Introduction
With regard to the global incidence of cancer, lung
cancer is currently ranked first and is the leading cause of
cancer-related mortality, with an overall 5-year survival rate of
only 17% (1,2). This malignant tumor occurs with an
annually increasing trend. Non-small cell lung cancer (NSCLC)
accounts for ~80% of all lung cancer types (3). NSCLC can be classified as two major
histological subtypes: Adenocarcinoma (AC) and squamous cell
carcinoma (SCC) (4). Approximately
65–75% of patients with NSCLC possess unresectable advanced or
metastatic disease at diagnosis (5).
However, there remains a lack of an effective biomarker to
diagnosis the malignant disease at an early stage. Furthermore,
NSCLC presents with insidious onset, but with a high level of
malignancy and fast progression (2).
Thus, the most viable method by which to improve the 5-year
survival rate in NSCLC patients is early detection, and subsequent
effective treatment.
A novel receptor-ligand system belonging to the
tumor necrosis factor (NF) superfamily, termed receptor activator
of nuclear factor (NF)-κB ligand (RANKL) and osteoprotegerin (OPG),
has recently become a focus of attention (6). RANKL is a membrane-bound protein that is
expressed mainly on the surface of osteoblasts and bone marrow
stromal cells, and binds to RANK on the surface of osteoclast
precursors, thus stimulating their differentiation into mature
osteoclasts (7). The RANKL/OPG system
was the first pathway to be found to be involved in bone regulation
(8). More significantly, recent
studies have indicated that this system is also important in the
pathogenesis of lung cancer, particularly the regulation of bone
tumor metastasis (9,10). The present study aimed to determine
the levels of OPG and soluble RANKL (sRANKL) in NSCLC patients, to
investigate the diagnostic significance of the sRANKL level and the
RANKL/OPG ratio, and to further analyze the role of this system in
the progression of NSCLC.
Materials and methods
Subjects
A total of 50 NSCLC patients who underwent a radical
resection for lung cancer between October 2011 and February 2013
were enrolled in the present study. All subjects were classified
pathologically with SCC (20 cases) or AC (30 cases) (11). The patients with diseases such as
liver and kidney disease, heart failure, diabetes, lupus and
rheumatoid arthritis, as well as those with fractures, were
excluded. Furthermore, 25 patients with benign space-occupying
pulmonary lesions were also enrolled. The detailed medical
histories, including smoking history and medical history relevant
to exclusion criteria, were retrieved for all enrolled patients.
The pathological results in the NSCLC patients and the patients
with benign space-occupying pulmonary lesions were confirmed by the
Department of Pathology in the Changhai Hospital of Shanghai
Affiliated to the Second Military Medical University (Shanghai,
China). Moreover, 25 healthy subjects who were examined and found
to be without any disease were also enrolled as controls. This
study was approved by the Ethics Committee of the First Affiliated
Hospital of Nanjing Medical University (Nanchang, China). All
enrolled patients/subjects signed informed consent forms.
Measurement of indicators
Venous blood samples were collected from the fasting
NSCLC patients and the patients with benign pulmonary lesions prior
to surgery, and the remaining blood samples were collected from
routine blood examinations in the healthy subjects. Serum was
separated by centrifugation as soon as possible and reserved in a
freezer at −20°C until use. Serum OPG and sRANKL levels were
detected by enzyme-linked immunosorbent assay (ELISA) according to
the manufacturer's instructions. The ELISA kit was purchased from
BioVendor (Brno, Czech Republic; the detection sensitivity for OPG
and sRANKL were 0.1 and 0.4 pmol/l, respectively).
