M, Schmücker U,
Renzing-Köhler K, Hölter T, Hüttmann A and Dührsen U: CD38
expression is an important prognostic marker in chronic lymphocytic
leukaemia. Leukemia. 16:30–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guse AH: Regulation of calcium signaling
by the second messenger cyclic adenosine diphosphoribose (cADPR).
Curr Mol Med. 4:239–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aksoy P, Escande C, White TA, Thompson M,
Soares S, Benech JC and Chini EN: Regulation of SIRT 1 mediated NAD
dependent deacetylation: A novel role for the multifunctional
enzyme CD38. Biochem Biophys Res Commun. 349:353–359. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z and Chen W: Emerging roles of SIRT1
in cancer drug resistance. Genes Cancer. 4:82–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu F, Chou PM, Zheng X, Mirkin BL and
Rebbaa A: Control of multidrug resistance gene mdr1 and cancer
resistance to chemotherapy by the longevity gene sirt1. Cancer Res.
65:10183–10187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oh WK, Cho KB, Hien TT, Kim TH, Kim HS,
Dao TT, Han HK, Kwon SM, Ahn SG, Yoon JH, et al: Amurensin G, a
potent natural SIRT1 inhibitor, rescues doxorubicin responsiveness
via down-regulation of multidrug resistance 1. Mol Pharmacol.
78:855–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Liu Z, Wu X, Chu S, Wang J, Yuan
H, Roth M, Yuan YC, Bhatia R and Chen WY: ATRA-induced cellular
differentiation and CD38 expression inhibits acquisition of BCR-ABL
mutations for CML acquired resistance. PLoS Genet. 10:e10044142014.
View Article : Google Scholar : PubMed/NCBI
|