Introduction
Liver cancer is a malignant tumor of the digestive
system with the highest morbidity and mortality rate in China.
Early symptoms are often not typical and clinical diagnoses usually
occur during middle and advanced stages. Consequently, the surgical
resection rate is extremely low at 5–20% (1). Local tumor intervention treatment has
become a viable option for the treatment of liver cancer. In
addition, ultrasound-guided percutaneous ethanol injection (PEI) in
tumors and percutaneous radiofrequency ablation (RFA) in early
stage and small hepatocellular carcinoma have become increasingly
applied. These methods were shown to be effective for obtaining a
comparable clinical effect compared to surgical resections
(2), and also have other advantages
such as negligible trauma, extremely low rates of complications and
are readily accepted by doctors and patients. However, in cases of
tumor ablation without the background of ‘pseudocapsule’ there is a
risk of alcohol diffusion during the injection which may damage the
normal liver tissue (3). RFA also has
a few advantages including ‘thermal subsidence’ and
‘three-dimensional leakage effect’. Nevertheless, it is not
effective in cases of larger tumors and tumors located in
particular areas of the body, such as the aorta, diaphragmatic
surface, gall bladder and heart (4).
Several studies on the application of PEI in
combination with RFA for the treatment of small hepatocellular
carcinoma and liver cancer are available (5). However, the application of PEI in
combination with RFA to treat liver cancer in middle and advanced
stages has yet to be examined.
Patients and methods
Patients
Between March 2013 and March 2015, 100 patients
diagnosed with stage III–IV (Union for International Cancer Control
stages) liver cancer were selected. The study conformed to
interventional treatment testimony. Approval was obtained from the
Ethics Committee of Zhengzhou Central Hospital Affiliated to
Zhengzhou University and informed consent was provided by patients
and their families.
The inclusion criteria for the study were: i)
Patients were mentally conscious; ii) all tumors were <8 cm in
diameter and the total number of tumors was <6; and iii) it was
the first time patients were treated using PEI and RFA. The
exclusion criteria for the study were: i) Patients with hepatic
metastasis; ii) pregnant patients and those with serious
coagulation mechanism disorders as well as those with medical
histories of liver cancer resection and chemoradiotherapy and
serious cachexia; iii) patients who had <6 months survival
expectancy; and iv) patients who were intolerant to surgery and
those with interrupted treatments, and missed follow ups.
According to the selective treatment plan, the
patients were divided into 3 groups. In group A (n=35), treatment
was initiated with PEI and after 1–2 weeks RFA was applied, while
in group B (n=33) treatment was initiated with RFA and after 1–2
weeks PEI was applied. Patients in group C received PEI and RFA
simultaneously. The clinical effects in the 3 groups were compared
after 6 months.
Group A, comprised 20 male and 15 female patients,
with an age range of 56–72 years and an average age of 63.4±10.5
years. The mean tumor size was 4.6±1.3 cm (range, 3.0–5.5 cm) and
the total number of tumors was 1–4 with an average of 2.7±1.3.
There were 16 patients with liver function classification
Child-Pugh B and 19 patients with Child-Pugh C, of which 5 cases
had tumors in paricular regions and 30 cases had other conditions.
In group B, there were 17 male and 16 female patients, with an age
range of 55–71 years and an average age of 61.7±12.3 years. The
mean tumor size was 4.3±1.4 cm (range, 2.8–5.6 cm) and the total
number of tumors was 1–4 with an average of 2.3±1.5. In group B
there were 18 patients with liver function classification
Child-Pugh B and 15 patients with Child-Pugh C, of which 4 cases
had tumors in particular areas and 29 patients had other
conditions. Group C, comprised of 19 male and 13 female patients
with an age range of 54–75 years and average age of 63.6±15.2
years. The mean tumor size was 4.7±1.2 cm (range, 3.5–6.0 cm) and
the total number of tumors was 1–4 with an average of 2.3±1.5.
There were 17 patients with liver function classification
Child-Pugh B and 15 patients with Child-Pugh C of which 5 cases of
tumors were in particular areas and 27 patients had other
conditions. The comparisons of baseline information for the 3
groups showed that the differences were not statistically
significant (P>0.05).
