Introduction
The most common primary tumor of the central nervous
system in adults is glioma, which demonstrates a variety of
histological tumor types and World Health Organization (WHO)
malignancy grades (1,2). Usually, the treatment strategy for
glioma consists of surgery combined with radiation therapy and
chemotherapy; however, the prognosis for patients with malignant
glioma remains poor (3). In general,
a therapeutic strategy is decided according to histopathological
results of the tumors (4). The
initiation and progression of glioma is generally hypothesized to
involve the accumulation of multiple genetic alterations;
therefore, the development of novel molecular markers is required
to improve glioma classification, the prediction of the prognosis
of patients and targeted therapeutic treatment strategies (5).
Ubiquitin-conjugating (E2) enzymes are members of a
family of structurally-associated proteins that mediate
ubiquitin-dependent proteolysis, including diverse cellular
processes, such as cell cycle progression, signal transduction and
differentiation (6,7). Ubiquitin-conjugating enzyme E2C (UBE2C)
belongs to the E2 family, and is involved in mitotic cyclin
destruction and cell cycle progression (8). UBE2C has been implicated in the
carcinogenesis of certain tumors (9,10), and is
overexpressed in various human cancers, including breast,
esophageal, colon, lung, liver and ovarian cancers (11–16).
In previous studies, the upregulation of UBE2C in
brain tumors has been reported (17,18). In
addition, the expression levels of UBE2C were elevated in
high-grade astrocytomas, but not low-grade astrocytomas (18). However, to the best of our knowledge,
the prognostic significance of the expression of UBE2C in glioma
has not been elucidated. The present study used a large cohort of
glioma patients and aimed to perform an analysis of UBE2C
expression in glioma tumors and to investigate the association of
glioma with clinicopathological variables and the prognosis of
patients.
Materials and methods
Patients and tissue samples
The present study was approved by the Ethics
Committee of Beijing Tiantan Hospital (Beijing, China). Written
informed consent was obtained from all patients. Tissue samples
were obtained from patients during surgery at Beijing Tiantan
Hospital between January 2006 and December 2009. In total, 220
adult patients diagnosed with glioma were enrolled in the present
study. The glioma tumor grade of patients was diagnosed
histologically according to the 2007 WHO classification (2), as follows: 97 patients possessed grade
II disease; 34 patients possessed grade III disease; and 89
patients possessed grade IV disease. Control non-cancerous brain
tissue specimens were obtained from 5 patients with craniocerebral
injuries.
All patients had undergone prior surgical resection,
followed by adjuvant radiation and alkylating agent-based
chemotherapy. Post-operative follow-up data was gathered for all
patients, and the clinical characteristics and pathological
findings, including the age at diagnosis, gender, extent of
resection, isocitrate dehydrogenase-1 (IDH1) mutation status and
temozolomide (TMZ) chemotherapy, were obtained.
RNA extraction and gene expression
profiling
Total RNA was extracted from frozen tumor tissues
using the Ambion mirVana™ miRNA Isolation kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. RNA concentration and quality were
measured using the NanoDrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Inc., Wilmington, DE, USA), and its integrity
was determined using the Agilent Bioanalyzer 2100 (Agilent
Technologies, Inc., Santa Clara, CA, USA). Gene expression analysis
was performed using Agilent Gene Expression oligo microarrays
(Agilent Technologies, Inc.) containing >41,000 probe sets for
~27,958 human genes using the Agilent One-Color Microarray-Based
Gene Expression Analysis (catalog no., G4140–90040; Agilent
Technologies, Inc.), according to the manufacturer's protocol.
Average values of the replicate spots for each gene were background
subtracted, normalized, log2-transformed and subjected
to additional analysis.
Immunohistochemistry analysis
Formalin-fixed, paraffin-embedded (FFPE), 5 µm-thick
tissue sections were used for immunohistochemical analysis, which
was performed using a streptavidin-biotin immunoperoxidase assay,
as previously described (19).
Immunohistochemical staining was performed using anti-human mouse
monoclonal anti-UBE2C antibody (catalog no., ab201979; dilution,
1:100; Abcam, Cambridge, MA, USA). Staining for UBE2C in the
nucleus or the cytoplasm was scored as absent, weak, moderate or
strong. Absent or weak staining was categorized as negative, while
moderate or strong staining was categorized as positive. Controls
consisted of tissues without primary antibody and non-cancerous
control tissues, and were included in all experiments to ensure the
quality of staining. In total, 2 pathologists performed
immunohistochemistry scoring independently and blindly.
