Introduction
Plexiform fibromyxoma is a one of the very few types
of mesenchymal neoplasm that predilect the stomach. Miettinen et
al (1) published the largest
series on the topic in 2009, coining the term ‘plexiform
fibromyxoma’ for this benign spindle cell tumor. Although
alternative designations for the process, including ‘plexiform
angiomyxoid myofibroblastic tumor’ (2–9) and
‘plexiform angiomyxoid tumor’ (10),
have been proposed, these appellations fail to adequately convey
the benign nature of the lesion. Indeed, plexiform fibromyxoma is
the term currently accepted by the World Health Organization for
this tumor (11).
Microscopically, the tumor exhibits a characteristic
plexiform growth pattern of small spindled cells in a myxoid or
fibromyxoid stroma. The tumor shares a myofibroblastic
immunophenotype with gastrointestinal stromal tumors (GISTs), which
are the most common mesenchymal tumor of the stomach with an
estimated incidence of >150 cases for every 1 case of plexiform
fibromyxoma; although immunohistochemical positivity of CD117 and
Discovered on GIST-1 (DOG-1) in GISTs aids in distinguishing the
two entities (1). This distinction is
important as GISTs possess a greater potential for aggressive
pathobiological behavior, whereas plexiform fibromyxomas are
considered to be largely benign.
The present study reports the clinicopathological
features of a plexiform fibromyxoma, excised from a 28-year-old
female, that grew as multiple, variably sized, bulbous
myxoedematous polypoid projections from the serosal surface. In
supplement, a review of the pertinent English language literature
regarding this rare neoplasm is presented.
Case report
A 28-year-old Vietnamese female presented to
Northwestern Memorial Hospital (Chicago, IL, USA) in June 2013 with
acute, severe abdominal pain and worsening anemia. Computed
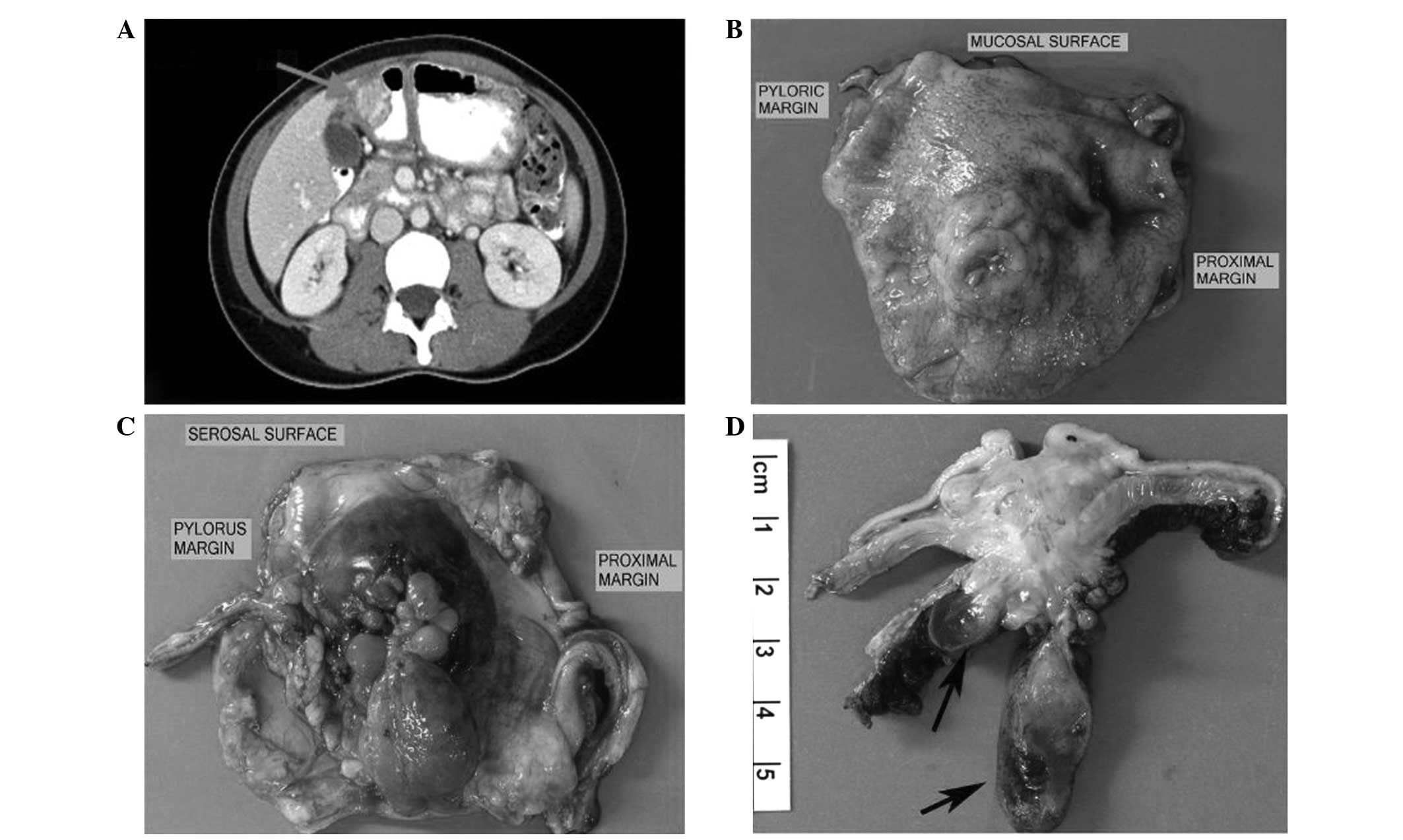
tomography (CT) of the abdomen and pelvis revealed a lobulated,
heterogeneously-enhancing mass with both a solid and cystic
component in the wall of the gastric antrum (Fig. 1A). A fine-needle aspiration and needle
core biopsy of the gastric mass demonstrated a myxoid spindle cell
lesion with features suggestive of a GIST. As a result of these
findings, the patient underwent a distal gastrectomy with a
Roux-en-Y gastrojejunostomy.
The distal gastrectomy specimen contained a
5.5×3.5-cm, stellate-shaped, multinodular, yellow-tan-colored mass
centered in the antral wall and which ulcerated the overlying
mucosa (Fig. 1B) and extended onto