Treatments
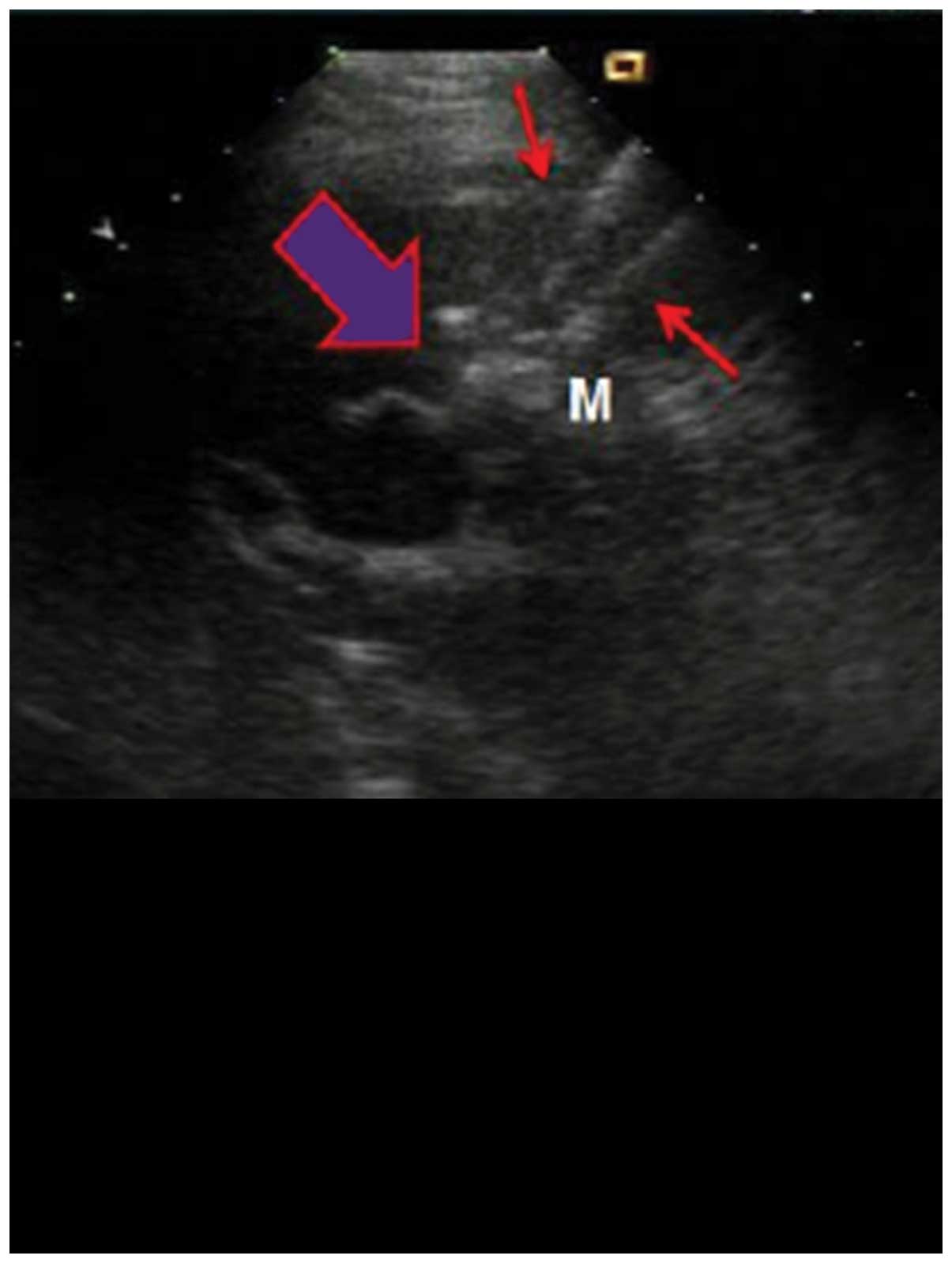
The patient was placed in supine position and PEI
was performed under general anesthesia, using percutaneous puncture
needling guided by ultrasound (Aloka SSD-1100 Color Doppler
Ultrasound; Siemens AG, Munich, Germany). Local anesthesia was
performed using lidocaine. A 22G EV needle (Hakko Co., Ltd., Tokyo,
Japan) was entered into the tumor by percutaneous liver biopsy, no
blood and gail were evident when the syringe was withdrawn. Using a
10 ml syringe, anhydrous alcohol was uniformly injected into the
tumor within 30–60 sec. After the alcohol injection, a high
echogenic mass was observed under ultrasound that spread from the
pinpoint. Injections were stopped when the high echogenic mass
fully covered the tumor and surpassed the edge by >0.5 cm. The
injection volume was calculated using the formula: V=4/3π
(r+0.5)3 where V is total volume, and r is the radius of
lesion. After completion of injection, the puncture needle was
gradually withdrawn. Drug overflow was avoided as the procedure was
observed under ultrasound. After withdrawal of the puncture needle,
the puncture point was covered with gauze and pressured was applied
on the dressing (Fig. 1).