Statistical analysis
Statistical analyses were performed using a
χ2 test or Fisher's exact test with 2×2 tables.
Kaplan-Meier survival time analysis was used to estimate the
survival time distributions, and the log-rank test was used to
assess the statistical significance between different groups.
Multivariate analysis was performed using Cox's proportional
hazards model, and the risk ratio and 95% confidence intervals were
calculated for each characteristic. Statistical analysis was
performed using SPSS version 13.0 for Windows (SPSS, Inc., Chicago,
IL, USA). Two-tailed tests were performed. P<0.05 was considered
to indicate a statistically significant difference.
Results
UBE2C expression in glioma
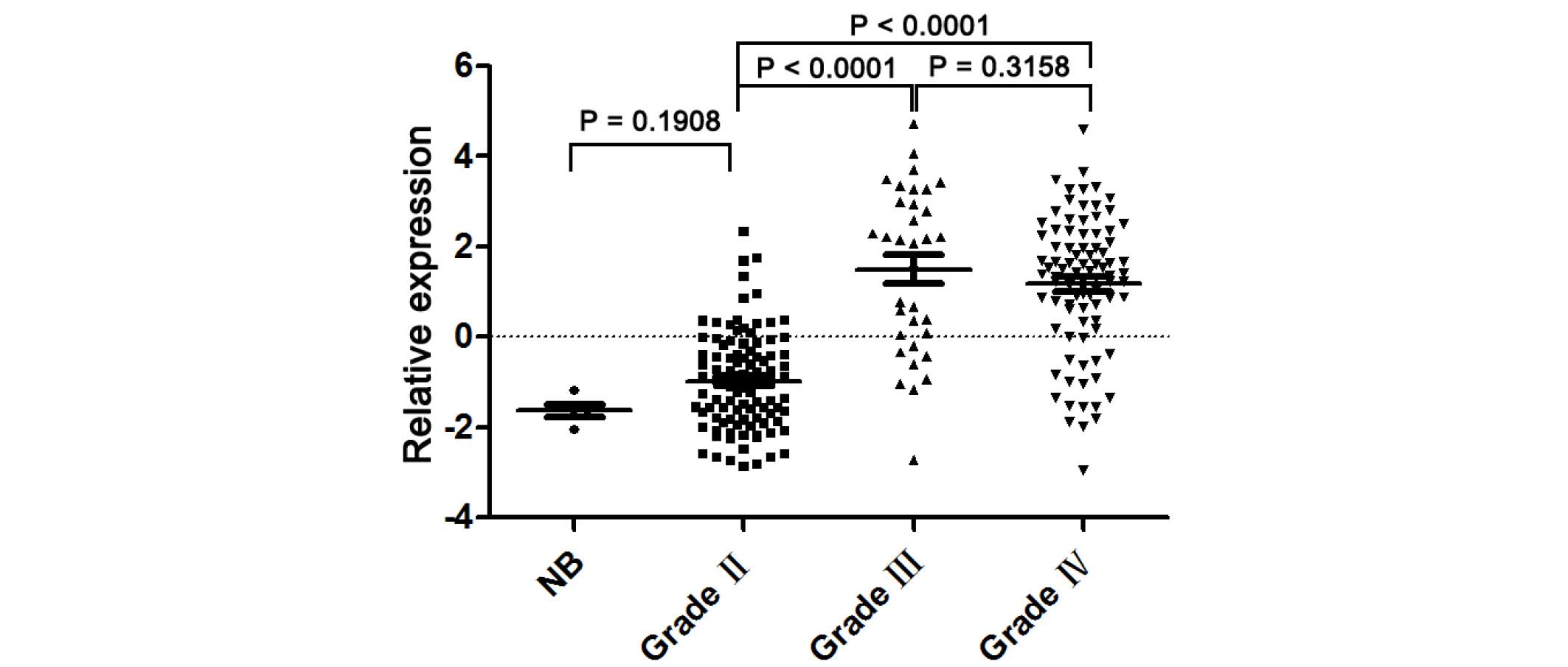
A microarray was performed to detect the UBE2C mRNA
expression in 220 glioma tissues and 5 non-cancerous brain
specimens. The present study observed significant differences in
the UBE2C expression between non-cancerous brain, low-grade glioma
and high-grade glioma tissues. Fig. 1
reveals that the expression levels of UBE2C mRNA in grade II tumor
tissues were significantly decreased compared to grade III
(P<0.0001) and IV (P<0.0001) glioma tissues. However, there
is no significant difference in UBE2C expression levels between
grade III and IV gliomas.
