Introduction
Hepatocellular carcinoma (HCC) is a significant
worldwide health issue. In developing countries, the overall
incidence of HCC remains extremely high, and in the majority of
developed countries, the incidence of HCC has steadily increased;
this is due to the spread of hepatitis B and C (1,2).
Currently, the main treatment strategies for patients with HCC
consist of surgical resection, liver transplantation and local
ablation therapies (3). A large
number of the patients diagnosed with advanced-stage HCC may only
be treated with palliative care (2,4), as there
is no available effective palliative chemotherapy. Therefore, the
prognosis of patients with advanced-stage HCC is poor (5). Consequently, novel effective medications
for the treatment of HCC are required. Recently, the study into
treatments for HCC has focused on natural products, which are
pharmacologically effective, with little toxicity and few side
effects.
Solanine is mainly located in the tuber of the
potato plant (Solanum tuberosum L.) and in the whole
nightshade plant (S. nigrum L.), which belong to the
Solanaceae family (6–10). Solanine expression is relatively high
in the green peel and the sprouts of a potato tuber and is the main
toxic substance produced (11), with
a molecular formula of C45H73NO15
(12,13). The whole nightshade plant contains
numerous steroid alkaloids, including solamargine, solasonine,
solanine and saponin, and may be used for antitumor therapeutics,
as it has a clear inhibitory effect on tumor growth in animal
models and a toxic effect on tumor cells (14,15). The
ethanol extract of nightshade is able to inhibit the growth of
breast cancer and induce apoptosis in tumor cells (16).
Previous studies have demonstrated that solanine
affects tumor growth through several possible biological
mechanisms. An et al (17)
revealed that solanine significantly reduces the membrane fluidity
and protein levels of tumor cells in xenograft mice with hepatoma
H22 tumors. In addition, solanine effectively reduced the sialic
acid level and membrane closing ability of the tumor cells. A
previous study demonstrated that the mechanism by which solanine
induces human hepatocellular carcinoma HepG2 cells to undergo
apoptosis appeared to be mediated by the inhibition of B-cell
lymphoma-2 expression (18).
Furthermore, solanine has been demonstrated to increase the
permeability of the mitochondrial membrane, which leads to the
release of apoptosis-associated proteins and inner mitochondrial
calcium ions, resulting in tumor cell apoptosis through the
mitochondrial pathway (19,20). Eom et al (21) reported that the berberine-induced
apoptosis of human glioblastoma cells was mediated by reactive
oxygen species (ROS) and mitochondrial dysfunction, which indicates
that ROS are important in cellular apoptosis. The present study
investigated the molecular mechanism of solanine-induced HCC cell
apoptosis, in particular ROS that are involved in the apoptotic
pathway of tumor cells. The present study cultured HepG2 cells
treated with solanine in vitro, and subsequently monitored
the production of ROS from the mitochondria and cytoplasm of HepG2
cells using probes for ROS.
Materials and methods
Cells and solanine treatment
The human hepatocellular carcinoma HepG2 cell line
was provided by the Shanghai Cancer Institute (Shanghai, China).
The HepG2 cells were cultured in HyClone™ Dulbecco's modified
Eagle's medium (GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% inactivated fetal bovine serum
(Sigma-Aldrich, St. Louis, MO, USA) in a 5% CO2 Forma™
humidified incubator (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C. The cells were seeded onto a 6-well plate
(4×104 cells/well), allowed to adhere overnight, and
subsequently treated with solanine (95% mass fraction;
Sigma-Aldrich) with a final concentration of 2 mg/ml. The cells
were incubated for 24–48 h. A negative control [dimethyl sulfoxide
(DMSO); Sangon Biotech Co., Ltd., Shanghai, China] and a positive
control (camptothecin, 0.5 mg/ml; Sigma-Aldrich) were used.
ROS detection using flow
cytometry
Various concentrations of hydrogen peroxide
(H2O2; 0.5 mM; Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) was added to each well, followed by an
incubation period of 30 min prior to uniformly blending the cells
in each well. The cells were washed twice with phosphate-buffered
saline (PBS) and the Invitrogen™ fluorescent probes (Thermo Fisher
Scientific, Inc.), 2′,7′-dichlorofluorescin diacetate (DCFDA),
dihydrorhodamine 123 (DHR 123), dihydroethidium (DHE) and MitoSOX™
(MITSOX; a mitochondrial superoxide probe), were added to the cells
according to the manufacturer's protocols. The cells were incubated
at 37°C for 30 min in the dark. Subsequently, the cells were washed
twice with PBS and analyzed using flow cytometry (BD LSR II Flow
Cytometer; BD Biosciences, San Jose, CA, USA).