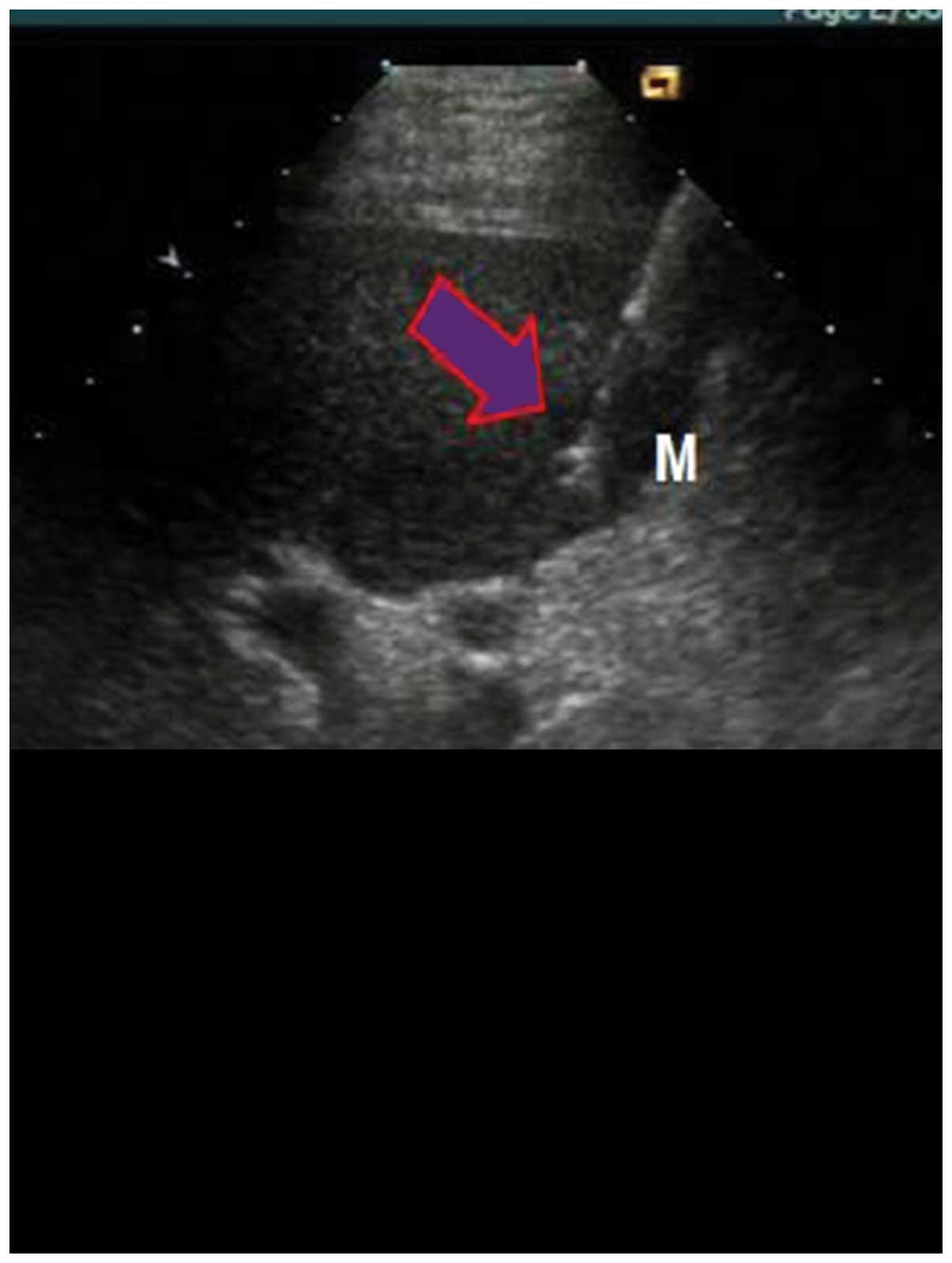
RFA was performed under general anesthesia once the
patient was in supine position, using percutaneous puncture
needling guided by ultrasound (Aloka SSD-1100 Color Doppler
Ultrasound; Siemens AG). Local anesthesia was performed using
lidocaine. The cool-tip RF System (Valleylab, Boulder, CO, USA),
uni-polar cold circulation system, mating electrode wire and plate
electrode were used during the process. Output frequency was
adjusted to (1±5%) 460 kHz, the maximum power was 150 W and the
needle used was a 14 G trocar. A total of 7–12 fine needle
electrodes were set on top of the inner needles, which formed a 5.0
cm spherical heat coagulation after stretching energization. The
unit was set to 90 W for RFA actual burning power and the
temperature was set at 100°C. After the temperature reached 100°C,
it was maintained for 15 min. Following completion of a single RFA,
the echo of the ablation area under the observation of ultrasound
was higher, the position of the needle electrodes was adjusted
according to the tumor size for repeated ablation, in order to
cover the whole target tumor in the echo area, and the ablation
area surpassed tumor lesions by 0.5–1 cm. Following completion of
the treatment, the stretching electrodes were withdrawn, and the
needles were withdrawn after burning of closed needles. The
adjustments of radiofrequency power started from 60 W each time,
and it were gradually elevated until impedance markedly increased
and power was automatically reduced. The scope of vaporization of
strong echo covering all boundaries of the tumors was used to
detrermine when to terminate the treatment. To prevent hemorrhage
and implantation metastasis, needles were heated under high
temperature each time prior to use (Fig.
2).
Observation indices
The volume of tumor ablation necrosis, volume after
ablation and complete ablation rate, quantity of alcohol and
radiofrequency energy used and liver function damage indices,
including raising levels of glutamic-pyruvic transaminase and total
bilirubin were recorded. Differences of survival rates were
compared 6 months after follow-ups. To determine tumor volume the
formula used was: V(cm3)=4/3 × r1(cm) × r2 (cm) ×
r3(cm), (r1=longest diameter/2, r2=shortest diameter/2,
r3=height/2). The volume of tumor necrosis was calculated by
subtracting the volume after ablation from the volume before