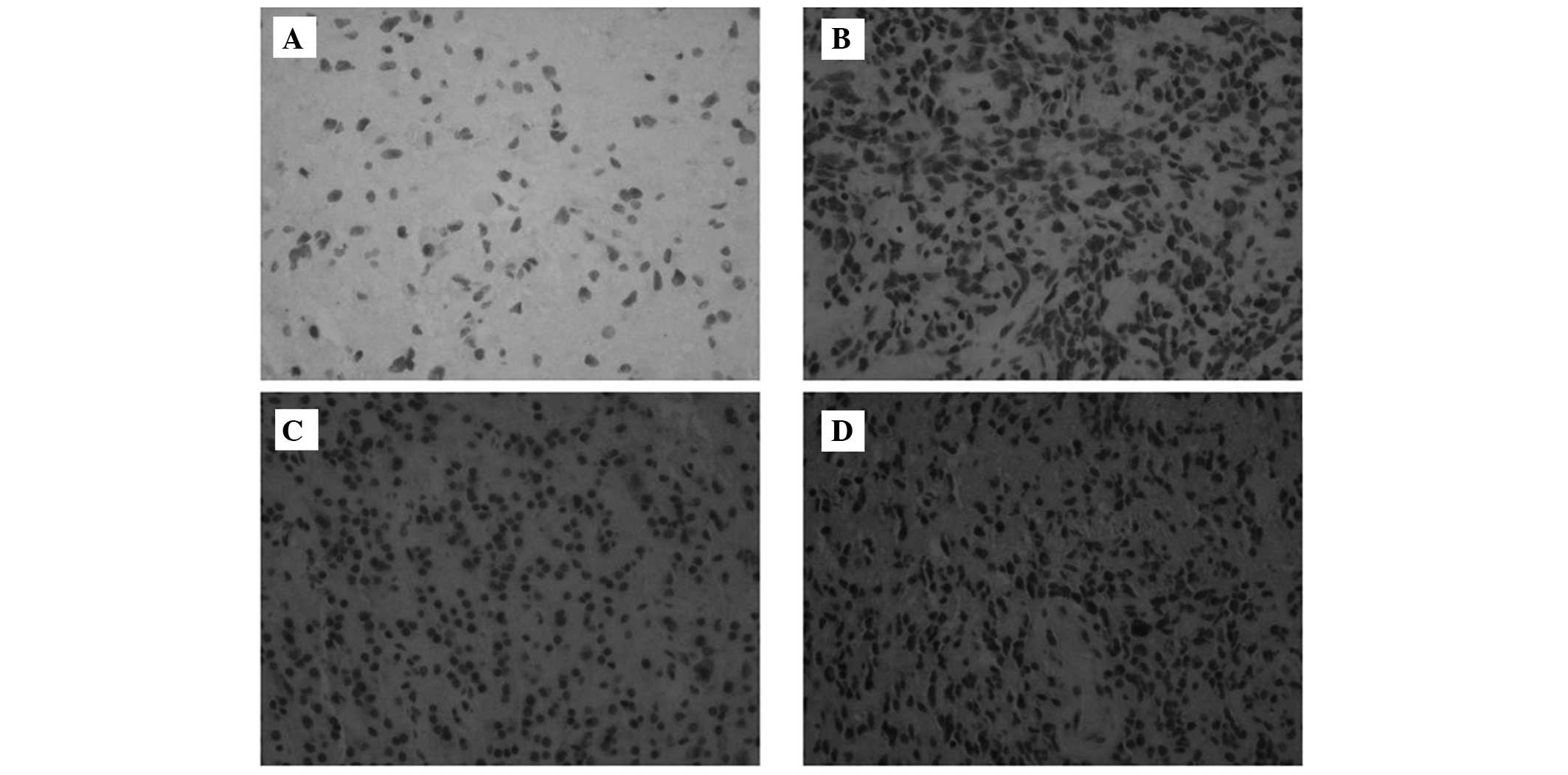
To additionally detect the expression of the UBE2C
protein in glioma tissues, immunohistochemistry was performed. As
illustrated in Fig. 2, UBE2C was not
expressed in non-cancerous brain tissues. There was significant
UBE2C protein expression in 46 of 97 (47.4%) low-grade gliomas. In
total, 73.5% of patients (25 out of 34 patients) with anaplastic
(grade III) gliomas demonstrated significant UBE2C protein
expression, whereas 78.8% (63 of 80) of glioblastomas (GBM; grade
IV) exhibited significant UBE2C staining (2).