Western blotting
In total, 48 h after solanine treatment, total
protein was extracted from the HepG2 cells using RIPA Lysis and
Extraction Buffer (Thermo Fisher Scientific, Inc.), which contained
proteinase and phosphatase inhibitors, at 4°C for 30 min. The cell
lysates were centrifuged at 12,000 × g for 20 min at 4°C. The
protein concentrations of the resulting supernatants were
determined using a Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). The supernatants were
mixed with 5X sodium dodecyl sulfate (SDS) loading buffer (Thermo
Fisher Scientific, Inc.) and heated at 95°C for 5 min. In total, 40
µg of protein was loaded onto and separated by 12% Tris-Glycine SDS
gel (Sangon Biotech Co., Ltd.), which was subsequently transferred
onto polyvinylidene fluoride membranes (pore size, 0.22 µm; Merck
Millipore, Darmstadt, Germany). The membranes were blocked with 5%
skimmed milk in Tris-buffered saline and Tween 20 (Sangon Biotech
Co., Ltd.), and incubated overnight with primary antibodies. The
primary antibodies were as follows: Anti-apoptosis
signal-regulating kinase 1 (ASK1; polyclonal rabbit anti-human;
dilution, 1:1,000; catalog no., 3762; Cell Signaling Technology
Inc., Danvers, MA, USA), anti-thioredoxin binding protein 2 (TBP-2;
monoclonal mouse anti-human; dilution, 1:1,000; catalog no.,
K02053; MBL International Co., Woburn, MA, USA) and anti-histone
deacetylase 1 (HDAC1; polyclonal rabbit anti-human; dilution,
1:1,000; catalog no., 2062; Cell Signaling Technology Inc.).
Subsequently, the membranes were incubated with a horseradish
peroxidase-labeled secondary antibody (dilution, 1:2,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). Anti-β-actin monoclonal
antibody (Santa Cruz Biotechnology, Inc.) was used as an endogenous
control. The results were analyzed by AlphaView software version
3.3.0.0 (ProteinSimple, Santa Clara, CA, USA). The integral optical
density (IOD) of each band was determined. The relative protein
levels were used to evaluate the differences between the
solanine-treated group and the control group, using the following
equation: The relative protein level = IOD ratio between the target
gene product bands and the β-actin protein bands in the
solanine-treated group/IOD ratio between the target gene product
bands and the β-actin protein bands in the control group.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) analysis
The HepG2 cells were harvested 48 h after solanine
treatment. The percentage of cells undergoing apoptosis was
detected using a FITC Annexin V Apoptosis Detection kit II (BD
Pharmingen, San Diego, CA, USA) and flow cytometry (BD LSR II Flow
Cytometer).
Cell counting and morphological
observation
The cells were harvested at the logarithmic growth
phase and counted using a Countess II FL Automated Cell Counter
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of
4×104 cells were added to each well of a 6-well plate
containing 2 ml medium, and the plate was placed into a 5%
CO2 humidified incubator at 37°C overnight (16–24 h).
Subsequently, DMSO, 2 mg/ml solanine or 0.5 mg/ml camptothecin was
added to the cells and incubated for an additional 24–48 h. The
morphological alterations in the cells were observed using an
optical microscope (Olympus IX70 Inverted Microscope; Olympus
Corporation, Tokyo, Japan) following staining with Wright's stain
(Sangon Biotech Co., Ltd.).
Statistical analysis
All experiments were repeated three times
independently. The results are presented as the mean ± standard
deviation. One-way analysis of variance was performed using SPSS
version 11.0 software (SPSS, Inc., Chicago, IL, USA) in order to
detect significant differences in measured variables between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Solanine induces the production of ROS
in the cytoplasm and mitochondria of HepG2 cells
The HepG2 cells were treated with various
concentrations of H2O2 for the same amount of
time (30 min), and separately with the same concentration (0.5
mmol/l) for various time periods. The cells were incubated with
DCFDA for 30 min. The results demonstrated that the optimal
concentration and effective time for H2O2
stimulation of the cells were 0.5 mM and 30 min, respectively.
Following solanine pretreatment, the HepG2 cells
were stimulated by H2O2 according to the
pretested parameters, and various probes were used that were
specific to certain ROS. The results revealed that ROS probes DCFDA
and DHR 123 detected abundant ROS, including hydroxyl radical
(OH−) and H2O2, in the cytoplasm
and mitochondria of the HepG2 cells pretreated with solanine
compared with the control group [DCFDA (n=5), P=0.0389; DHR 123
(n=5), P=0.0215]. The amount of ROS produced by the
solanine-treated cells was decreased compared with the ROS produced
by camptothecin-treated cells, which was observed using the DCFDA
probe. By contrast, the amount of ROS produced by the
solanine-treated cells was increased compared with the ROS produced
by the camptothecin-treated cells, as determined using the DHR123
probe. This suggests that ROS production in various cellular
locations may be by differential mechanisms. Increased levels of
ROS production were also observed post-H2O2
stimulation, as detected by the DHR123 probe (n=5; P=0.0043).
Superoxide anion (O2−) ROS, which was
generated by solanine pretreatment, was also detected by
O2−-specific DHE and MITSOX probes. However,
there was no significant difference between the solanine-treated
and control groups [DHE (n=5), P=0.606; MITSOX (n=5), P=0.107]
(Fig. 1).