ablation. A contrast-enhanced ultrasound examination was conducted
two weeks after the treatment. Arterial portal and delayed phases
of the ablation area with no contrast agent observed was regarded
as complete ablation; radiofrequency energy (J)=watt (W) × curative
time (sec).
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analyses. Measurement data were presented by
mean ± standard deviation. Analysis of variance was used for
comparisons among the groups and enumeration data were presented by
%, while the χ2 test was used for comparison among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparisons of ablation
parameters
The volume of tumor ablation necrosis in group A was
significantly larger than that in groups B and C, while the volume
was significantly smaller than that in groups B and C after
ablation. The complete ablation rate in group A was significantly
higher than that in groups B and C, and the differences were
statistically significant (P<0.05). The comparisons of quantity
of alcohol used and radiofrequency energy in the three groups
showed that the differences were not statistically significant
(P>0.05) (Table I).
 | Table I.Comparisons among different ablation
parameters. |
Table I.
Comparisons among different ablation
parameters.
| Groups | Cases | Volume before
ablation (cm3) | Volume after
ablation | Volume of necrosis
ablation | Complete ablation
rate (%) | Usage volume of
alcohol, ml | Radiofrequency energy
(×103 J) |
|---|
| A | 35 | 15.5±3.3 | 1.5±0.8 | 13.8±4.2 | 30 (85.7) | 8.5±1.3 | 456.7±33.4 |
| B | 33 | 14.7±3.4 | 6.6±1.2 | 10.6±3.7 | 20 (60.6) | 8.2±1.5 | 432.6±45.2 |
| C | 32 | 15.3±3.5 | 7.2±1.5 | 10.5±3.8 | 20 (62.5) | 8.3±1.6 | 423.4±43.3 |
| F
(χ2) |
| 0.632 | 6.926 | 6.524 | 6.360 | 0.423 | 0.938 |
| P-value |
| 0.425 | 0.032 | 0.038 | 0.042 | 0.725 | 0.546 |
Comparisons of liver function damage
indices
The comparisons of glutamic-pyruvic transaminase and
total bilirubin levels prior to the treatment in the 3 groups
showed that the differences were not statistically significant
(P>0.05). By contrast, after the treatment, the raising levels
of glutamic-pyruvic transaminase and total bilirubin in group A
were significantly lower than that in groups B and C, and the
differences were statistically significant (P<0.05) (Table II).
 | Table II.Comparisons among liver function
damage indices. |
Table II.