Statistical analysis
All data were analyzed using SPSS statistical
software, version 17.0 (SPS, Inc., Chicago, IL, USA). The category
variables are represented by the number of cases, and the
significance between two groups was tested using the χ2
test. Quantitative data are presented as the mean ± standard
deviation. The difference between two groups was analyzed by the
Mann-Whitney U-test. Meanwhile, the variance in serum sRANKL
levels, OPG levels and sRANKL/OPG ratios among the three groups
were determined with a one-way analysis of variance. Receiver
operating characteristic (ROC) curves were used to evaluate the
performance of sRANKL and sRANKL/OPG, and the optimal cut-off
points were determined based on Youden's index (12). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical parameters
As summarized in Table
I, the relevant clinical parameters of the patients with NSCLC
were compared against the patients with benign space-occupying
pulmonary lesions or the healthy controls. The results showed that
there was no significant difference between the NSCLC and control
groups with regard to gender, age or smoking history. The remaining
incomparable clinical pathological characteristics of NSCLC are
also described in Table I.
 | Table I.Clinical parameters of the NSCLC group
and the combined control group. |
Table I.
Clinical parameters of the NSCLC group
and the combined control group.
| Category | NSCLC (n=50) | Controls (n=50) | P-value |
|---|
| Gender (male/female),
n | 34/16 | 34/16 | 1.000 |
| Age,
yearsa | 62.4±10.0 | 62.8±15.3 | 0.878 |
| Positive smoking
history, n | 17 | 13 | 0.383 |
| Pathology | Adenocarcinoma
(n=30) | Normal physical
examination (n=25) | – |
|
| Squamous cell
carcinoma (n=20) | Benign
space-occupying lesion (n=25) |
|
| Tumor size,
mma | 32.1±17.9 | – | – |
| Clinical phase
(I/II/III), n | 22/18/10 | – | – |
| Pathological grading
(medium-severe/low), n | 34/16 | – | – |
| Lymph node metastasis
(positive/negative), n | 25/25 | – | – |
Comparison of serum sRANKL and OPG
levels, and sRANKL/OPG ratio
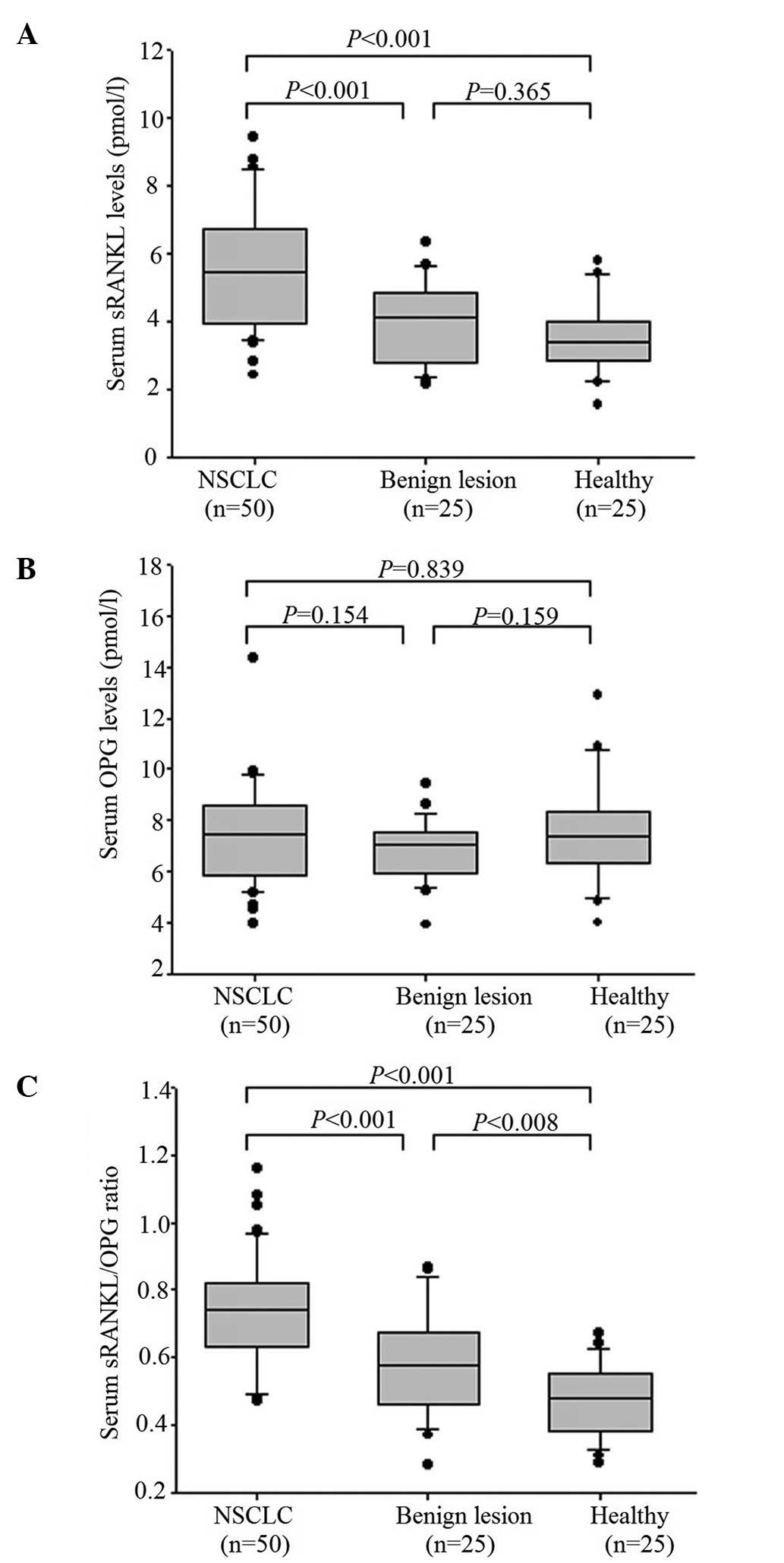
As shown in Fig. 1,
the serum sRANKL level in the patients with NSCLC (5.51±1.75