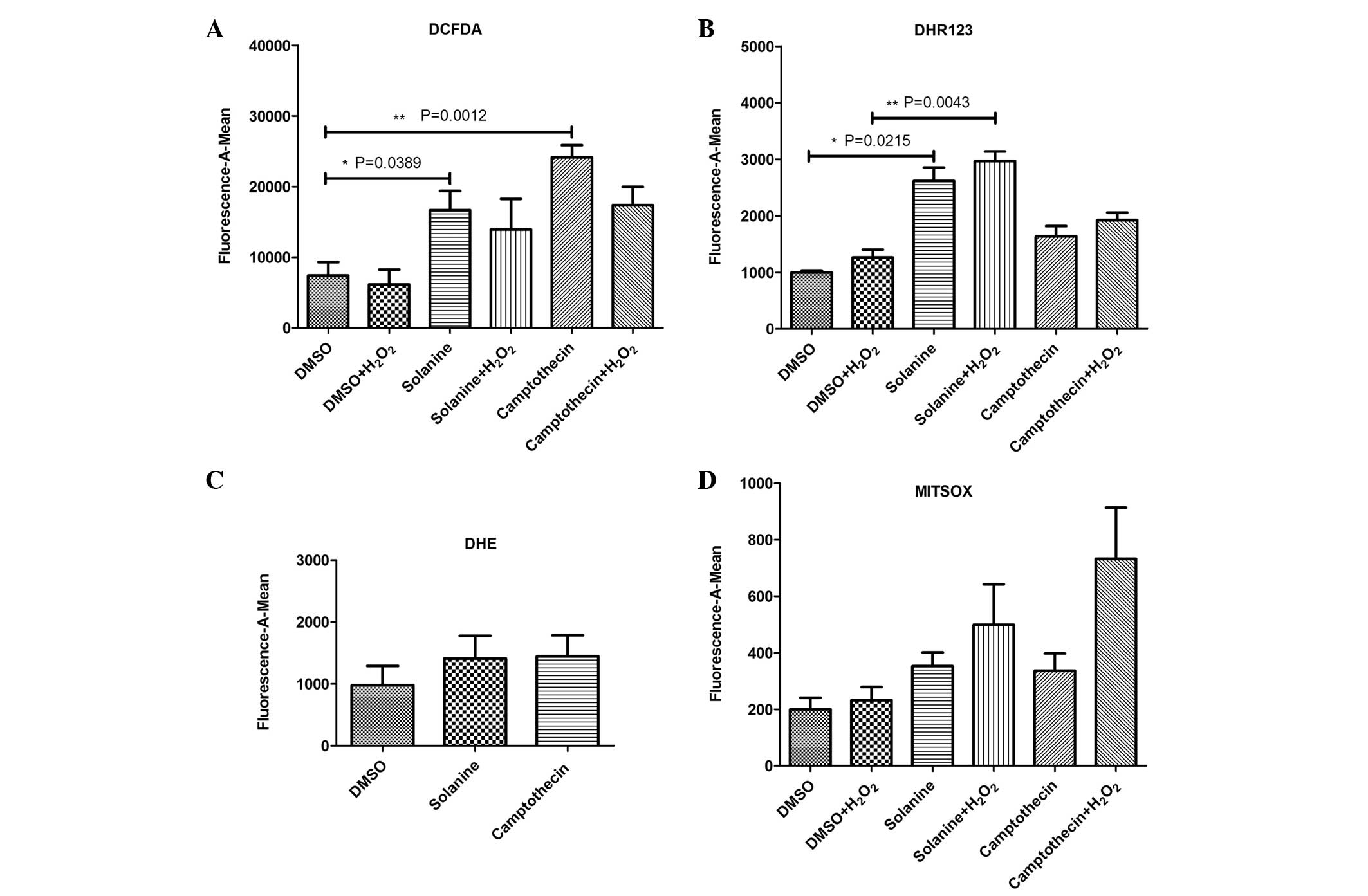 | Figure 1.Various probes were used to detect
reactive oxygen species produced by solanine-induced human
hepatocellular carcinoma HepG2 cells. (A) DCFDA probe was used to
detect OH− and H2O2 in the
cytoplasm, (B) DHR 123 probe was used to detect OH− and
H2O2 in the mitochondria, (C) DHE probe was
used to detect O2− in the cytoplasm, and (D)
MITSOX probe was used to detect O2− in the
mitochondria. DCFDA, 2′,7′-dichlorofluorescin diacetate; DHR 123,
dihydrorhodamine 123; DHE, dihydroethidium; MITSOX, MitoSOX™ Red
Mitochondrial Superoxide Indicator; OH−, hydroxyl
radical; H2O2, hydrogen peroxide;
O2−, superoxide anion; DMSO, dimethyl
sulfoxide. |
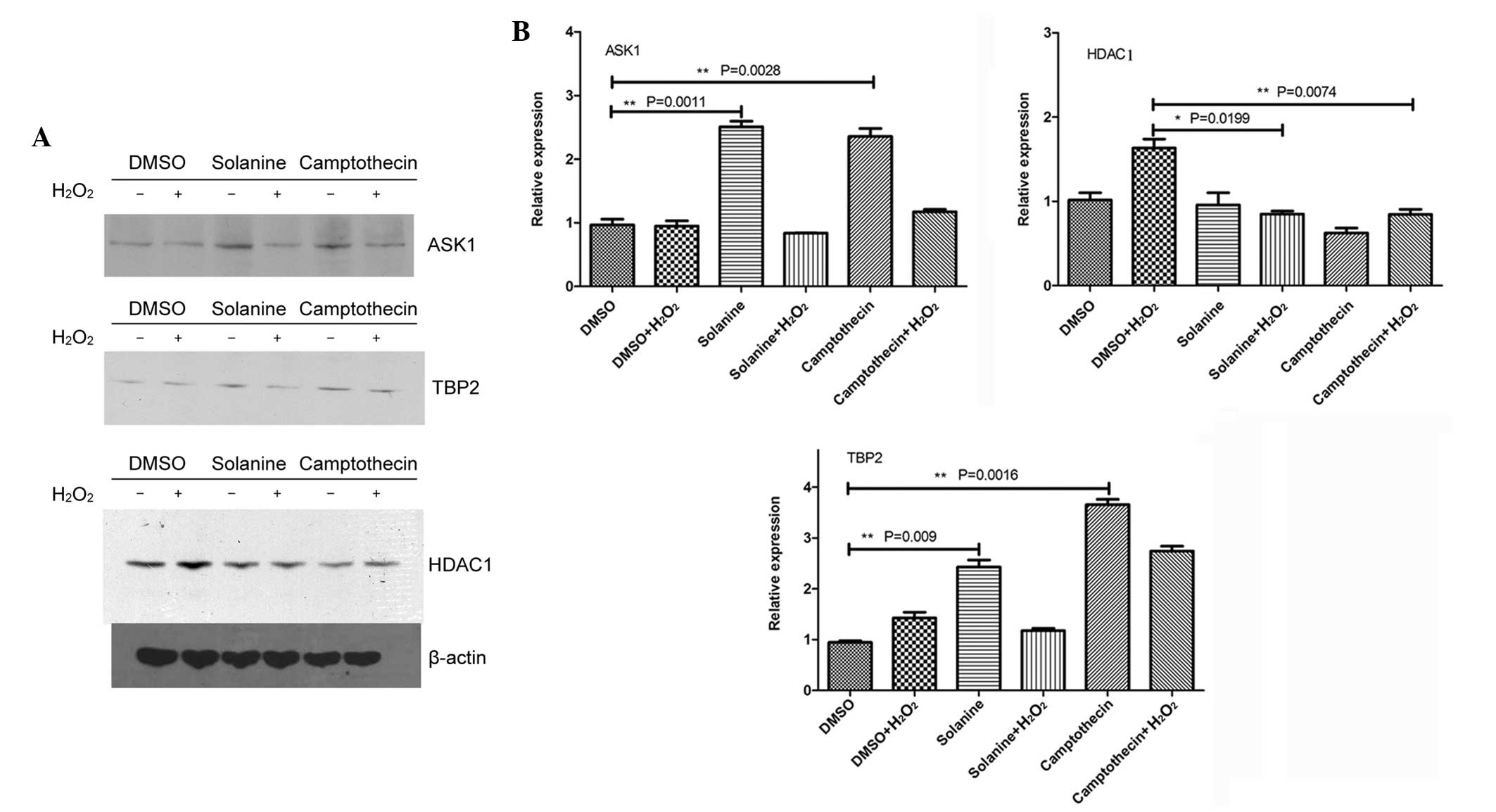
Solanine induces the upregulation of
ASK1 and TBP-2, and the downregulation of HDAC1
It has previously been suggested that ASK1 is an
important protein involved in regulating apoptosis (22). Another apoptosis-associated protein is
TBP-2, also termed vitamin D-upregulated protein 1, which
effectively combines with thioredoxin, eventually leading to
cellular apoptosis (23). The
increased expression of HDAC1 increases the proliferation of tumor
cells and alters the extracellular matrix; therefore, significantly
promoting tumor cell migration and aggression (24). In addition, HDAC1 removes the
acetyl-groups on histones, a more advanced chromosome structure,
and inhibits basic transcription complex assembly; therefore,
inhibiting transcription (25). The
present results demonstrated that solanine and camptothecin
increased the expression of ASK1 and TBP-2, but reduced the
expression of HDAC1 (Fig. 2).