Comparisons among liver function
damage indices.
| Groups | Glutamic-pyruvic
transaminase level before treatments, U/l | Raising
levelsa | Total bilirubin
before treatments, µmol/l | Raising
levelsa |
|---|
| A | 78.5±13.6 | 13.6±4.2 | 27.9±5.3 | 6.3±1.2 |
| B | 75.6±14.2 | 18.2±4.7 | 25.5±4.2 | 10.2±2.5 |
| C | 76.7±13.5 | 17.5±4.3 | 23.4±4.6 | 8.9±2.4 |
| F-value | 0.632 | 6.325 | 0.759 | 6.856 |
| P-value | 0.421 | 0.039 | 0.532 | 0.027 |
Comparisons of survival rates
Eight patients succumbed in group A, of whom 2 had
tumor recurrence in situ, 1 patient succumbed to tumor
rupture hemorrhage, 3 succumbed to tumor emboli blocking the
hepatic artery or portal vein hemorrhage, 3 patients succumbed to
liver failure, and 1 patient had extrahepatic tumor metastasis.
Mortalities occured 2 weeks to 6 months after the treatment
(average, 4.2 months) and the survival rate was 77.1%. In group B,
17 patients succumbed of whom 5 patients had tumor recurrence in
situ, 3 patients succumbed to tumor rupture hemorrhage, 7
patients to tumor emboli blocking the hepatic artery or portal vein
hemorrhage, 5 due to liver failure, and 2 patients succumbed to
extrahepatic tumor metastasis. In group B, mortalities occured 1
week to 5.5 months after treatment (average, 3.5 months), and the
survival rate was 48.5%. In group C, 15 mortalities occured of whom
6 patients succumbed to tumor recurrence in situ, 4 to tumor
rupture hemorrhage, 6 to tumor emboli blocking the hepatic artery
or portal vein hemorrhage, 3 to liver failure, and 2 patients
succumbed to extrahepatic tumor metastasis. Patients in group C
succumbed to the disease 1.5 weeks to 5.6 months following
treatment (average, 3.7 months), and the survival rate was 53.1%.
The survival rate in group A increased significantly, and the
differences were statistically significant (χ2=6.739,
P=0.034).
Discussion
Ultrasound guidance technology has the advantages of
real-time monitoring, accurate guidance, negligible trauma, safety
and effectiveness, easy operation and repeatability (6). These advantages have led this technology
to be considered as one of the three major types of treatment for
liver cancer together with surgery and regional vascular
intervention (7). Ultrasound guidance
technology aims to effectively treat tumors or cytoreduction,
reducing symptoms, improving life quality and extending survival
time.
Ultrasound-guided local interstitial chemical
ablation consists of injecting substances into tumors to induce
necrosis. Frequently used injections include anhydrous alcohol,
acetic acid, hot saline water or hot distilled water, radionuclide,
biological agents and cod liver oil acid sodium anhydrous alcohol
solution (8). Sugiura et al
(9) clinically applied PEI in
treating liver cancer for the first time, and now PEI is the most
widely used method for chemical ablation. However, due to the
disadvantages of non-uniform diffusion and uncontrollability of
injected alcohol observed in numerous treatments of larger tumors,
PEI is mainly applied in the treatment of small hepatocellular
carcinoma. However, Livraghi et al (10) reported that PEI could be applied in ≥5
cm liver tumors. They showed that for the 1,066 patients
participating in their study, the 3-, 5- and 7-year survival rates
were 72.3, 43.2 and 27.0%, respectively. The ultrasound-guided
local interstitial thermal ablation method consists of conducting
energy into the tumor to inactivate tumor cells in situ.
Thermal ablation includes radiofrequency, microwave, laser,
refrigeration and focused ultrasound. Rossi et al (11) were the first group who reported
treating small hepatocellular carcinoma using ultrasound-guided
RFA, with comparable results compared to PEI. RFA can also be used
as a supplementary method for the treatment of large liver tumors.