the serosal surface as multiple, variably sized, myxoedematous
polyps imparting a cotelyedon-like appearance to the serosal aspect
of the stomach (Fig. 1C and D).
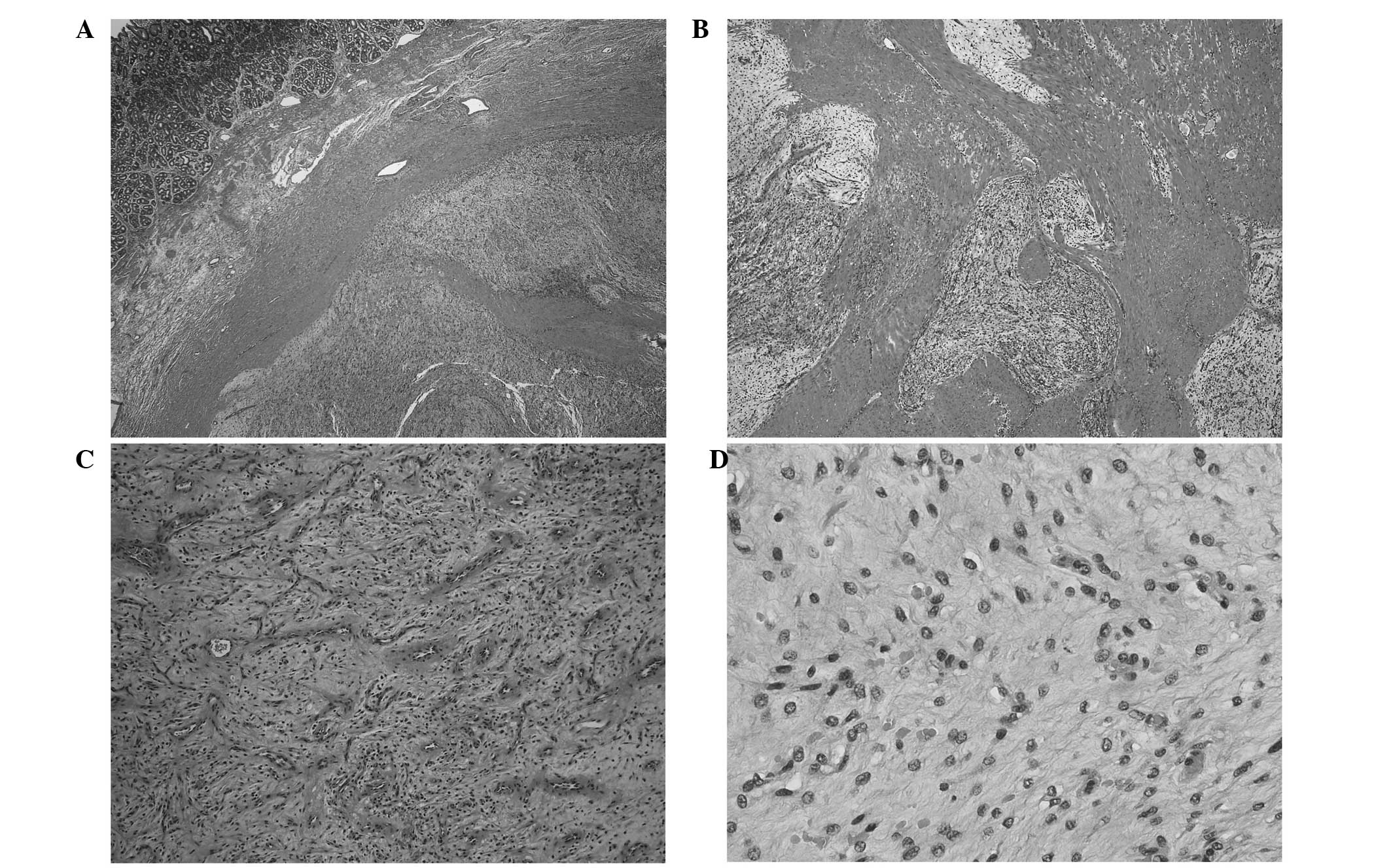
At low-power microscopic magnification, intertwined
pale, myxocollagenous nodules and fascicles of tumor infiltrated
the entire thickness of the gastric wall (Fig. 2A and B) and formed highly
myxoedematous polypoid structures on the serosal surface. The
myxocollagenous fascicles and nodules of tumor possessed an
accentuated vascular component composed of non-branching
capillaries and scattered mast cells (Fig. 2C). High-power microscopic evaluation
revealed mildly cellular fascicles of cytologically bland spindled
cells with scanty, pale, eosinophilic cytoplasm and indistinct cell
borders (Fig. 2D). The mitotic count
was 7 mitoses per 50 high-power fields, with no atypical mitotic
figures identified. No necrosis was observed within the lesional
tissue. All margins of resection were free of tumor. The intact
mucosa overlying the tumor exhibited superficial chronic
inflammation, but no Helicobacter pylori organisms were
visually identified.
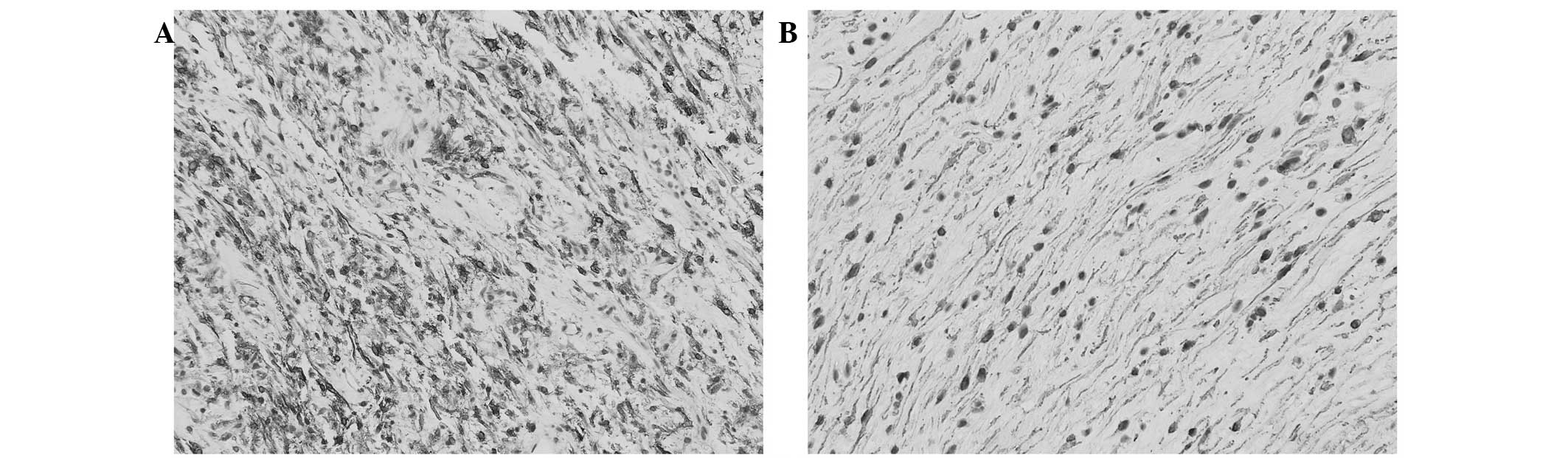
Immunohistochemically, the spindle cells diffusely
expressed smooth muscle actin (SMA) (Fig.