Association between UBE2C expression
and the clinicopathological parameters
The present study evaluated the association of UBE2C
expression with clinicopathological variables (Table I). According to the strength of
staining for UBE2C in cells, which was classified as absent, weak,
moderate or strong staining, the glioma tissues were divided into
two groups, negative and positive. UBE2C expression was not
associated with the patient age, patient gender, extent of
resection, IDH1 mutation status and TMZ chemotherapy.
 | Table I.Association between UBE2C protein
expression and clinical characteristics of patients with
glioblastoma. |
Table I.
Association between UBE2C protein
expression and clinical characteristics of patients with
glioblastoma.
| Characteristic | Negative | Positive | P-value |
|---|
| Total, n | 17 | 63 |
|
| Age, years |
|
|
|
| <48 | 10 | 23 | 0.16 |
|
>48 | 7 | 40 |
|
| Gender |
|
|
|
| Male | 12 | 38 | 0.58 |
|
Female | 5 | 25 |
|
| Extent of
resection |
|
|
|
|
Total | 4 | 28 | 0.17 |
|
Subtotal | 13 | 35 |
|
| IDH1 mutation
status |
|
|
|
| No
mutation | 4 | 7 | 0.23 |
|
Mutation | 13 | 56 |
|
| TMZ chemotherapy |
|
|
|
| No
TMZ | 12 | 46 | 1.00 |
| TMZ | 5 | 17 |
|
Association between UBE2C expression
and survival time
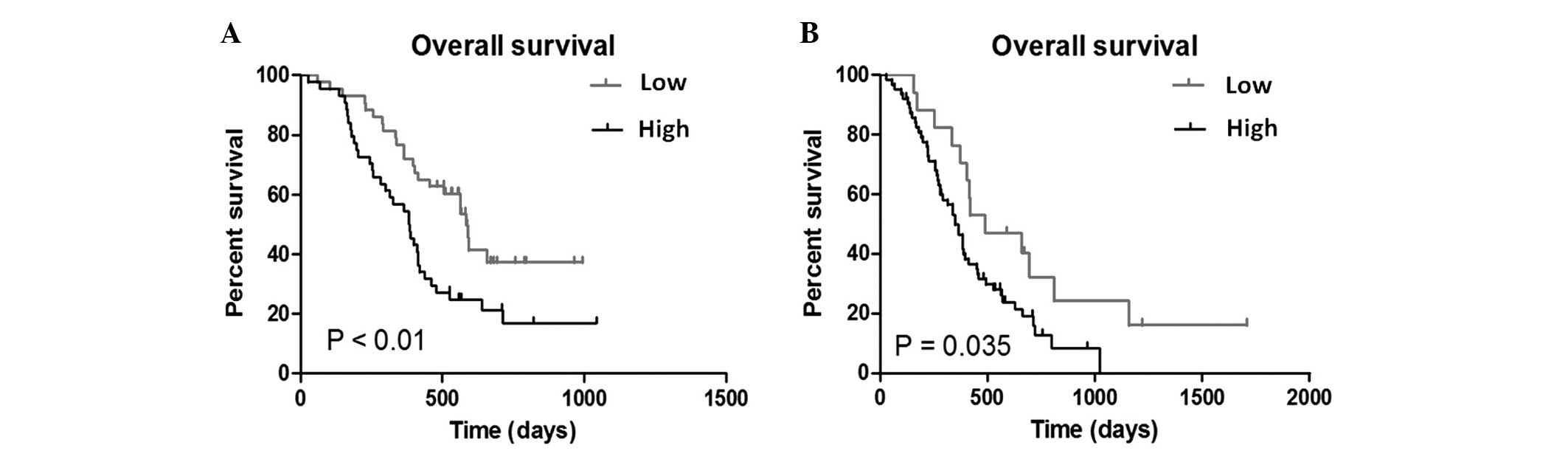
The Kaplan-Meier method was performed to elucidate
the association of UBE2C expression with the prognosis of glioma
patients. The median expression of UBE2C in anaplastic glioma
patients was 2.1061 and the median expression of UBE2C in GBM
patients was 1.3937. When the median expression values of UBE2C
(the relative expression level detected by microarray) was used as
the cut-off point, patients with a higher UBE2C expression
possessed a significantly decreased overall survival time in
patients with anaplastic glioma (P<0.01; Fig. 3A) and those with GBM (P=0.035;
Fig. 3B).