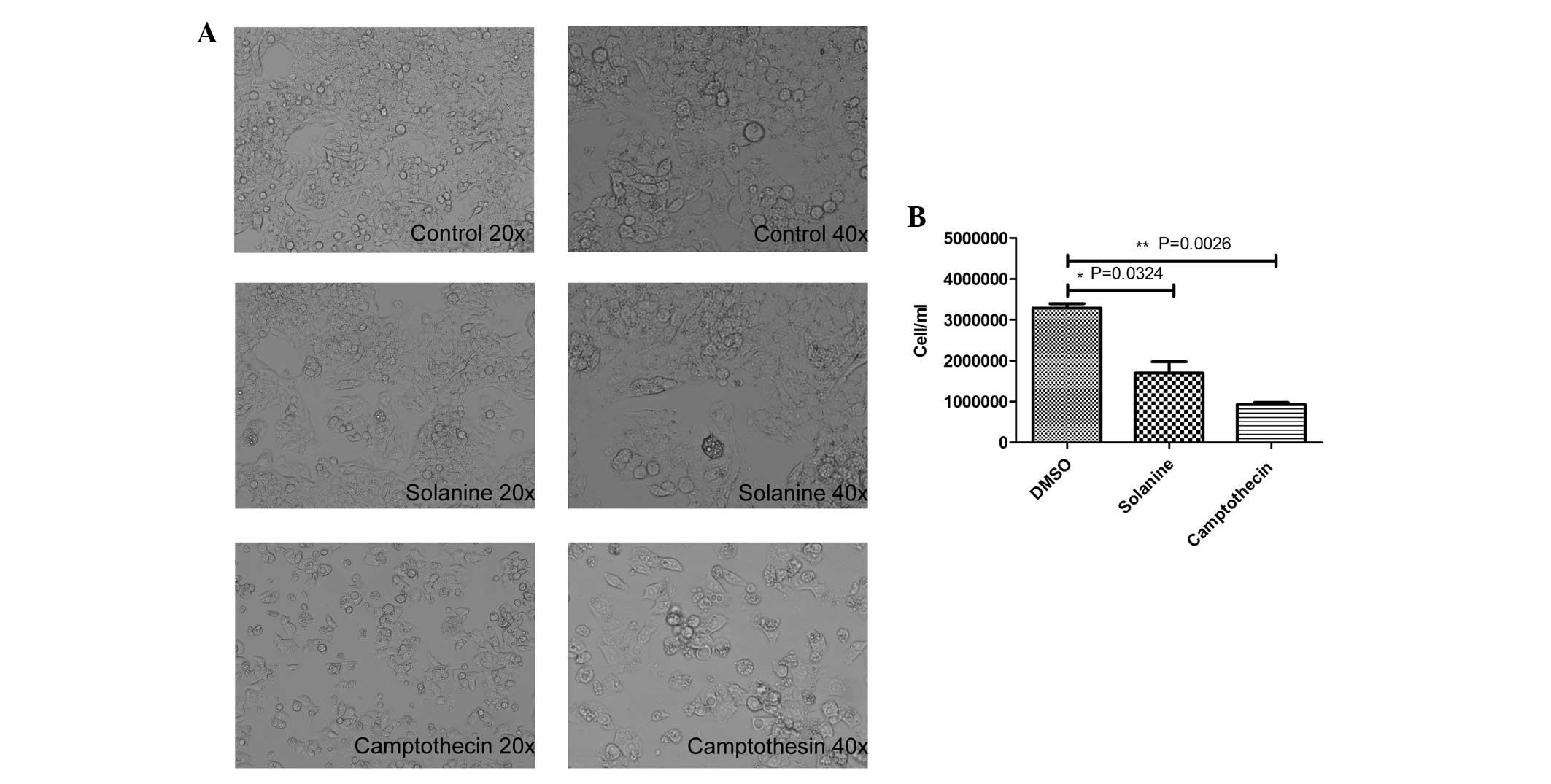
Solanine significantly inhibits the
growth of HepG2 cells and triggers cell apoptosis
Next, the present study investigated the effect of
solanine on the growth and apoptosis of the HepG2 cells. As
revealed by Fig. 3A, the HepG2 cells
treated with solanine exhibited notable morphological alterations
in contrast to the control cells, which exhibited no alterations in
morphology. The solanine-treated cells exhibited numerous
cytoplasmic vacuoles, and the cells were round, shrunken and
detached. The number of cells was significantly decreased in the
solanine-treated (n=3; P=0.0324) and camptothecin-treated groups
(n=3; P=0.0026) compared with the control group (Fig. 3B).
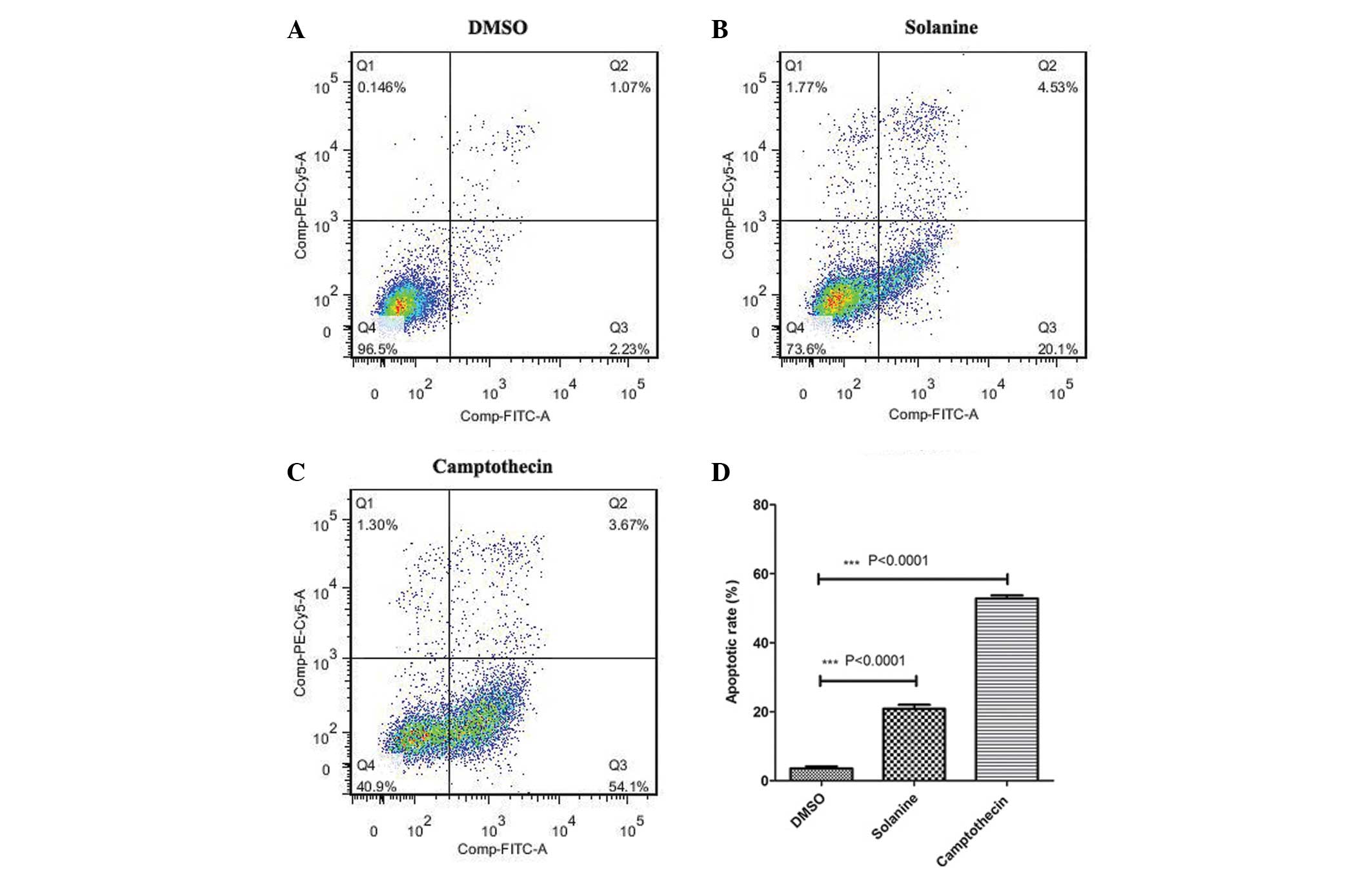
Consistent with the cell counting results, flow
cytometry analysis confirmed that there was an increased percentage
of cells undergoing apoptosis in the solanine and
camptothecin-treated groups compared with the control group
(Fig. 4). This indicates that
following treatment with solanine, the reduced cell growth observed
may be due to enhanced cellular apoptosis.
Discussion
ROS, including O2−,
H2O2 and OH−, are products of
aerobic respiration in eukaryotic cells. ROS have a cytotoxic
effect and are also intracellular signal transduction and gene
expression regulation molecules, with key roles in the regulation
of cell growth, survival and apoptosis (26–28). The
mitochondria of a eukaryotic cell are the main site for the
production of ROS, and a large amount of ROS may be produced in the
process of oxidative phosphorylation. The impact of reactive oxygen
on the mitochondria results in the dissipation of the mitochondrial
membrane potential, the release of cytochrome c and causes
the mitochondrial membrane permeability transition pore (PTP) to
open (29). A variety of proapoptotic
signals, including physical damage, radiation, chemotherapy,
excitatory amino acids and death ligands, may cause an increase in
cellular endogenous or exogenous ROS, or alter the redox
equilibrium, and these signals may trigger cellular apoptosis. Once
an apoptotic pathway is activated, an increase in the expression of