3A) and focally expressed CD10 (Fig.
3B), but were negative for CD117, DOG-1, CD34, S-100 protein,
D2-40, keratin AE1/3 and nuclear β-catenin.
A follow-up CT scan performed one month after the
surgery revealed no intra-abdominal pathology, and an
esophagogastroduodenoscopy performed due to symptoms of dyspepsia
23 months following surgery revealed moderate stenosis of the
gastrojejunal anastomosis, but no evidence of residual or recurring
tumor. Any future follow-up for this benign tumor is presently on
an ‘as needed’ basis.
Discussion
Plexiform fibromyxoma is a rare mesenchymal neoplasm
that predilects the stomach and, as demonstrated in the present
case, has the potential for misdiagnosis as the more common and
often aggressive GIST.
In the English language literature, 32 previously
reported cases of plexiform fibromyxoma were identified (Table I) (1–10,12–18). The
tumor has been documented in 19 females and 13 males, ranging in
age from 7 to 75 years (mean, 40 years; median, 43 years). Symptoms
reported at presentation vary and are non-specific, but most
commonly include abdominal pain, ulceration, anemia and mass
effects. The antrum of the stomach is involved in 88% of reported
cases, with contiguous involvement of the pylorus and/or duodenum
in an additional 7 cases. Isolated examples of plexiform
fibromyxoma have been documented in the gastric fundus (14), cecum (13), pyloroduodenal region (16) and posterior mediastinum (16).
 | Table I.Clinicopathological findings and
outcome data for 33 cases of plexiform fibromyxoma reported in the
English language literature. |
Table I.
Clinicopathological findings and
outcome data for 33 cases of plexiform fibromyxoma reported in the
English language literature.
| Author, year
(reference) | No. of cases | Patient age,
years | Patient gender | Presenting
symptoms | Tumor location | Tumor size, cm | Mitoses/50 HPFs | Surgical
intervention | Outcome |
|---|
| Shockman et
al, 1965(12) | 1 | 45 | M | Abd. pain | Antrum | 3 | NG | Resection, NOS | NG |
| Takahashi et
al, 2007 (6) | 2 | 50 | M | Perforated
stomach | Antrum | 4 | 0 | D. gastrec. | NG |
|
|
| 68 | M | Incidental
finding | Antrum and
pylorus | 4.5 | 0 | D. gastrec. | ANED, 12 mth |
| Galant et
al, 2008 (2) | 1 | 61 | M | Hematemesis | Antrum | 3.7 | 0 | D. gastrec. | ANED, 6 mth |
| Rau et al,
2008 (3) | 1 | 50 | F | Nausea | Antrum | 1.9 | 0 | Wedge
resection | ANED, 12 mth |
| Yoshida et
al, 2008 (10) | 2 | 46 | M | UGI bleed | Antrum | 3.5 | 0 | D. gastrec. | ANED, 4 mth |
|
|
| 19 | F | Mass | Antrum | 4.5 | 0 | D. gastrec. | ANED, 9 mth |
| Miettinen et
al, 2009 (1) | 12 | 7–75 | 5 × M; 7 × F | 3 × UGI bleed; 3 ×
ulcer; 2 × weight loss; 2 × anemia; 2 × NG; 1 × mass | 12 × antrum (6 ×
contiguous involvement of pylorus and/or duodenum) | 3–15 | 0–4 | 10 × P./D.