Multivariate analysis of 80 patients with GBM was
performed for UBE2C expression and pathological predictors for
survival time using Cox's proportional hazard regression analysis.
The results demonstrated that the UBE2C expression, extent of
resection, TMZ chemotherapy and IDH1 mutation status were
independent prognostic factors of patient prognosis (Table II). Therefore, the expression level
of UBEC1 may be a valuable prognostic marker for glioblastoma
patients.
 | Table II.Association between UBE2C expression
and clinical characteristics and the clinical outcome in 80
glioblastoma patients. |
Table II.
Association between UBE2C expression
and clinical characteristics and the clinical outcome in 80
glioblastoma patients.
| Characteristic | HR | 95% CI | P-value |
|---|
| Gender |
|
|
|
| Male | 1.00 |
|
|
|
Female | 0.84 | 0.50–1.41 | 0.51 |
| Age, years |
|
|
|
| <48 | 1.00 |
|
|
|
>48 | 0.82 | 0.47–1.44 | 0.49 |
| Extent of
resection |
|
|
|
|
Subtotal | 1.00 |
|
|
|
Total | 0.44 | 0.25–0.77 | <0.01 |
| UBE2C
expression |
|
|
|
|
Low | 1.00 |
|
|
|
High | 2.40 | 1.23–4.69 |
0.01 |
| IDH1 mutation
status |
|
|
|
| No
mutation | 1.00 |
|
|
| Mutation | 0.34 | 0.14–0.83 |
0.02 |
| TMZ
chemotherapy |
|
|
|
| No
TMZ | 1.00 |
|
|
|
TMZ | 0.37 | 0.20–0.70 | <0.01 |
Discussion
Halting cell-cycle progression is crucial for the
development of tumorigenesis and tumor progression (20). UBE2C is a human homolog of the
cyclin-selective E2, which is involved in the destruction of
mitotic cyclins (21). Previous
studies have identified that, at the early G1 phase of the cell
cycle, UBE2C undergoes anaphase-promoting, complex-dependent
degradation (22,23). This cell cycle-dependent expression of
UBE2C may be a unique autoregulatory feedback loop for the
regulation of antigen-presenting cells (24). Therefore, an impairment of UBE2C
expression may inhibit the autoregulatory feedback loop and lead to
the dysregulation of cell growth (22,24,25). A
previous study by van Ree et al (26) identified that UBE2C overexpression
leads to early degradation of cyclin B, supernumerary centrioles,
lagging chromosomes and aneuploidy. In addition, UBE2C transgenic
mice were prone to carcinogen-induced lung tumors and a broad
spectrum of spontaneous tumors (26).
These results indicate that UBE2C is a prominent protooncogene,
which causes instability of the whole chromosome and leads to tumor
formation.
The present study evaluated the expression levels of
UBE2C in 220 human glioma tissues and 5 non-cancerous brain tissue
specimens. The present study revealed that UBE2C was expressed at
low levels in non-cancerous and low-grade glioma tissues, whereas
high-grade gliomas expressed UBE2C at increased levels. This result
was consistent with previous studies, which have revealed the
involvement of UBE2C in the malignant transformation of tissues and
tumor progression (8,27,28).
Okamoto et al (24) observed
that the expression levels of UBE2C were extremely low in numerous
normal tissues, but prominent in the majority of primary tumors and
cancerous cell lines. Additionally, an overexpression of UBE2C
stimulated growth and colony-forming activity in NIH3T3 cells
(24). In astrocytic tumors, UBE2C
gene expression has been reported to increase with advancing
pathological stages (17). Therefore,
UBE2C may be vital in the aggressive progression of glioma.