pmol/l) was significantly higher than that of the patients with
benign lesions (3.98±1.20 pmol/l) (P<0.001). Moreover, the
sRANKL level of the NSCLC patients was significantly elevated in
comparison with the healthy subjects (3.50±1.04 pmol/l)
(P<0.001). However, there was no significant difference between
the patients with benign lesions and the healthy subjects (Fig. 1A). When analyzing the differences in
serum OPG levels, there was no statistical significance among the
three groups; the values for the patients with NSCLC, the patients
with benign lesions and the healthy subjects were 7.43±1.86,
6.81±1.20 and 7.52±2.03 pmol/l, respectively (Fig. 1B). Whereas the sRANKL/OPG ratio was
increased in the patients with NSCLC (0.74±0.16) in comparison to
the patients with benign lesions (0.59±0.16, P<0.001) and the
healthy subjects (0.47±0.11, P<0.001). When comparing the
sRANKL/OPG ratio in the patients with benign lesions and the
healthy subjects, no significant differences were found (Fig. 1C).
Efficacies of serum sRANKL level and
sRANKL/OPG ratio in the diagnosis of NSCLC
To further determine the diagnostic significance of
the sRANKL level and the sRANKL/OPN ratio, ROC curves were used to
analyze the efficacy in discerning NSCLC patients from those with
benign lesions or the healthy controls. As shown in Table II, based on the greatest Youden's
index, the optimal cut-off points for sRANKL level and sRANKL/OPG
ratio were 4.20 pmol/l and 0.60, respectively. The area under the
curve (AUC) for sRANKL level and sRANKL/OPG ratio was 0.837 [95%
confidence interval (CI), 0.746–0.927] and 0.922 (95% CI,
0.863–0.980), respectively. The sensitivity, specificity and
accuracy for sRANKL levels at the optimal cut-off point were 74.0,
84.0 and 77.3% respectively. Furthermore, the performance of the
sRANKL/OPG ratio was better than the sRANKL levels in the diagnosis
of NSCLC. The sensitivity, specificity and accuracy for the
sRANKL/OPG ratio was 84.0, 88.0 and 85.3%, respectively.
 | Table II.Efficacy of sRANKL level and
sRANKL/OPG ratio in the diagnosis of non-small cell lung cancer
using an ROC. |
Table II.