gastrec.; 1 × subtotal gastrec.; 1 × gastric wall resection | 4 × ANED, 108–239
mth; 3 × DUNK, 2.306 mth; 2 × ATSU, 36.264 mth; 3 × NG |
| Pailoor et
al, 2009 (4) | 1 | 23 | F | Melena | Antrum | 8 | 0 | P. gastrec. | ANED, 2 mth |
| Daum et al,
2010 (13) | 1 | 44 | F | NG | Cecum | 5 | 0 | NG | NG |
| Sing et al,
2010 (5) | 1 | 35 | F | ‘Cushingoid’
appearance | Antrum | 4 | 0 | Wide local
excision | ANED, 12 mth |
| Tan et al,
2010 (7) | 1 | 34 | M | Abd. discomfort and
mass | Antrum | 3.5 | 0 | D. gastrec. | ANED, 2 mth |
| Wang et al,
2010 (14) | 1 | 54 | F | Abd. distension,
loss of appetite, ‘heartburn’ | Fundus | 1.5 | NG | Endoscopic
resection | ANED, 6 mth |
| Kim et al,
2011 (8) | 1 | 52 | M | Dyspepsia | Antrum | 3.5 | 0 | Wedge
resection | ANED, 5 mth |
| Kang et al,
2012 (9) | 2 | 47 | M | Mass | Antrum | 3 | 3 | Wedge
resection; | NG |
|
|
| 63 | F | None | Antrum | 2.2 | 0 | Endoscopic
resection | NG |
| Lee et al,
2013 (15) | 1 | 42 | F | Abd. pain, fever,
anemia, fistulating abscess | Antrum | 12.9 | 0 | D. gastrec. | ANED, <1
mth |
| Duckworth et
al, 2014 (16) | 2 | 16 | F | Chest pain | Posterior
mediastinum | 3.2 | 0 | Resection | ANED, 14 mth |
|
|
| 11 | F | Anemia | Pyloroduodenal
junction | 3.5 | 0 | D. gastrec. | ANED, 15 mth |
| Ikemura et
al, 2014 (17) | 1 | 27 | F | Abd. pain,
melena | Antrum | 4.6 | NG | P. gastrec. | ANED, 40 mth |
| Li et al,
2014 (18) | 1 | 32 | F | Incidental
finding | Antrum | 3.4 | NG | P. gastrec | ANED, 36 mth |
| Present case,
2015 | 1 | 28 | F | Abd. pain,
anemia | Antrum | 5.5 | 7 | D. gastrec. | ANED, 23 mth |
Grossly, the neoplasm ranges in size from 1.5 to 15
cm (median, 4.3 cm; mean, 5.2 cm). The cut surface of the lesion
reveals a multinodular mass centered in the muscularis propria,
with dome-shaped elevation and often ulceration of the mucosal
surface. Subserosal nodules (1,2) and
polypoid projections (10) have been
briefly mentioned in a few reports detailing the gross appearance
of plexiform fibromyxoma. Although GISTs may present primarily as
an extramural polypoid mass in the stomach (19), the presence of multiple subserosal
myxoedematous polyps, as in the current case, is not a macroscopic
feature of GISTs, whereas as it is in plexiform fibromyxoma and
therefore may be used to differentiate the two tumor entities on a
macroscopic level.
We contend that the light microscopic features of
plexiform fibromyxoma are relatively unique and allow an
unequivocal diagnosis in the majority of cases. The process is
characterized by an interwoven growth pattern of pale-appearing,
mildly cellular fascicles and nodules of cytologically bland, short
spindled cells proliferating in the myxoid and/or myxocollagenous
stroma with an enriched network of simple capillary-sized
vessels.
