Introduction
Breast cancer is one of the most common malignancies
and leading causes of cancer-associated mortalities among females
(1). Based on DNA microarray
techniques, breast cancer is classified into five subtypes: Luminal
A; luminal B; normal breast-like; human epidermal growth factor
receptor 2 (HER2/neu) overexpressing; and basal-like (2). The subtype that is immunohistochemically
characterized by the lack of expression of the estrogen receptor
(ER), progesterone receptor (PR) and HER2 is defined as triple
negative breast cancer (TNBC) (3).
TNBC, which accounts for 10–15% of breast cancers,
is considered to exhibit an aggressive clinical behavior and poor
prognosis, due to the insensitivity of the cancer to endocrine and
targeted therapy (4–12). Therefore, chemotherapy is a
significant therapy for such cancers. The treatment options for
TNBC include anthracyclines, taxanes, platinum and alkylating
agents (13–16). However, there is no standard
chemotherapy regimen for TNBC.
Based on National Comprehensive Cancer Network
guidelines, the adjuvant 5-fluorouracil, epirubicin and
cyclophosphamide (FEC) chemotherapy regimen is the suggested
regimen for breast cancer. However, there is no conclusion
regarding the clinicopathological and demographical factors of TNBC
patients that are suitable for adjuvant FEC chemotherapy.
Therefore, the aim of the present retrospective study is to analyze
the association between the clinicopathological and demographical
characteristics and the survival of TNBC patients that receive FEC
adjuvant chemotherapy.
Materials and methods
Patients
In total, 956 patients were diagnosed with TNBC, and
25.0% (239/956) of these patients had received adjuvant FEC
chemotherapy with surgery, modified radical mastectomy or
breast-conserving surgery plus a sentinel lymph node examination at
the Harbin Medical University (Harbin, Heilongjiang, China) between
April 2001 and December 2008. These 239 patients were included in
the present study. Primary cancers were evaluated in accordance
with the 7th edition of the American Joint Committee on Cancer
(AJCC) tumor-node-metastasis (TNM) staging system. The inclusion
criteria included females with histologically confirmed ER, PR and
HER2-negative breast cancer, between stages I and IIIA. The
criteria for TNBC were 0% expression for ER, 0% expression for PR
and HER2 expression of 0 or 1+ only. Patients with an
immunohistochemical score for HER-2 neu of 2+
demonstrated no HER2 gene amplification by fluorescence in situ
hybridization. These patients had not undergone neoadjuvant
chemotherapy and radiotherapy prior to surgery. The exclusion
criteria included stage IV disease and a history of other cancers.
Pathology reports were obtained from the medical record room of the
Harbin Medical University. Data on the demographical and clinical
characteristics, extent of disease, and types of surgery,
chemotherapy and radiotherapy were collected from the medical
records. The data also included the menopausal status based on the
hormone level and age, family history of cancer in first and
second-degree relatives, tumor size and lymph node status. The
number of pregnancies consisted of full-term pregnancies,
non-full-term pregnancies and miscarriages. Clinical data of these
patients were used for survival analysis.
Treatment
All patients underwent conservative surgery or a
modified radical mastectomy. Chemotherapy consisting of adjuvant
FEC was administered to patients (500 mg/m2
cyclophosphamide, day 1; 75 mg/m2 epirubicin, day 1; 500
mg/m2 5-fluorouracil, days 1 and 8, every 3 weeks, for
at least 4 cycles).
Statistical Analysis
The vital status of the study group was assessed
from the medical record room. January 30, 2013 was the follow-up
completion date. Univariate and multivariate Cox proportional
hazard models were used to assess the effects of variables on
patient survival (Table I). The
parameters were then tested using the multivariate Cox proportional
hazards model, which was performed to identify the independent
variables for predicting survival. Hazard ratios (HR) and the 95%
confidence intervals (CIs) were recorded for each factor.
Disease-free survival (DFS) time was calculated from the date of
surgery resection to the date of the final follow-up or relapse.