Efficacy of sRANKL level and
sRANKL/OPG ratio in the diagnosis of non-small cell lung cancer
using an ROC.
| Indicator | Cut-off | AUC (95% CI) | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| sRANKL | 4.20 pmol/l | 0.837
(0.746–0.927) | 74.0 | 84.0 | 77.3 |
| sRANKL/OPG | 0.60 | 0.922
(0.863–0.980) | 84.0 | 88.0 | 85.3 |
Diagnostic significance of serum
sRANKL level and sRANKL/OPG ratio in distinguishing between benign
and malignant space-occupying pulmonary lesions
In addition, it was difficult to distinguish between
the patients with malignant lesions and those with benign lesions.
The present study also analyzed the diagnostic efficacy of the
sRANKL level and the sRANKL/OPG ratio in discerning malignant and
benign lesions. Based on the greatest Youden's index, the optimal
diagnostic cut-off point for the sRANKL level and the sRANKL/OPG
ratio were 5.24 pmol/l and 0.63, respectively (Table III). The AUC for each indictor was
0.744 (95% CI, 0.632–0.855) and 0.751 (95% CI, 0.631–0.871),
respectively. The sensitivity, specificity and accuracy of the
sRANKL level in the diagnosis of benign or malignant pulmonary
lesions were 66.0, 84.0 and 68.0%, respectively. With regard to the
sRANKL/OPG ratio, the sensitivity, specificity and accuracy were
78.0, 64.0 and 73.3%, respectively. Therefore, the sRANKL/OPG ratio
exhibited a better performance compared with the sRANKL level.
 | Table III.Efficacy of serum sRANKL level and
sRANKL/OPG ratio in distinguishing between benign and malignant
space-occupying pulmonary lesions using an ROC. |
Table III.
Efficacy of serum sRANKL level and
sRANKL/OPG ratio in distinguishing between benign and malignant
space-occupying pulmonary lesions using an ROC.
| Indicator | Optimal cut-off
point | AUC (95% CI) | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| sRANKL | 5.24 pmol/l | 0.744
(0.632–0.855) | 60.0 | 84.0 | 68.0 |
| sRANKL/OPG | 0.63 | 0.751
(0.631–0.871) | 78.0 | 64.0 | 73.3 |
Discussion
Conventional diagnostic markers, such as
carcinoembryonic antigen (CEA) and CA199, have limited sensitivity
(CEA, 52%; CA199, 48.2%) and specificity (CEA, 80%; CA199, 90%)
with regard to discerning patients with lung cancer, particularly
those with advanced stage (13).
Although patients who undergo a surgical resection are able to
achieve a favorable clinical outcome, it is impossible to avoid
cancer metastasis and the associated poor outcome, which greatly
reduces the middle and long-term patient survival rates. Hence,
there has been an urgent requirement to identify effective
diagnostic approaches. Recently, following the development of
molecular biology, studies have begun to investigate convenient or
inexpensive markers to aid in the diagnosis and prognosis of
malignant tumors (14). Tumor markers
are those molecules that cancer cells secrete into the body fluids
or tissue. Generally, the levels of these biomarkers are
significant different in comparison with healthy controls.
Clinicians can use these markers to diagnose patients with cancer,
monitor tumor progress, guide treatment, monitor recurrence or
metastasis, and predict prognosis. Currently, CEA, neuron-specific
enolase, carbohydrate antigen 125 (CA-125) and CA19-9 have been
applied in the clinic to diagnose or monitor the progress of lung
cancer, with limited success (15). A
long-term observation of these markers revealed that none of them
were able to accurately determine the patient's disease state
(16).
As aforementioned, the roles of OPG and RANKL have
been extensively investigated in the regulation of tumor
progression (16,17). The two molecules were shown to be
significant in bone regulation (18).