RFA is also suitable for those patients that cannot tolerate
surgery or those unwilling to undergo surgery. The results obtained
from prior studies showed that in cancer tissues, HSP-70 expression
level, AFP expression level, clinical staging, liver function
classifications and the inactivated degree of tumors in liver
cancer tissues, were are all closely associated with RFA treatment
effects in large liver tumors (12).
RFA was certified by the Food and Drug Association in early 1996
and currently is cosidered the most widely applied ablation method
(13). There are some limitations
associated with RFA such as high costs, limited popularity and
technological difficulties.
The application of PEI in combination with RFA for
the treatment of large liver cancer and liver cancer of particular
areas has become a hot topic of research. This method is designed
to reduce the tumor size of through dehydration and solidification
using anhydrous alcohol, and thus increase the success rate of RFA
ablation and reduce the frequency of ablation (14). Multi-polar RFA treatment devices (RITA
Medical System; Rita Medical, Mountain View, CA, USA;
Radio-Therapeutics, Sunnyvale, CA, USA) have 3–5 electrodes that
are similar to ‘eagle claw’ or ‘umbrella’. The cold circulation in
radiofrequency electrode is designed to prevent the ebullition and
the formation of cavities of tissues around the pinpoint of the
electrodes because of the heat (15).
There are no absolute conclusions regarding whether PRI and RFA
should be carried out seperately or simultaneously, and there are
few studies on the quantitative comparison of the volume of alcohol
usage and radiofrequency power. Through large numbers of clinical
practices, it was identified that although the basis of liver
cancer in middle and advanced stages is poor, survival time cannot
be improved via conservative treatments (16). The clinical effects of simple PRI and
RFA treatments are not substantial, however, the present study
concluded that adimistering PEI treatment 1–2 weeks prior to RFA
treatment can significantly improve the patients' condition by
intensification of the volume of tumor ablation necrosis and reduce
tumor volume following ablation. This method can also improve
complete the ablation rate, reduce the raising levels of
glutamic-pyruvic transaminase and total bilirubin and improve the
survival rate. The volume of alcohol usage and radiofrequency power
was not increased in this method. Possible reasons for this
include: i) The stress damage of anhydrous alcohol to organic
bodies is higher than that of RFA, and PEI was used for the first
time (17); ii) usually there is the
existence of ‘pseudocapsule’ in primary liver cancer, and PEI being
used for the first time, which influences the dispersion effects of
anhydrous alcohol (18); iii) the
working mechanism of RFA is to use ion in tissues to create
vibrations of the same frequency around electrodes, which turns it
into heat energy to produce coagulative necrosis. The pretreatment
of anhydrous alcohol can make the frequency of tumor tissues more
concentrated, which shortens the duration of RFA (19); and iv) the simultaneous
implementations of PEI and RFA treatments cannot produce a
superimposed effect, which in turn creates the phenomenon of offset
(20).
In conclusion, for patients with liver cancer in
middle and advanced stages, the treatment method using PEI followed
by RFA is more beneficial in terms of improving tumor ablation
rate, alleviating liver damage and increasing survival rates.
References
|
1
|
Hou W and Zhu X: Extra vascular
interventional treatment of liver cancer, present and future. Drug
Discov Ther. 9:335–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu LT, Zhou ZH, Lin JH, Chen Z, Wang K,
Wang P, Zhu XY, Shen YH, Meng ZQ and Liu LM: Clinical study of
transarterial chemoembolization combined with 3-dimensional
conformal radiotherapy for hepatocellular carcinoma. Eur J Surg
Oncol. 37:245–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurokohchi K, Watanabe S, Masaki T, Hosomi
N, Miyauchi Y, Himoto T, Kimura Y, Nakai S, Deguchi A, Yoneyama H,
et al: Comparison between combination therapy of percutaneous
ethanol injection and radiofrequency ablation and radiofrequency
ablation alone for patients with hepatocellular carcinoma. World J
Gastroenterol. 11:1426–1432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulier S, Ni Y, Jamart J, Ruers T, Marchal
G and Michel L: Local recurrence after hepatic radiofrequency
coagulation: Multivariate meta-analysis and review of contributing
factors. Ann Surg. 242:158–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarnagin WR: Management of small
hepatocellular carcinoma: A review of transplantation, resection,
and ablation. Ann Surg Oncol. 17:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JW, Shin SS, Heo SH, Hong JH, Lim HS,
Seon HJ, Hur YH, Park CH, Jeong YY and Kang HK: Ultrasound-guided
percutaneous radiofrequency ablation of liver tumors: how we do it
safely and completely. Korean J Radiol. 16:1226–1239. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang TT, Luo HC, Cui X, Zhang W, Zhang
LY, Chen XP and Li KY: Ultrasound-guided percutaneous microwave
ablation treatment of initial recurrent hepatocellular carcinoma
after hepatic resection: long-term outcomes. Ultrasound Med Biol.