Overall survival (OS) time was defined as the elapsed time between
the date of the surgery and the date of last follow-up or
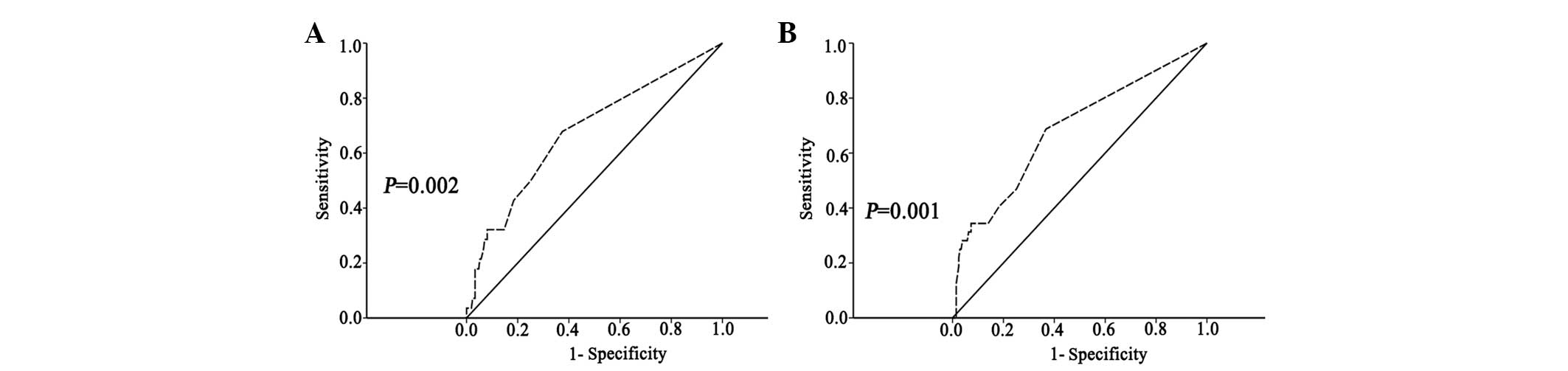
mortality. The cut-off value was selected using the receiver
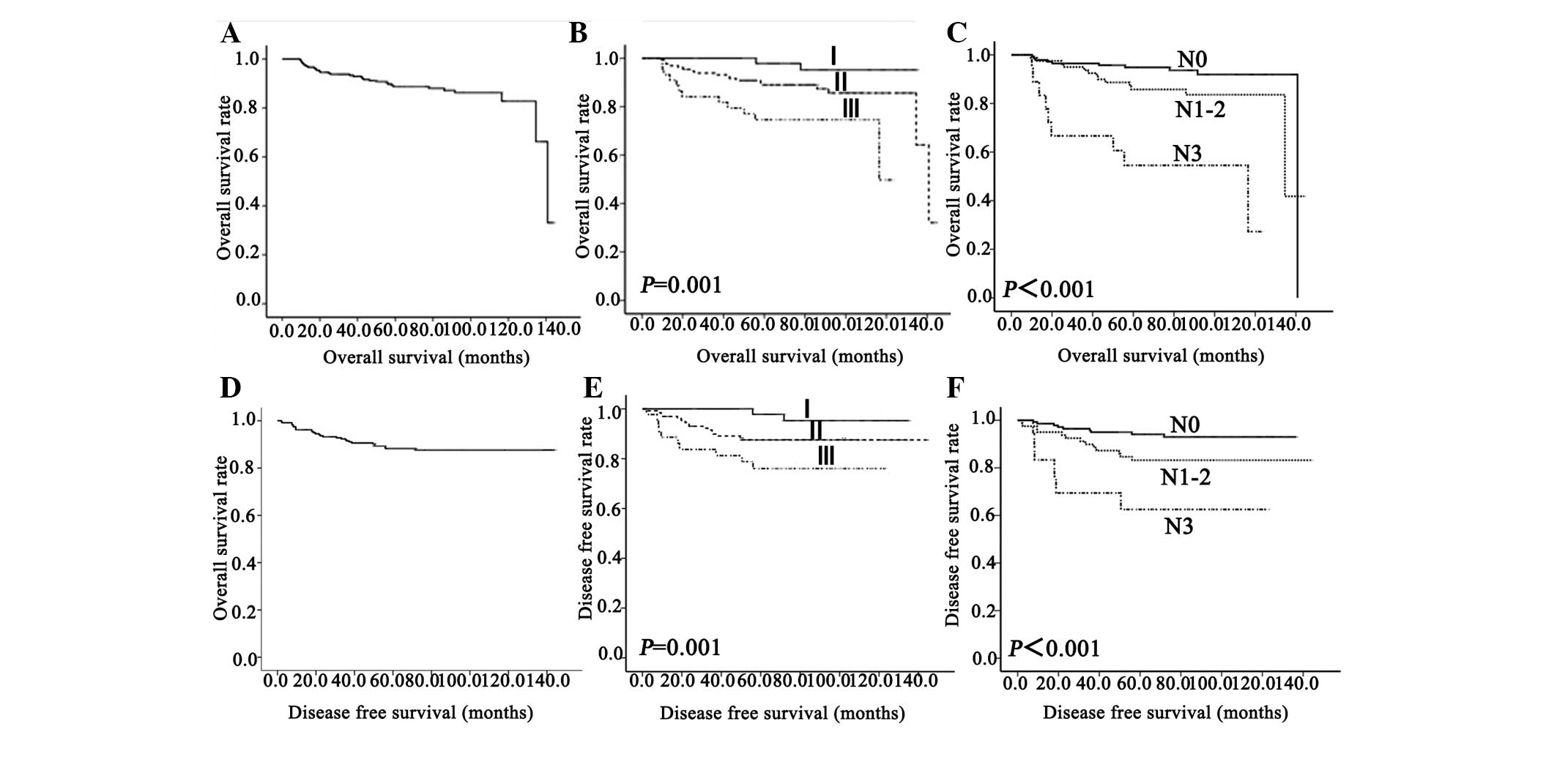
operating characteristic (ROC) curve analysis (Fig. 1). Survival was estimated using the
Kaplan-Meier method (Fig. 2).
Spearman's χ2 test was used to analyze categorical
variables (Table II). P<0.05 was
considered to indicate a statistically significant difference. SPSS
19.0 software for Windows (IBM SPSS, Armonk, NY, USA) was used for
statistical analysis.
 | Table I.The clinicopathological and
demographical factors of triple-negative breast cancer patients
with adjuvant fluorouracil, epirubicin and cyclophosphamide
chemotherapy. |
Table I.
The clinicopathological and
demographical factors of triple-negative breast cancer patients
with adjuvant fluorouracil, epirubicin and cyclophosphamide
chemotherapy.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.861
(0.430–1.725) |
0.673 |
|
0.473 | 1.308
(0.613–2.793) |
0.488 |
|
0.757 |