More importantly, OPG and RANKL were indicated to be involved in
cancer pathogenesis, development and metastasis (9,10).
Furthermore, the RANKL/OPG ratio was shown to exhibit a positive
correlation with NF-κB activation (19). This finding suggested that OPG/RANKL
activated NF-κB, which may in turn stimulate a series of signaling
pathways. Thereby, RANKL and OPG could regulate cell
differentiation, function or apoptosis. In view of this, either
RANKL or OPG would promote the occurrence of NSCLC. However, these
hypotheses require confirmation in future studies.
In the present study, the serum sRANKL and OPG
levels were initially determined. The sRANKL level and sRANKL/OPG
ratio were indicated to be potential NSCLC biomarkers. With the
exception of the aforementioned studies, few studies have been
published that are associated with RANKL or OPG and the progression
of lung cancer. The present study found that the sRANKL levels were
significantly higher in the NSCLC patients compared with the
healthy controls or patients with lung benign space-occupying
lesions. In accordance with these results, the study by
Karapanagiotou et al also demonstrated higher sRANKL levels
in NSCLC patients (20). However,
there was no difference in the OPG levels among the three groups in
the present study. This finding was not consist with the results in
the study by Karapanagiotou et al, where it was concluded
that higher OPG levels promoted the development of lung cancer
metastasis (20). This may be due to
the relatively small sample size resulting in selective bias.
Moreover, the present study and that of Karapanagiotou et al
demonstrated that the sRANKL/OPG ratio was significantly higher in
the NSCLC patients. All these findings indicated that sRANKL or OPG
promoted the progression of cancer. Another previous study proved
that blocking OPG or attenuating sRANKL/OPG inhibited tumor cell
proliferation (21).
Significantly, the present study identified that the
sRANKL level and the sRANKL/OPG ratio were potential biomarker
candidates for lung cancer. According to the ROC curve analysis,
the sensitivity, specificity and accuracy of the indictors
exhibited superior performance in comparison with previously
reported clinical lung cancer biomarkers, including CEA and CA19-9
(none of which were specific biomarkers of lung cancer) (22). The AUC values for the sRANKL level and
the sRANKL/OPG ratio were 0.837 and 0.922, respectively.
Additionally, the sRANKL level and the sRANKL/OPG ratio exhibit
potential as biomarkers with the ability to discern between
malignant or benign lesions. Clinically, the majority of lung
cancer patients show no signs of disease in the early stages. For
instance, certain patients with lung nodules have atypical imaging
pictures or a tumor diameter <2.5 cm (23). The present study also analyzed the
performance of the sRANKL level and the sRANKL/OPG ratio in the
diagnosis of malignant versus benign lesions. To date, few studies
have proposed any available factors to apply in the clinic.
However, the present study only determined the performance of the
sRANKL level and the sRANKL/OPG ratio in relatively small groups,
therefore, further analysis will be required as a large-scale
validation that will also include the comparison of the sRANKL
level and the sRANKL/OPG ratio against other currently used
markers, such as CEA or CA19–9.
In summary, the serum sRANKL level and the
sRANKL/OPG ratio may have potential as novel biomarkers for
diagnosis of NSCLC. OPG and RANKL may be involved in the
pathogenesis of NSCLC. The present results may provide important
biomarker candidates for early diagnosis of NSCLC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Gu J, Roth JA, Hildebrandt MA,
Lippman SM, Ye Y, Minna JD and Wu X: Pathway-based serum microRNA
profiling and survival in patients with advanced stage non-small
cell lung cancer. Cancer Res. 73:4801–4809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ourari-Dhahri B, Ben Slima H, Ben Amar J,
El Gharbi L, Ali M, Azzabi Baccar S, Aouina H and Bouacha H:
Management of non small cell lung cancer. Tunis Med. 90:847–851.