41:2391–2399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark TW: Chemical ablation of liver
cancer. Tech Vasc Interv Radiol. 10:58–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiura Y, Iwasaka T and Tarumi N:
Percutaneous ethanol injection therapy: A new treatment for
hepatocellular carcinoma. Radiaology. 90:53–57. 1983.
|
|
10
|
Livraghi T, Giorgio A, Marin G, Salmi A,
de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S and
Torzilli G: Hepatocellular carcinoma and cirrhosis in 746 patients:
Long-term results of percutaneous ethanol injection. Radiology.
197:101–108. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rossi S, Di Stasi M, Buscarini E, Cavanna
L, Quaretti P, Squassante E, Garbagnati F and Buscarini L:
Percutaneous radiofrequency interstitial thermal ablation in the
treatment of small hepatocellular carcinoma. Cancer J Sci Am.
1:73–81. 1995.PubMed/NCBI
|
|
12
|
Kudo M: Radiofrequency ablation for
hepatocellular carcinoma: Updated review in 2010. Oncology.
78(Suppl 1): 113–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wahl DR, Stenmark MH, Tao Y, Pollom EL,
Caoili EM, Lawrence TS, Schipper MJ and Feng M: Outcomes After
Stereotactic Body Radiotherapy or Radiofrequency Ablation for
Hepatocellular Carcinoma. J Clin Oncol. Nov 30–2015.(Epub ahead of
print). PubMed/NCBI
|
|
14
|
Kai L, Jia L, Zhi-Gang W and Lei Y:
Ultrasonic guided percutaneous ethanol injection with or without
combined radiofrequency ablation for hepatocellular carcinomas.
Indian J Cancer. 52(Suppl): E102–E104. 2015.PubMed/NCBI
|
|
15
|
Rehman J, Landman J, Lee D, Venkatesh R,
Bostwick DG, Sundaram C and Clayman RV: Needle-based ablation of
renal parenchyma using microwave, cryoablation, impedance- and
temperature-based monopolar and bipolar radiofrequency, and liquid
and gel chemoablation: laboratory studies and review of the
literature. J Endourol. 18:83–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogasawara S, Chiba T, Ooka Y, Suzuki E,
Kanogawa N, Saito T, Motoyama T, Tawada A, Kanai F and Yokosuka O:
Post-progression survival in patients with advanced hepatocellular
carcinoma resistant to sorafenib. Invest New Drugs. 14:1–3.
2016.
|
|
17
|
Shi F, Tan Z, An H, Wang X, Xu Y and Wang
S: Hepatocellular carcinoma ≤4 cm treated with radiofrequency
ablation with or without percutaneous ethanol injection. Ann
Hepatol. 15:61–70. 2015.2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Y, Zhao X, Yun Q, Zhu X, Zhu Y, Li Q,
Hu K, Wang J and Qiao Z: Transarterial chemoembolization (TACE)
plus percutaneous ethanol injection (PEI) for the treatment of
unresectable hepatocellular carcinoma: A meta-analysis of
randomized controlled trials. Int J Clin Exp Med. 8:10388–10400.
2015.PubMed/NCBI
|
|
19
|
Yang B, Zan RY, Wang SY, Li XL, Wei ML,
Guo WH, You X, Li J and Liao ZY: Radiofrequency ablation versus
percutaneous ethanol injection for hepatocellular carcinoma: a
meta-analysis of randomized controlled trials. World J Surg Oncol.
13:962015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Liang P, Yu XL, Cheng ZG, Han ZY,
Yu J and Liu FY: Safety assessment and therapeutic efficacy of
percutaneous microwave ablation therapy combined with percutaneous
ethanol injection for hepatocellular carcinoma adjacent to the
gallbladder. Int J Hyperthermia. 31:40–47. 2015. View Article : Google Scholar : PubMed/NCBI
|