| Menopausal
status | 1.225
(0.603–2.489) |
0.574 |
|
0.369 | 0.836
(0.399–1.754) |
0.636 |
|
0.974 |
| Number of
pregnancies | 1.246
(0.613–2.533) |
0.544 |
|
0.841 | 2.454
(1.149–5.239) |
0.020 |
|
0.077 |
| Family history | 1.094
(0.438–2.731) |
0.848 |
|
0.825 | 0.921
(0.350–2.423) |
0.868 |
|
0.667 |
| Tumor
histology | 0.767
(0.183–3.220) |
0.717 |
|
0.863 | 0.412
(0.056–3.034) |
0.384 |
|
0.454 |
| Adjuvant
radiotherapy | 1.394
(0.422–4.601) |
0.585 |
|
0.704 | 2.066
(0.716–5.959) |
0.179 |
|
0.574 |
| Primary
surgery | 1.411
(0.380–5.244) |
0.607 |
|
0.246 | 0.708
(0.106–4.717) |
0.721 |
|
0.924 |
| pT stage | 1.254
(0.657–2.393) |
0.493 |
|
0.848 | 1.238
(0.626–2.450) |
0.539 |
|
0.957 |
| pN stage | 1.996
(1.465–2.720) | <0.001 | 1.996
(1.465–2.720) | <0.001 | 1.824
(1.315–2.531) | <0.001 | 1.824
(1.315–2.531) | <0.001 |
| TNM stage | 2.901
(1.640–5.132) | <0.001 |
|
0.540 | 2.442
(1.357–4.395) |
0.003 |
|
0.375 |
 | Table II.Correlation between the pT, pN and
TNM stages, and the clinicopathological and demographic
characteristics of patients with triple-negative breast cancer
patients. |
Table II.