Immunohistochemically, plexiform fibromyxoma
demonstrates a myofibroblastic phenotype with documented expression
of SMA (n=26 cases) (1,2,4–10,14–18),
vimentin (n=8) (2,4,6,13,14,16,18),
muscle specific actin (n=6) (5,6,10,15),
desmin (n=9) (4,5,7,8,10,16–18),
caldesmon (n=5) (5,6,10,18) and calponin (n=3) (5,10,16). Immunoexpression of CD10 (n=2)
(1), nestin (n=1) (16) and progesterone receptor protein (n=1)
(5) have also been documented. In the
majority of reports (3,6,7,9,10,14,17,18), Ki-67
immunoexpression is generally very low (≤2%). Notably, tumor cells
do not express CD117 (cKIT), CD34, DOG-1, neurofilament, S-100
protein, nuclear β-catenin, epithelial membrane antigen, activin
receptor-like kinase 1 (ALK-1), CDK4, Muc-4, estrogen receptor
protein or keratins.
Mutations in key exons (‘hotspots’) of the CD117 and
platelet-derived growth factor receptor-α (PDGFα) genes are
important in the pathogenesis and treatment of the GIST, and are
found in ~85% of cases (20).
Molecular evaluation of GIST mutational ‘hotspots’ in these two
genes has revealed ‘wild-type’ sequences in all cases of plexiform
fibromyxoma tested (1,7,9,10,13).
Plexiform fibromyxoma acts in a benign fashion with
surgical excision despite the occasional presence of extragastric
extension and/or lymphatic/vascular-space invasion by the tumor
(1). To date, 21 patients have been
reported to be alive without recurrent/persistent disease during
follow-up periods ranging from 1 to 239 months (mean, 48 months;
median, 12 months) (1–10,12–18).
The differential diagnosis of plexiform fibromyxoma
includes other myxoid spindle cell processes that involve the
stomach. The myxoid variant of GIST is the predominant entity to
consider in the differential diagnosis. Apart from plexiform
epithelioid cell GIST variants (21),
conventional GISTs typically form a solitary nodular or, in certain
cases, a dumbbell-shaped mass. Microscopically, GISTs are composed
of larger spindled cells with more abundant, lightly eosinophilic
cytoplasm that are arranged in more compact and cellular fascicles.
An exaggerated degree of nuclear pallisading and/or perinuclear
vacuolization is identified in certain spindle cell variants.
Despite immunoexpression of muscle actins in both entities, GISTs
typically express CD117 and DOG-1 (>90% of cases) (22) and CD34 (>80% of cases) (23).
The inflammatory fibroid polyp is a benign lesion
that has a predilection for the gastric antrum (24). The process arises from the submucosa
and may also involve the muscularis propria. In contrast to
plexiform fibromyxoma, the lesion grows as a solitary and, often,
polypoid mass. It is composed of cytologically bland epithelioid
and short spindled cells typically arranged concentrically around
vessels in a loose fibromyxoid stroma. The accompanying
inflammatory infiltrate, rich in eosinophils, is a hallmark of the
process and further helps to distinguish the lesion from
fibromyxoma. Immunohistochemically, the inflammatory fibroid polyp
is positive for CD34, but not SMA (20). PDGFα gene mutations are common in the
lesion (25).
Gastrointestinal schwannoma is a benign peripheral
nerve sheath tumor that arises primarily as an intramural gastric
or colonic mass (26,27). Unlike plexiform fibromyxoma,
gastrointestinal schwannoma grows as a solitary, unencapsulated
nodule and frequently exhibits lymphoid aggregates or follicles at
its periphery. The cells of schwannoma are typically larger than
those of fibromyxoma and exhibit characteristic nuclear contour
irregularities. Gastrointestinal schwannomas demonstrate strong and
diffuse immunoexpression of S-100 protein, and little to no
expression of SMA.
The myxoid variant of leiomyoma, on occasion, may
exhibit plexiform growth. In contrast to fibromyxoma, the cells of
leiomyoma are typically larger with centrally located, blunt-ended
nuclei and brightly eosinophilic fibrillary cytoplasm, and
frequently grow in a packeted or trabecular pattern. The
immunoprofiles of the two lesions include similar expression of
myoid markers.
Intra-abdominal fibromatosis is a locally aggressive
myofibroblastic neoplasm that may arise from the pelvis or
abdominal soft tissue, and can secondarily invade the walls of
gastrointestinal viscera, thereby mimicking a primary process. It
has a distinctly infiltrative growth pattern and does not exhibit
true plexiform architecture. Although myxoid change may occur in
fibromatosis, the lesion differs from fibromyxoma by its possession
of larger cells that are evenly distributed within distinct,
elongated fascicles. Along with SMA immunoexpression, fibromatosis
characteristically exhibits nuclear expression of β-catenin in
75–90% of cases (28,29).