ROS may accelerate the apoptosis process (30–32).
Therefore, the release of ROS and the process of apoptosis are
mutually affected.
In recent years, solanine has become notable due to
its antitumor properties. The main components of solanine that have
these antitumor properties are the alkaloids, which lead to
cytotoxicity and anti-nuclear splitting in tumor cells (33). Previous studies have demonstrated that
solanine has a clear cytotoxic effect on human HepG2 cells through
the direct inhibition of the activity of matrix metalloproteinase
(MMP)-2 and MMP-9 and tumor angiogenesis; therefore, inhibiting
tumor metastasis (34,35). In addition, solanine reduces the
mitochondrial membrane potential, by increasing the opening of the
PTP. Opening of the PTP leads to an increase in calcium ions, which
initiates cell apoptosis resulting in an antitumor effect (36).
The present results demonstrated that solanine
induces HepG2 cells to significantly produce ROS, including
OH− and H2O2, in the cytoplasm and
mitochondria of the cell, to a similar level as the positive
control, compared with the negative control (P<0.05). In the
present study, the specific probes targeting differential-localized
ROS identified that ROS components remained in the cytoplasm and
mitochondria of the cells; however, the main location for the
production of ROS was observed to be in the mitochondria. The ROS
produced in the mitochondria may directly diffuse to the cytoplasm
due to the increasing permeability of the mitochondrial membrane.
However, the increased expression of ROS was not detected by the
DCFDA and DHR123 probes, specific for OH− and
H2O2, respectively,
post-H2O2 stimulation. This may be due to the
following possible explanations: Apoptosis, induced by antitumor
drugs, may have a different apoptosis response compared with
non-chemotherapy-induced apoptosis due to cell cycle sensitivity
(37,38). The permeability of the membrane of the
HepG2 cells remains relatively high when exposed to antitumor
drugs, indicating a potential possibility of an intracellular
pattern of diffusion as H2O2 enters the cell.