Correlation between the pT, pN and
TNM stages, and the clinicopathological and demographic
characteristics of patients with triple-negative breast cancer
patients.
|
|
| pT classification,
n | pN stage, n | TNM stage, n |
|---|
|
|
|
|
|
|
|---|
| Variables | Patients, n | T1 | T2 | T3 | Correlation
(P-value) | N0 | N1 | N2 | N3 | Correlation
(P-value) | I | II | III | Correlation
(P-value) |
|---|
| All cases | 239 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Age |
|
|
|
| −0.100 (0.122) |
|
|
|
| 0.078
(0.232) |
|
|
| 0.031
(0.632) |
| <48 years | 109 | 34 | 69 | 6 |
| 67 | 30 | 6 | 6 |
| 27 | 67 | 15 |
|
| ≥48 years | 130 | 52 | 74 | 4 |
| 74 | 28 | 16 | 12 |
| 37 | 64 | 29 |
|
| Menopausal
status |
|
|
|
| 0.051
(0.431) |
|
|
|
| −0.085 (0.191) |
|
|
| −0.048 (0.463) |
|
Premenopausal | 130 | 44 | 80 | 6 |
| 61 | 24 | 3 | 11 |
| 34 | 77 | 19 |
|
|
Postmenopausal | 109 | 42 | 63 | 4 |
| 80 | 34 | 9 | 7 |
| 30 | 54 | 25 |
|
| Number of
pregnancies |
|
|
|
| 0.146
(0.024)a |
|
|
|
| 0.177
(0.006)b |
|
|
|
0.241
(<0.001)b |
| ≥2 | 95 | 26 | 64 | 5 |
| 47 | 25 | 12 | 11 |
| 16 | 52 | 27 |
|
| ≤1 | 144 | 60 | 79 | 5 |
| 94 | 33 | 10 | 7 |
| 48 | 79 | 17 |
|
| Family history |
|
|
|
| 0.023
(0.725) |
|
|
|
| 0.042
(0.519) |
|
|
| 0.046
(0.479) |
|
Yes | 40 | 15 | 24 | 1 |
| 25 | 9 | 6 | 0 |
| 13 | 20 | 7 |
|
| No | 199 | 71 | 119 | 9 |
| 116 | 49 | 16 | 18 |
| 51 | 111 | 37 |
|
| Tumor
histology |
|
|
|
| −0.011 (0.871) |
|
|
|
| −0.016 (0.807) |
|
|
| −0.006 (0.925) |
| Ductal
adenocarcinoma | 219 | 86 | 133 | 0 |
| 129 | 53 | 19 | 18 |
| 59 | 119 | 41 |
|
|
Non-ductal adenocarcinoma | 20 | 0 | 10 | 10 |
| 12 | 5 | 3 | 0 |
| 5 | 12 | 3 |
|
| Adjuvant
radiotherapy |
|
|
|
| −0.021(0.750) |
|
|
|
| 0.199
(0.002)b |
|
|
| 0.122
(0.060) |
|
Yes | 19 | 7 | 12 | 0 |
|
6 | 4 | 6 | 3 |
| 5 |
5 | 9 |
|
| No | 220 | 79 | 131 | 10 |
| 135 | 54 | 16 | 15 |
| 59 | 126 | 35 |
|
| Primary
surgery |
|
|
|
| −0.186
(0.004)b |
|
|
|
| −0.041 (0.529) |
|
|
| −0.147
(0.023)a |
|
Modified radical
mastectomy | 227 | 77 | 140 | 10 |
| 133 | 55 | 22 | 17 |
| 57 | 127 | 43 |
|
|
Conservative surgery | 12 | 9 |
3 | 0 |
|
8 | 3 | 0 | 1 |
| 7 |
4 | 1 |
|
Results
The demographic and clinical characteristics and the
treatment options of the patients are presented in Table I. The average age of patients was
48.3±9.4 years (median, 49.0 years; range, 26.0–76.0 years). The
average follow-up time was 80.1±30.4 months (range, 9.5–144.1
months). The number of pre-menopausal patients was 130 patients,
54.4% of the total. Of the patients, 6 (2.5%) suffered bone
metastases and 25 patients (10.5%) suffered visceral metastases.