Mesenteric inflammatory myofibroblastic tumor has
the potential to involve the gastric wall, simulating a primary
lesion. The lesion may exhibit myxoid areas with spindled tumor
cells growing in a fasciitis-like pattern. This process differs
from fibromyxoma by exhibiting multi-patterned growth, a distinct
population of larger cells with eosinophilic cytoplasm, and a
conspicuous lymphoplasmacytic infiltrate (30). In addition to SMA, >40% of cases
exhibit ALK-1 immunoexpression (31).
In summary, the current study reports a case of
plexiform fibromyxoma, a rare mesenchymal gastric neoplasm that
requires distinction from the more aggressive GIST. Attention to
the characteristic gross and microscopic growth patterns, as well
as the absence of CD117 and DOG-1 immunoexpression, may aid in
separating these two entities. We also contend that the macroscopic
presence of multiple, myxoedematous, serosal polyps should strongly
suggest a diagnosis of plexiform fibromyxoma as opposed to GIST or
myoid spindle cell lesions.
References
|
1
|
Miettinen M, Makhlouf HR, Sobin LH and
Lasota J: Plexiform fibromyxoma: A distinctive benign gastric
antral neoplasm not to be confused with a myxoid GIST. Am J Surg
Pathol. 33:1624–1632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galant C, Rousseau E, Minh Ho, Duc DK and
Pauwels P: Re: Plexiform angiomyxoid myofibroblastic tumor of the
stomach. Am J Surg Pathol. 32:1910author reply 1912, 1913. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rau TT, Hartmann A, Dietmaier W, Schmitz
J, Hohenberger W, Hofstaedter F and Katenkamp K: Plexiform
angiomyxoid myofibroblastic tumour: Differential diagnosis of
gastrointestinal stromal tumour in the stomach. J Clin Pathol.
61:1136–1137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pailoor J, Mun KS, Chen CT and Pillay B:
Plexiform angiomyxoid myofibroblastic tumour of the stomach.
Pathology. 41:698–699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sing Y, Subrayan S, Mqadi B, Ramdial PK,
Reddy J, Moodley MS and Bux S: Gastric plexiform angiomyxoid
myofibroblastic tumor. Pathol Int. 60:621–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi Y, Suzuki M and Fukusato T:
Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J
Gastroenterol. 16:2835–2840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan CYS, Santos LD and Biankin A:
Plexiform angiomyxoid myofibroblastic tumour of the stomach: A case
report. Pathology. 42:581–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim A, Bae YK, Shin HC and Choi JH:
Plexiform angiomyxoid myofibroblastic tumor of the stomach: A case
report. J Korean Med Sci. 26:1508–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang Y, Jung W, Do IG, Lee EJ, Lee MH, Kim
KM and Choi J: Plexiform angiomyxoid myofibroblastic tumor of the
stomach: Report of two cases and review of the literature. Korean J
Pathol. 46:292–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida A, Klimstra DS and Antonescu CR:
Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol.
32:1910–1912; author reply 1912–1913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miettinen M, Blay JY, Kindblom LG and
Sobin LH: Mesenchymal tumours of the colon and rectum. World Health
Organization Classification of Tumours - Pathology and Genetics of
Tumours of the Digestive System. Hamilton SR and Aaltonen LA:
(Lyon, France). IARC Press. 142.2000.
|
|
12
|
Shockman AT and Rosen JH: Fibromyxoma of
the stomach. Del Med J. 37:225–228. 1965.PubMed/NCBI
|
|
13
|
Daum O, Jirasek T, Grossmann P, Mukensnabl
P and Michal M: Plexiform fibroma of the colon. Appl
Immunohistochem Mol Morphol. 18:483–484. 2010.PubMed/NCBI
|
|
14
|
Wang WY, Li JN and Li GD: Plexiform
angiomyxoid myofibroblastic tumour of the gastric fundus:
Successful diagnosis and treatment by endoscopy. J Clin Pathol.