2012.(In French). PubMed/NCBI
|
|
4
|
Kuner R, Muley T, Meister M, Ruschhaupt M,
Buness A, Xu EC, Schnabel P, Warth A, Poustka A, Sültmann H and
Hoffmann H: Global gene expression analysis reveals specific
patterns of cell junctions in non-small cell lung cancer subtypes.
Lung Cancer. 63:32–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esteban E, Casillas M and Cassinello A:
Pemetrexed in first-line treatment of non-small cell lung cancer.
Cancer Treat Rev. 35:364–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang
S, Lu Q and Sun Y: Differential expression of the RANKL/RANK/OPG
system is associated with bone metastasis in human non-small cell
lung cancer. PLoS One. 8:e583612013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong YY, Feige U, Sarosi I, Bolon B,
Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al:
Activated T cells regulate bone loss and joint destruction in
adjuvant arthritis through osteoprotegerin ligand. Nature.
402:304–309. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harada S and Takahashi N: Control of bone
resorption by RANKL-RANK system. Clin Calcium. 21:1121–1130.
2011.(In Japanese). PubMed/NCBI
|
|
9
|
Hanada R, Hanada T, Sigl V, Schramek D and
Penninger JM: RANKL/RANK-beyond bones. J Mol Med (Berl).
89:647–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peters S and Meylan E: Targeting receptor
activator of nuclear factor-kappa B as a new therapy for bone
metastasis in non-small cell lung cancer. Curr Opin Oncol.
25:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fluss R, Faraggi D and Reiser B:
Estimation of the Youden Index and its associated cutoff point.
Biom J. 47:458–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diamandis EP, Goodglick L, Planque C and
Thornquist MD: Pentraxin-3 is a novel biomarker of lung carcinoma.
Clin Cancer Res. 17:2395–2399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiedemann GJ: Biomarker screening for
early detection of cance. Dtsch Med Wochenschr. 138:43–45. 2013.(In
German). PubMed/NCBI
|
|
15
|
Ferrigno D, Buccheri G and Giordano C:
Neuron-specific enolase is an effective tumour marker in non-small
cell lung cancer (NSCLC). Lung Cancer. 41:311–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strathmann FG, Schulte S, Goerl K and
Petron DJ: Blood-based biomarkers for traumatic brain injury:
Evaluation of research approaches, available methods and potential
utility from the clinician and clinical laboratory perspectives.
Clin Biochem. 47:876–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ibrahim T, Sacanna E, Gaudio M, Mercatali
L, Scarpi E, Zoli W, Serra P, Ricci R, Serra L, Kang Y and Amadori
D: Role of RANK, RANKL, OPG and CXCR4 tissue markers in predicting
bone metastases in breast cancer patients. Clin Breast Cancer.
11:369–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dougall WC and Chaisson M: The
RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer
Metastasis Rev. 25:541–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owen S, Ye L, Sanders AJ, Mason MD and
Jiang WG: Expression profile of receptor activator of nuclear-κB
(RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast
cancer. Anticancer Res. 33:199–206. 2013.PubMed/NCBI
|
|
20
|
Karapanagiotou EM, Terpos E, Dilana KD,
Alamara C, Gkiozos I, Polyzos A and Syrigos KN: Serum bone turnover
markers may be involved in the metastatic potential of lung cancer
patients. Med Oncol. 27:332–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silva I and Branco JC: Rank/Rankl/opg:
Literature review. Acta Reumatol Port. 36:209–218. 2011.PubMed/NCBI
|
|
22
|
Shu J, Li CG, Liu YC, Yan XC, Xu X, Huang
XE, Cao J, Li Y, Lu YY, Wu XY, et al: Comparison of serum tumor
associated material (TAM) with conventional biomarkers in cancer
patients. Asian Pac J Cancer Prev. 13:2399–2403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prisadov GT, Uchikov AP, Welker K,
Wallimann H, Murdzhev KA and Uzunova VN: Size of tumour as a risk
factor for malignancy in patients with peripheral pulmonary
nodules. Folia Med (Plovdiv). 54:17–21. 2012.PubMed/NCBI
|