The lymph node metastatic rate of patients with tumor sizes of
<2 cm was 27.9% (24/86); however, for tumor sizes of >2 cm,
the rate was as high as 48.4% (74/153).
The results of univariate analysis are shown in
Table I. The univariate analysis
showed that the lymph node status (P<0.001) and stage of disease
(P<0.001) were significantly associated with the OS time. The OS
time showed no significant difference for age (P=0.673), menopausal
status (P=0.574), number of pregnancies (P=0.544), family history
(P=0.848), tumor histology (P=0.717), adjuvant radiotherapy
(P=0.585), primary surgery (P=0.607) or tumor size (P=0.493). The
DFS was significantly associated with the lymph node status
(P<0.001) and stage of disease (P=0.003), but not with the other
factors. In the multivariate statistical analysis, the significant
independent prognostic variable affecting survival, including OS
and DFS time, was lymph node status rather than the stage of
disease, despite the stage of disease being a well-characterized
independent prognostic factor (Table
I)
The prognostic value of lymph node status on TNBC
recurrence and mortality was assessed using ROC analysis. The
association of lymph node status and tumor recurrence or mortality
was identified. The cut-off points for OS and DFS in the study
population were each 0.5. The area under the curves (AUCs) of the
lymph node status for TNBC recurrence and mortality were 0.676
(P=0.002; 95% CI, 0.580–0.791) and 0.685 (P=0.001; 95% CI,
0.565–0.788), respectively (Fig
1.).
The OS rates of patients following diagnosis were
97.5, 92.1 and 71.1% at 12, 36 and 60 months, respectively; the DFS
rates were 95.0, 88.3 and 69.0%, respectively (Fig. 2). The Kaplan-Meier survival curves
stratified for lymph node status and stage of disease are exhibited
in Fig. 2. Patients with N3 or stage
III disease tended to demonstrate a shorter OS and DFS compared
with patients with N0-2 (OS, P<0.001; DFS, P<0.001) or stage
I–II disease (OS, P=0.001; DFS, P=0.005) (Fig. 2.).
The tumor size, lymph node status and stage of
disease were all associated with the number of pregnancies.
Additionally, the lymph node status and stage of disease were
associated with primary surgery, and the lymph node status was
associated with adjuvant radiotherapy (Table II).
Discussion
The results of the present study demonstrated that
age (45.6 vs. 54.4%) and menopausal status (54.4 vs. 45.6%) did not
significantly affect the incidence of TNBC in China. This is not in
accordance with the findings of previous studies, which report the
prevalence of TNBC among non-African female breast cancer patients
as between 10 and 17% and report that TNBC is increased in
menopausal females compared with pre-menopausal African females
(17–19). Numerous studies have demonstrated that
premenopausal African-American females were more prone to develop
TNBC (5,9,17,18). Carey et al reported that the
morbidity rate of the TNBC subtype among African-American breast
cancer patients <50 years old is as high as 39%, whereas among
Caucasian females and post-menopausal African-American females, the
morbidity rate is 16 and 14% respectively (5,9,18).