Previous findings have demonstrated that growth of tumor cells may
be inhibited when they are pretreated with chemotherapeutics.
Subsequent simulation by H2O2 may therefore
result in tumor shedding and necrosis, which has a lower
fluorescence signal. In addition, the present study identified that
there was no difference in the expression of
O2− with specific probes DHE and MITSOX in
the solanine, camptothecin and control cell groups (P>0.05).
This suggests that solanine may induce the cytoplasm and
mitochondria of HepG2 cells to produce ROS, which consists of
OH− and H2O2, but not
O2−. Therefore, the effects of solanine on
tumor cells was generated by the production of OH− and
H2O2.
In addition, the present study investigated the
alterations in the expression of certain apoptosis- and
proliferation-associated proteins in solanine-induced cellular
apoptosis. The present results demonstrated that an increase in the
expression levels of ASK1 and TBP-2, which activate downstream
signaling pathways, including c-Jun N-terminal kinases (JNK) and
p38 mitogen-activated protein kinases (p38), may be the main
mechanism through which the HepG2 cells underwent apoptosis.
Solanine also significantly inhibited the expression of the
proliferation-associated protein HDAC1, which is another possible
mechanism of the antitumor effects of solanine treatment. To
additionally elucidate the molecular mechanisms of solanine-induced
cellular apoptosis, the specific inhibition of mitochondrial
O2− should be performed to investigate
whether solanine-induced apoptosis is associated with mitochondrial
O2−. Studies are ongoing to investigate this
hypothesis.
In conclusion, solanine induces HepG2 cells to
produce ROS, mainly OH− and H2O2,
and increases the expression and activity of the
apoptosis-associated proteins ASK1 and TBP-2, which activate
downstream signaling pathways, including JNK and p38, eventually
leading to cell apoptosis. Therefore, solanine is a potential
anticancer drug, which may have a clinical application in the
future.
Acknowledgements
The present study was partially supported by the
Administration of Traditional Chinese Medicine of Zhejiang Province
(grant no. 2011ZA046), the Major Program of Science and Technology
Bureau of Zhejiang Province (grant no. 2013C37002) and the National
Natural Science Foundation of China (grant no. 81401319). The
abstract was presented at the International Liver Transplantation
Society 21st Annual International Congress July 8–11 2015 in
Chicago (IL, USA) and published as abstract no. P-110 on the
Abstracts2View™ Portal (http://www.abstracts2view.com/ilts/).
References
|
1
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stefaniuk P, Cianciara J and
Wiercinska-Drapalo A: Present and future possibilities for early
diagnosis of hepatocellular carcinoma. World J Gastroenterol.
16:418–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Wang N, Cheung F, Lao L, Li C and
Feng Y: Chinese medicines for prevention and treatment of human
hepatocellular carcinoma: Current progress on pharmacological
actions and mechanisms. J Integr Med. 13:142–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kodamatani H, Saito K, Niina N, Yamazaki S
and Tanaka Y: Simple and sensitive method for determination of
glycoalkaloids in potato tubers by high-performance liquid
chromatography with chemiluminescence detection. J Chromatogr A.
1100:26–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman M: Potato glycoalkaloids and
metabolites: Roles in the plant and in the diet. J Agric Food Chem.
54:8655–8681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bodart P and Noirfalise A: Glycoalkaloids
in potatoes. Rev Med Liege. 58:25–32. 2003.(In French). PubMed/NCBI
|
|
9
|
Shindo T, Ushiyama H, Kan K, Yasuda K and
Saito K: Contents and its change during storage of alpha-solanine
and alpha-chaconine in potatoes. Shokuhin Eiseigaku Zasshi.
45:277–282. 2004.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zywicki B, Catchpole G, Draper J and Fiehn
O: Comparison of rapid liquid chromatography-electrospray
ionization-tandem mass spectrometry methods for determination of
glycoalkaloids in transgenic field-grown potatoes. Anal Biochem.
336:178–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korpan YI, Nazarenko EA, Skryshevskaya IV,
Martelet C, Jaffrezic-Renault N and El'skaya AV: Potato
glycoalkaloids: True safety or false sense of security? Trends
Biotechnol. 22:147–151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glossman-Mitnik D: CHIH-DFT determination
of the molecular structure and infrared and ultraviolet spectra of
gamma-solanine. Spectrochim Acta A Mol Biomol Spectrosc.
66:208–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stobiecki M, Matysiak-Kata I, Frański R,
Skała J and Szopa J: Monitoring changes in anthocyanin and steroid
alkaloid glycoside content in lines of transgenic potato plants
using liquid chromatography/mass spectrometry. Phytochemistry.
62:959–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Panter KE, Gaffield W, Evans RC
and Bunch TD: Effects of steroidal glycoalkaloids from potatoes
(Solanum tuberosum) on in vitro bovine embryo development.
Anim Reprod Sci. 85:243–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji YB: Antitumor effects in traditional
Chinese medicine. Pharmacological Action, Application of Available
Composition of Traditional Chinese Medicine (1st). (Harbin,
Heilongjiang). Heilongjiang Science and Technology Press.