However, the association between UBE2C expression
and the clinical prognosis of glioma patients has not been defined.
The present study demonstrated for the first time, to the best of
our knowledge, that a high expression of UBE2C was associated with
a poor outcome for patients with malignant gliomas. In the present
study, 34 anaplastic gliomas and 89 GBM tissues were analyzed for
UBE2C expression and survival time. The results demonstrated that
anaplastic glioma and GBM patients with an increased UBE2C
expression possessed a significantly decreased overall survival
time. Multivariate analysis of 80 GBM patients indicated that UBE2C
expression is an independent prognostic factor. These findings
suggest that UBE2C may be a reliable indicator for prognosis in
malignant glioma patients.
In summary, the present study demonstrated that
UBE2C is overexpressed in malignant glioma. This result is based on
a large sample of frozen and FFPE tissue samples. The present study
identified for the first time, to the best of our knowledge, that
UBE2C is associated with an aggressive clinical outcome and
decreased overall survival time. Multivariate regression analysis
indicated the independence of UBE2C overexpression as a predictor
for poor prognosis in malignant glioma patients. Additional
investigation is required to confirm the findings prior to the
clinical application of UBE2C.
Acknowledgements
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7154193).
Glossary
Abbreviations
Abbreviations:
|
UBE2C
|
ubiquitin-conjugating enzyme E2C
|
|
GBM
|
glioblastoma
|
|
IDH1
|
isocitrate dehydrogenase-1
|
|
TMZ
|
temozolomide
|
References
|
1
|
Ricard D, Idbaih A, Ducray F, Lahutte M,
Hoang-Xuan K and Delattre JY: Primary brain tumours in adults.
Lancet. 379:1984–1996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fuller GN and Scheithauer BW: The 2007
revised World Health Organization (WHO) classification of tumours
of the central nervous system: Newly codified entities. Brain
Pathol. 17:304–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talacchi A, Turazzi S, Locatelli F, Sala
F, Beltramello A, Alessandrini F, Manganotti P, Lanteri P, Gambin
R, Ganau M, et al: Surgical treatment of high-grade gliomas in
motor areas. The impact of different supportive technologies: A
171-patient series. J Neurooncol. 100:417–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nitta M, Muragaki Y, Maruyama T, Iseki H,
Ikuta S, Konishi Y, Saito T, Tamura M, Chemov M, Watanabe A, et al:
Updated therapeutic strategy for adult low-grade glioma stratified
by resection and tumor subtype. Neurol Med Chir (Tokyo).
53:447–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martinho O, Granja S, Jaraquemada T,
Caeiro C, Miranda-Gonçalves V, Honavar M, Costa P, Damasceno M,
Rosner MR, Lopes JM and Reid RM: Downregulation of RKIP is
associated with poor outcome and malignant progression in gliomas.
PLoS One. 7:e307692012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joazeiro CA and Weissman AM: RING finger
proteins: Mediators of ubiquitin ligase activity. Cell.
102:549–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen Z, Jiang X, Zeng C, Zheng S, Luo B,
Zeng Y, Ding R, Jiang H, He Q, Guo J and Jie W: High expression of
ubiquitin-conjugating enzyme 2C (UBE2C) correlates with
nasopharyngeal carcinoma progression. BMC Cancer. 13:1922013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rape M and Kirschner MW: Autonomous
regulation of the anaphase-promoting complex couples mitosis to
S-phase entry. Nature. 432:588–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rape M, Reddy SK and Kirschner MW: The
processivity of multiubiquitination by the APC determines the order
of substrate degradation. Cell. 124:89–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parris TZ, Danielsson A, Nemes S, Kovács
A, Delle U, Fallenius G, Möllerström E, Karlsson P and Helou K:
Clinical implications of gene dosage and gene expression patterns
in diploid breast carcinoma. Clin Cancer Res. 16:3860–3874. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Raoof DA, Wang Z, Lin MY, Thomas
DG, Greenson JK, Giordano TJ, Orringer MB, Chang AC, Beer DG and
Lin L: Expression and effect of inhibition of the
ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma.