TNBC is prone to local recurrence and distant
metastasis. In the present study, 6 patients (2.5%) suffered bone
metastases and 25 patients (10.5%) suffered visceral metastases.
TNBC has an increased risk of local recurrence or distant
metastasis following the final diagnosis (5,17,18,20–22). In
the present study, the general disease progression rate is 12.1%
(29/239, the local recurrence rate is 6.7% (16/239) and the distant
metastasis rate is 8.8% (21/239). These findings suggest that
distant metastasis may exhibit a certain organ tendency in TNBC
(23–25) and that the specific target organ
metastasis may be associated with specific gene expression
(26–28). Dent et al observed that the
frequency of distant metastasis was significantly increased among
patients with TNBC compared with non-TNBC patients (33.9 and 22.4%,
respectively), and the risk of distant metastasis was found to be
increased in the TNBC group (relative risk = 2.6) (18). There was a gradual increase in the
risk of distant metastasis in the TNBC group, with a peak in the
2nd and 3rd years (18), followed by
a rapid decline, with a lower risk in the 5th year and no distant
metastasis in the 8th year of follow up (29). Previous studies also reported that
TNBC was associated with an increased risk of visceral metastasis
and a decreased risk of bone metastasis (30,31). These
results are similar to those indicated in the present study.
The variation in the tumor size was not of notable
importance in patients with TNBC that possessed no distant
metastasis, and had received adjuvant FEC chemotherapy. The benefit
of cyclophosphamide, methotrexate and 5-fluorouracil (CMF)
chemotherapy to patients with triple-negative, node-negative breast
cancer is notable (32). One previous
study indicated that there was no additional benefit associated
with the cyclophosphamide, epirubicin and 5-fluorouracil (CEF)
regimen over CMF, suggesting that non-anthracycline regimens may be
sufficient in intrinsic subtypes; however, additional studies are
required (33).
Therefore, the lymph node status, not stage of
disease, was regarded as an independent prognostic indicator for OS
and DFS. For this reason, the present study considered that the
patients with large numbers of lymph node metastasis may not be
suitable for FEC adjuvant chemotherapy, but may receive
taxane-containing chemotherapy regimens. A previous study reported
that patients with node-positive breast cancer responded better to
docetaxel compared with fluorouracil (34).
In total, 40 of the 239 TNBC patients (16.7%) in the
present study had a family history of breast cancer, which was not
significantly increased compared with the non-TNBC subgroup total
of 13% reported by Bhatti et al (35). Haffty et al concluded that TNBC
exhibits an increased proportion of positive family history of
breast cancer (36). Zhang et
al reported that, in China, there was no statistically
significant difference in the family history of TNBC and non-TNBC
groups (37).
For tumor sizes of <2 cm, the rate of lymph node
metastasis was 27.9% (24/86), compared with 48.4% for tumor sizes
of >2 cm (74/153). Therefore, tumor size was not indicated to be
associated with lymph node metastasis, which is in agreement with
several studies (17,36,38). The
present study, which assessed the association between tumor size
and lymph node metastasis, produced varying results. Kandel et
al demonstrated that 50% of patients with TNBC developed lymph
node metastases when the average tumor size of TNBC was 2 cm
(39). However, Haffty et al
suggested that tumor size was not associated with lymph node
metastasis (36).
Additionally, in the first year after diagnosis in
the present study, the DFS rate for patients with TNBC was 95.0%
(227/239), which was increased compared with that in the non-TNBC
group of another study (18). This
finding may be attributed to the indication that TNBC appears to be
more sensitive to chemotherapy compared to non-TNBC (40). Therefore, patients with TNBC may
achieve increased short-term DFS rates. In brief, TNBC patients
have a poorer OS time and tend to relapse sooner compared with
patients with other breast cancer subtypes (41). The present study reports that lymph
node status is an effective prognostic parameter for TNBC patients,
particularly for those that exhibit the N3 stage of disease;
however, the effect of stages of disease is decreased. Therefore, a
larger sample size is required in order to verify the results of
the present study. In summary, the present study presents evidence
that lymph node status may predict the prognosis of TNBC patients
receiving FEC adjuvant chemotherapy in China. Patients with N0-2
may obtain the most benefit from FEC.