4331995.(In Chinese).
|
|
16
|
Son YO, Kim J, Lim JC, Chung Y, Chung GH
and Lee JC: Ripe fruit of Solanum nigrum L. inhibits cell
growth and induces apoptosis in MCF-7 cells. Food Chem Toxicol.
41:1421–1428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An L, Tang JT, Liu XM and Gao NN: Research
progress on mechanism of anti-tumor effect of Solanum
nigrum. Zhongguo Zhong Yao Za Zhi. 31:1225–1226. 2006.(In
Chinese). PubMed/NCBI
|
|
18
|
Ji YB, Gao SY, Ji CF and Zou X: Induction
of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J
Ethnopharmacol. 115:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macho A, Hirsch T, Marzo I, Marchetti P,
Dallaporta B, Susin SA, Zamzami N and Kroemer G: Glutathione
depletion is an early and calcium elevation is a late event of
thymocyte apoptosis. J Immunol. 158:4612–4619. 1997.PubMed/NCBI
|
|
20
|
Gao SY, Wang QJ and Ji YB: Effect of
solanine on the membrane potential of mitochondria in HepG2 cells
and [Ca2+]i in the cells. WorId J Gastroenterol. 12:3359–3367.
2006.
|
|
21
|
Eom KS, Kim HJ, So HS, Park R and Kim TY:
Berberine-induced apoptosis in human glioblastoma T98G cells is
mediated by endoplasmic reticulum stress accompanying reactive
oxygen species and mitochondrial dysfunction. Biol Pharm Bull.
33:1644–1649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashiguchi K, Tsuchiya H, Tomita A, Ueda
C, Akechi Y, Sakabe T, Kurimasa A, Nozaki M, Yamada T, Tsuchida S
and Shiota G: Involvement of ETS1 in thioredoxin-binding protein 2
transcription induced by a synthetic retinoid CD437 in human
osteosarcoma cells. Biochem Biophys Res Commun. 391:621–626. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouchain G and Delorme D: Novel
hydroxamate and anilide derivatives as potent histone deacetylase
inhibitors: Synthesis and antiproliferative evaluation. Curr Med
Chem. 10:2359–2372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Phi DK, Mühlbauer E and Phi-van L:
Histone deacetylase HDAC1 downregulates transcription of the
serotonin transporter (5-HTT) gene in tumor cells. Biochim Biophys
Acta. 1849:909–918. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee WL, Huang JY and Shyur LF: Phytoagents
for cancer management: Regulation of nucleic acid oxidation, ROS
and related mechanisms. Oxid Med Cell Longev. 2013:9258042013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Y, Yang J, Liu X and Yuan J:
Relationship between reactive oxygen species and apoptosis in HepG2
cells induced by sodium selenite. Wei Sheng Yan Jiu. 36:272–274.
2007.(In Chinese). PubMed/NCBI
|
|
28
|
Wu XD, Jin ZX, Wan CL and Jin R: Effect of
herba agrimoniae in apoptosis of human hepatoma cell line HepG2
cells research. Zhong Guo Zhong Yi Yao Zi Xun. 2:82010.(In
Chinese).
|
|
29
|
Chen WQ, Shen W and Shen DM: The changes
of ROS and mitochondria membrane potential in HepG2 cells on the
pressure of cisplatin. Zhonghua Gan Zang Bing Za Zhi. 13:531–533.
2005.(In Chinese). PubMed/NCBI
|
|
30
|
Hu YZ, Dong YG, Zhai YF, Lu JH, Wu MX,
Zhou Y and He ZY: Effect of simvastatin on homocysteine - induced
endothelial dysfunction and inflammatory response and its molecular
mechanisms. Zhongguo Bingli Shengli Zazhi. 8:1594–1598. 2005.(In
Chinese).
|
|
31
|
Djordjević VB: Free radicals in cell
biology. Int Rev Cytol. 237:57–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Closa D and Folch-Puy E: Oxygen free
radicals and the systemic inflammatory response. IUBMB Life.
56:185–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao XQ: Solanum nigrum compound
syrup-induced hepatocyte apoptosis in murine I-122 mechanism.
Liaoning Zhongyi Zazhi. 9:974–975. 2005.(In Chinese).
|
|
34
|
Lu MK, Shih YW, Chien Chang TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu MK, Chen PH, Shih YW, Chang YT, Huang
ET, Liu CR and Chen PS: Alpha-chaconine inhibits angiogenesis in
vitro by reducing matrix metalloproteinase-2. Biol Pharm Bull.
33:622–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lépine S, Sulpice JC and Giraud F:
Signaling pathways involved in glucocorticoid-induced apoptosis of
thymocytes. Crit Rev Immunol. 25:263–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang B, Du J, Wang J, Tan G, Gao Z, Wang Z
and Wang L: Alpinetin suppresses proliferation of human hepatoma
cells by the activation of MKK7 and elevates sensitization to
cis-diammined dichloridoplatium. Oncol Rep. 27:1090–1096.
2012.PubMed/NCBI
|
|
38
|
Al-Qubaisi M, Rozita R, Yeap SK, Omar AR,
Ali AM and Alitheen NB: Selective cytotoxicity of goniothalamin
against hepatoblastoma HepG2 cells. Molecules. 16:2944–2959. 2011.
View Article : Google Scholar : PubMed/NCBI
|