63:569–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee PW, Yau DT, Lau PP and Chan JK:
Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor)
of stomach: An unusual presentation as a fistulating abscess. Int J
Surg Pathol. 22:286–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duckworth LV, Gonzalez RS, Martelli M, Liu
C, Coffin CM and Reith JD: Plexiform fibromyxoma: Report of two
pediatric cases and review of the literature. Pediatr Dev Pathol.
17:21–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikemura M, Maeda E, Hatao F, Aikou S, Seto
Y and Fukayama M: Plexiform angiomyxoid myofibroblastic tumor
(PAMT) of the stomach. A case report focusing on its characteristic
growth pattern. Int J Clin Exp Pathol. 7:685–689. 2014.PubMed/NCBI
|
|
18
|
Li P, Yang S, Wang C, Li Y and Geng M:
Presence of smooth muscle cell differentiation in plexiform
angiomyxoid myofibroblastic tumor of the stomach: A case report.
Int J Clin Exp Pathol. 7:823–827. 2014.PubMed/NCBI
|
|
19
|
Agaimy A and Wunsch PH: Gastrointestinal
stromal tumours: A regular origin in the muscularis propria, but an
extremely diverse gross presentation. A review of 200 cases to
critically re-evaluate the concept of so-called
extra-gastrointestinal stromal tumours = Langenbecks Arch Surg.
391:322–329. 2006.
|
|
20
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miettinen M, Wang ZF, Sarlomo-Rikala M,
Osuch C, Rutkowski P and Lasota J: Succinate
dehydrogenase-deficient GISTs: A clinicopathologic,
immunohistochemical, and molecular genetic study of 66 gastric
GISTs with predilection to young age. Am J Surg Pathol.
35:1712–1721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miettinen M, Sobin LH and Lasota J:
Gastrointestinal stromal tumors presenting as omental masses-a
clinicopathologic analysis of 95 cases. Am J Surg Pathol.
33:1267–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miettinen M, Sobin LH and Sarlomo-Rikala
M: Immunohistochemical spectrum of GISTs at different sites and
their differential diagnosis with a reference to CD117 (KIT). Mod
Pathol. 13:1134–1142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu TC, Lin MT, Montgomery EA and Singhi
AD: Inflammatory fibroid polyps of the gastrointestinal tract:
Spectrum of clinical, morphologic and immunohistochemistry
features. Am J Surg Pathol. 37:586–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lasota J, Wang ZF, Sobin LH and Miettinen
M: Gain-of-function PDGFRA mutations, earlier reported in
gastrointestinal stromal tumors, are common in small intestinal
inflammatory fibroid polyps. A study of 60 cases. Mod Pathol.
22:1049–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daimaru Y, Kido H, Hashimoto H and Enjoji
M: Benign schwannoma of the gastrointestinal tract: A
clinicopathologic and immunohistochemical study. Hum Pathol.
19:257–264. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miettinen M, Shekitka KM and Sobin LH:
Schwannomas in the colon and rectum: A clinicopathologic and
immunohistochemical study of 20 cases. Am J Surg Pathol.
25:846–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhattacharya B, Dilworth HP,
Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, Fisher C and
Montgomery E: Nuclear beta-catenin expression distinguishes deep
fibromatosis from other benign and malignant fibroblastic and
myofibroblastic lesions. Am J Surg Pathol. 29:653–659. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montgomery E, Torbenson MS, Kaushal M,
Fisher C and Abraham SC: Beta-catenin immunohistochemistry
separates mesenteric fibromatosis from gastrointestinal stromal
tumor and sclerosing mesenteritis. Am J Surg Pathol. 26:1296–1301.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coffin CM, Dehner LP and Meis-Kindblom JM:
Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma and
related lesions: An historical review with differential diagnostic
considerations. Semin Diagn Pathol. 15:102–110. 1998.PubMed/NCBI
|
|
31
|
Cessna MH, Zhou H, Sanger WG, Perkins SL,
Tripp S, Pickering D, Daines C and Coffin CM: Expression of ALK1
and p80 in inflammatory myofibroblastic tumor and its mesenchymal
mimics: A study of 135 cases. Mod Pathol. 15:931–938. 2002.
View Article : Google Scholar : PubMed/NCBI
|