Acknowledgements
The present study was supported by Harbin Medical
University Cancer Hospital (grant no., JJZ2011-02), Heilongjiang
Provincial Department of Education Project (grant no., 11541140)
and Heilongjiang Provincial Bureau of Health (grant no.,
2009-012).
Glossary
Abbreviations
Abbreviations:
|
TNBC
|
triple negative breast cancer
|
|
FEC
|
fluorouracil, epirubicin and
cyclophosphamide
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
AUC
|
area under curve
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
References
|
1
|
Alkis N, Durnali AG, Arslan UY, Kocer M,
Onder FO, Tokluoglu S, Celenkoglu G, Muallaoglu S, Utkan G, Ulas A,
et al: Optimal timing of adjuvant treatment in patients with early
breast cancer. Med Oncol. 28:1255–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono M, Tsuda H, Shimizu C, Yamamoto S,
Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, et
al: Tumor-infiltrating lymphocytes are correlated with response to
neoadjuvant chemotherapy in triple-negative breast cancer. Breast
Cancer Res Treat. 132:793–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abd El-Rehim DM, Pinder SE, Paish CE, Bell
J, Blamey RW, Robertson JF, Nicholson RI and Ellis IO: Expression
of luminal and basal cytokeratins in human breast carcinoma. J
Pathol. 203:661–671. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dawson SJ, Provenzano E and Caldas C:
Triple negative breast cancers: Clinical and prognostic
implications. Eur J Cancer. 45(Suppl 1): 27–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irvin WJ Jr and Carey LA: What is
triple-negative breast cancer? Eur J Cancer. 44:2799–2805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pal SK, Childs BH and Pegram M: Triple
negative breast cancer: Unmet medical needs. Breast Cancer Res
Treat. 125:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metzger-Filho O, Tutt A, de Azambuja E,
Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis
P, et al: Dissecting the heterogeneity of triple-negative breast
cancer. J Clin Oncol. 30:1879–1887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gucalp A and Traina TA: Triple-negative
breast cancer: Adjuvant therapeutic options. Chemother Res Pract.
2011:6962082011.PubMed/NCBI
|
|
15
|
De Laurentiis M, Cianniello D, Caputo R,
Stanzione B, Arpino G, Cinieri S, Lorusso V and De Placido S:
Treatment of triple negative breast cancer (TNBC): Current options
and future perspectives. Cancer Treat Rev. 36(Suppl 3): S80–S86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et
al: Efficacy of neoadjuvant Cisplatin in triple-negative breast
cancer. J Clin Oncol. 28:1145–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calza S, Hall P, Auer G, Bjöhle J, Klaar
S, Kronenwett U, Liu ET, Miller L, Ploner A, Smeds J, et al:
Intrinsic molecular signature of breast cancer in a
population-based cohort of 412 patients. Breast Cancer Res.
8:R342006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mersin H, Yildirim E, Berberoglu U and
Gülben K: The prognostic importance of triple negative breast
carcinoma. Breast. 17:341–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodríguez-Pinilla SM, Sarrió D, Honrado E,
Hardisson D, Calero F, Benitez J and Palacios J: Prognostic
significance of basal-like phenotype and fascin expression in
node-negative invasive breast carcinomas. Clin Cancer Res.
12:1533–1539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodríguez-Pinilla SM, Sarrió D, Honrado E,
Moreno-Bueno G, Hardisson D, Calero F, Benítez J and Palacios J:
Vimentin and laminin expression is associated with basal-like
phenotype in both sporadic and BRCA1-associated breast carcinomas.