Neoplasia. 8:1062–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bavi P, Uddin S, Ahmed M, Jehan Z, Bu R,
Abubaker J, Sultana M, Al-Sanea N, Abduljabbar A, Ashari LH, et al:
Bortezomib stabilizes mitotic cyclins and prevents cell cycle
progression via inhibition of UBE2C in colorectal carcinoma. Am J
Pathol. 178:2109–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner KW, Sapinoso LM, El-Rifai W,
Frierson HF, Butz N, Mestan J, Hofmann F, Deveraux QL and Hampton
GM: Overexpression, genomic amplification and therapeutic potential
of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of
diverse anatomic origin. Oncogene. 23:6621–6629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ieta K, Ojima E, Tanaka F, Nakamura Y,
Haraguchi N, Mimori K, Inoue H, Kuwano H and Mori M: Identification
of overexpressed genes in hepatocellular carcinoma, with special
reference to ubiquitin-conjugating enzyme E2C gene expression. Int
J Cancer. 121:33–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berlingieri MT, Pallante P, Guida M, Nappi
C, Masciullo V, Scambia G, Ferraro A, Leone V, Sboner A,
Barbareschi M, et al: UbcH10 expression may be a useful tool in the
prognosis of ovarian carcinomas. Oncogene. 26:2136–2140. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donato G, Iofrida G, Lavano A, Volpentesta
G, Signorelli F, Pallante PL, Berlingieri MT, Pierantoni MG,
Palmieri D, Conforti F, et al: Analysis of UbcH10 expression
represents a useful tool for the diagnosis and therapy of
astrocytic tumors. Clin Neuropathol. 27:219–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Huang CG, Lu YC, Luo C, Hu GH,
Liu HM, Chen JX and Han HX: Expression of ubiquitin-conjugating
enzyme E2C/UbcH10 in astrocytic tumors. Brain Res. 1201:161–166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Qiu XG, Chen BS, Li SW, Cui Y,
Ren H and Jiang T: Antiangiogenic therapy with bevacizumab in
recurrent malignant gliomas: Analysis of the response and core
pathway aberrations. Chin Med J (Engl). 122:1250–1254.
2009.PubMed/NCBI
|
|
20
|
Jiang L, Wang T, Bao Y, Qian J, Wu XJ, Hu
GH and Lu YC: A study of UbcH10 expression and its association with
recurrence of meningiomas. J Surg Oncol. 106:327–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Townsley FM, Aristarkhov A, Beck S,
Hershko A and Ruderman JV: Dominant-negative cyclin-selective
ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase.
Proc Natl Acad Sci USA. 94:2362–2367. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamanaka A, Hatakeyama S, Kominami K,
Kitagawa M, Matsumoto M and Nakayama K: Cell cycle-dependent
expression of mammalian E2-C regulated by the anaphase-promoting
complex/cyclosome. Mol Biol Cell. 11:2821–2831. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujita T, Ikeda H, Taira N, Hatoh S, Naito
M and Doihara H: Overexpression of UbcH10 alternates the cell cycle
profile and accelerate the tumor proliferation in colon cancer. BMC
Cancer. 9:872009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamoto Y, Ozaki T, Miyazaki K, Aoyama M,
Miyazaki M and Nakagawara A: UbcH10 is the cancer-related E2
ubiquitin-conjugating enzyme. Cancer Res. 63:4167–4173.
2003.PubMed/NCBI
|
|
25
|
Troncone G, Guerriero E, Pallante P,
Berlingieri MT, Ferraro A, Del Vecchio L, Gorrese M, Mariotti E,
Iaccarino A, Palmieri EA, et al: UbcH10 expression in human
lymphomas. Histopathology. 54:731–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Ree JH, Jeganathan KB, Malureanu L and
van Deursen JM: Overexpression of the E2 ubiquitin-conjugating
enzyme UbcH10 causes chromosome missegregation and tumor formation.
J Cell Biol. 188:83–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto A, Ishibashi Y, Urashima M,
Omura N, Nakada K, Nishikawa K, Shida A, Takada K, Kashiwagi H and
Yanaga K: High UBCH10 protein expression as a marker of poor
prognosis in esophageal squamous cell carcinoma. Anticancer Res.
34:955–961. 2014.PubMed/NCBI
|
|
28
|
Li SZ, Song Y, Zhang HH, Jin BX, Liu Y,
Liu WB, Zhang XD and Du RL: UbcH10 overexpression increases
carcinogenesis and blocks ALLN susceptibility in colorectal cancer.
Sci Rep. 4:69102014. View Article : Google Scholar : PubMed/NCBI
|