J Clin Pathol. 60:1006–1012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reis-Filho JS, Milanezi F, Steele D,
Savage K, Simpson PT, Nesland JM, Pereira EM, Lakhani SR and
Schmitt FC: Metaplastic breast carcinomas are basal-like tumours.
Histopathology. 49:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdulkarim BS, Cuartero J, Hanson J,
Deschênes J, Lesniak D and Sabri S: Increased risk of locoregional
recurrence for women with T1-2N0 triple-negative breast cancer
treated with modified radical mastectomy without adjuvant radiation
therapy compared with breast-conserving therapy. J Clin Oncol.
29:2852–2858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hernandez-Aya LF, Chavez-Macgregor M, Lei
X, Meric-Bernstam F, Buchholz TA, Hsu L, Sahin AA, Do KA, Valero V,
Hortobagyi GN, et al: Nodal status and clinical outcomes in a large
cohort of patients with triple-negative breast cancer. J Clin
Oncol. 29:2628–2634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertucci F, Finetti P, Cervera N,
Charafe-Jauffret E, Mamessier E, Adélaïde J, Debono S, Houvenaeghel
G, Maraninchi D, Viens P, et al: Gene expression profiling shows
medullary breast cancer is a subgroup of basal breast cancers.
Cancer Res. 66:4636–4644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fadare O, Wang SA and Hileeto D: The
expression of cytokeratin 5/6 in invasive lobular carcinoma of the
breast: Evidence of a basal-like subset? Hum Pathol. 39:331–336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sihto H, Lundin J, Lundin M, Lehtimäki T,
Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T,
Isola J, et al: Breast cancer biological subtypes and protein
expression predict for the preferential distant metastasis sites: A
nationwide cohort study. Breast Cancer Res. 13:R872011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Colleoni M, Cole BF, Viale G, Regan MM,
Price KN, Maiorano E, Mastropasqua MG, Crivellari D, Gelber RD,
Goldhirsch A, et al: Classical cyclophosphamide, methotrexate, and
fluorouracil chemotherapy is more effective in triple-negative,
node-negative breast cancer: Results from two randomized trials of
adjuvant chemoendocrine therapy for node-negative breast cancer. J
Clin Oncol. 28:2966–2973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheang MC, Voduc KD, Tu D, Jiang S, Leung
S, Chia SK, Shepherd LE, Levine MN, Pritchard KI, Davies S, et al:
Responsiveness of intrinsic subtypes to adjuvant anthracycline
substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer
Res. 18:2402–2412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hugh J, Hanson J, Cheang MC, Nielsen TO,
Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et
al: Breast cancer subtypes and response to docetaxel in
node-positive breast cancer: Use of an immunohistochemical
definition in the BCIRG 001 trial. J Clin Oncol. 27:1168–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhatti AB, Khan AI, Siddiqui N, Muzaffar
N, Syed AA, Shah MA and Jamshed A: Outcomes of triple-negative
versus non-triple-negative breast cancers managed with
breast-conserving therapy. Asian Pac J Cancer Prev. 15:2577–2581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Hao C, Dong G and Tong Z:
Analysis of Clinical Features and Outcome of 356 Triple-Negative
Breast Cancer Patients in China. Breast Care (Basel). 7:13–17.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tischkowitz M, Brunet JS, Bégin LR,
Huntsman DG, Cheang MC, Akslen LA, Nielsen TO and Foulkes WD: Use
of immunohistochemical markers can refine prognosis in triple
negative breast cancer. BMC Cancer. 7:1342007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kandel MJ, Stadler Z, Masciari S, Collins
L, Schnitt S, Harris L, Miron A, Richardson A and Garber JE:
Prevalence of BRCA1 mutations in triple negative breast cancer
(BC). J Clin Oncol. 24(Suppl 18): 5082006.
|
|
40
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaplan HG, Malmgren JA and Atwood M: T1N0
triple negative breast cancer: Risk of recurrence and adjuvant
chemotherapy. Breast J. 15:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|