Introduction
Tumefactive multiple sclerosis (MS) is an uncommon
tumor-like variant of MS that is characterized as a demyelinating
inflammatory CNS disease with large acute lesions of ≥2 cm in
diameter (1). In a large proportion
of cases, these lesions are accompanied by edema, ring enhancement
on imaging studies, and mass effects (2). As other space occupying lesions,
including primary brain tumors, abscesses, metastases and stroke,
may present similarly on magnetic resonance imaging (MRI), it is
often challenging to determine the correct diagnosis (1). Diagnosis is further hampered when the
patient has not yet been diagnosed with MS. In addition, although
even more uncommon, the coincidence of brain tumors and tumefactive
MS is possible, even within the same lesion (3,4).
Therefore, correct diagnosis of tumefactive MS is essential and
frequently requires biopsy (5).
However, biopsy may be reluctantly undertaken due to
its inherent small but non-negligible risks (6). As recently suggested, factors advocating
the diagnosis of tumefactive MS and supporting deferral of biopsy
include the additional presence of oligoclonal cerebrospinal fluid
(CSF) banding, and/or white matter lesions suggestive of MS, and/or
a sustained response to corticosteroids. In the presence of these
conditions and the absence of clinical deterioration, a ‘wait and
see’ strategy without biopsy and with frequent follow-up MRI may be
justifiable (7).
In cases of suspected MS with tumefactive lesions,
it has been suggested that further imaging modalities be utilized
to guide clinical decision-making (8). In particular, positron emission
tomography (PET) using radioactively labelled, metabolically active
tracers, such as 18F-fluorodeoxyglucose
(18F-FDG) and 11C-methionine, is considered
potentially useful. As 18F-FDG uptake has a high
background activity in the brain, there is a high false-negative
rate for the detection of an underlying glioma. This is not the
case with 11C-methionine (9). However, 11C-methionine has a
short half-life. Therefore, its use is restricted to centers with
an on-site cyclotron (9).
By contrast, 18F-fluoroethyl-L-tyrosine
(18F-FET), another commonly used radiolabelled amino
acid, is characterized by a longer half-life and is thus more
suitable for widespread clinical usage (10). 18F-FET uptake in glioma is
high even in the presence of an intact blood-brain barrier
(11). Contrast-enhancing non-tumoral
lesions usually exhibit a normal 18F-FET uptake
(12). Despite these properties,
18F-FET PET has so far not been systematically used with
regard to distinction between inflammatory and tumorous
lesions.
To the best of our knowledge, the current study
presents the first documented case of a patient with a large
space-occupying lesion initially diagnosed as tumefactive MS based
on clinical and imaging factors, in whom subsequent
18F-FET PET correctly predicted a diagnosis of a
glioma.
Case report
A 41-year-old Caucasian woman was admitted to
University of Bonn Medical Center (Bonn, Germany) with a
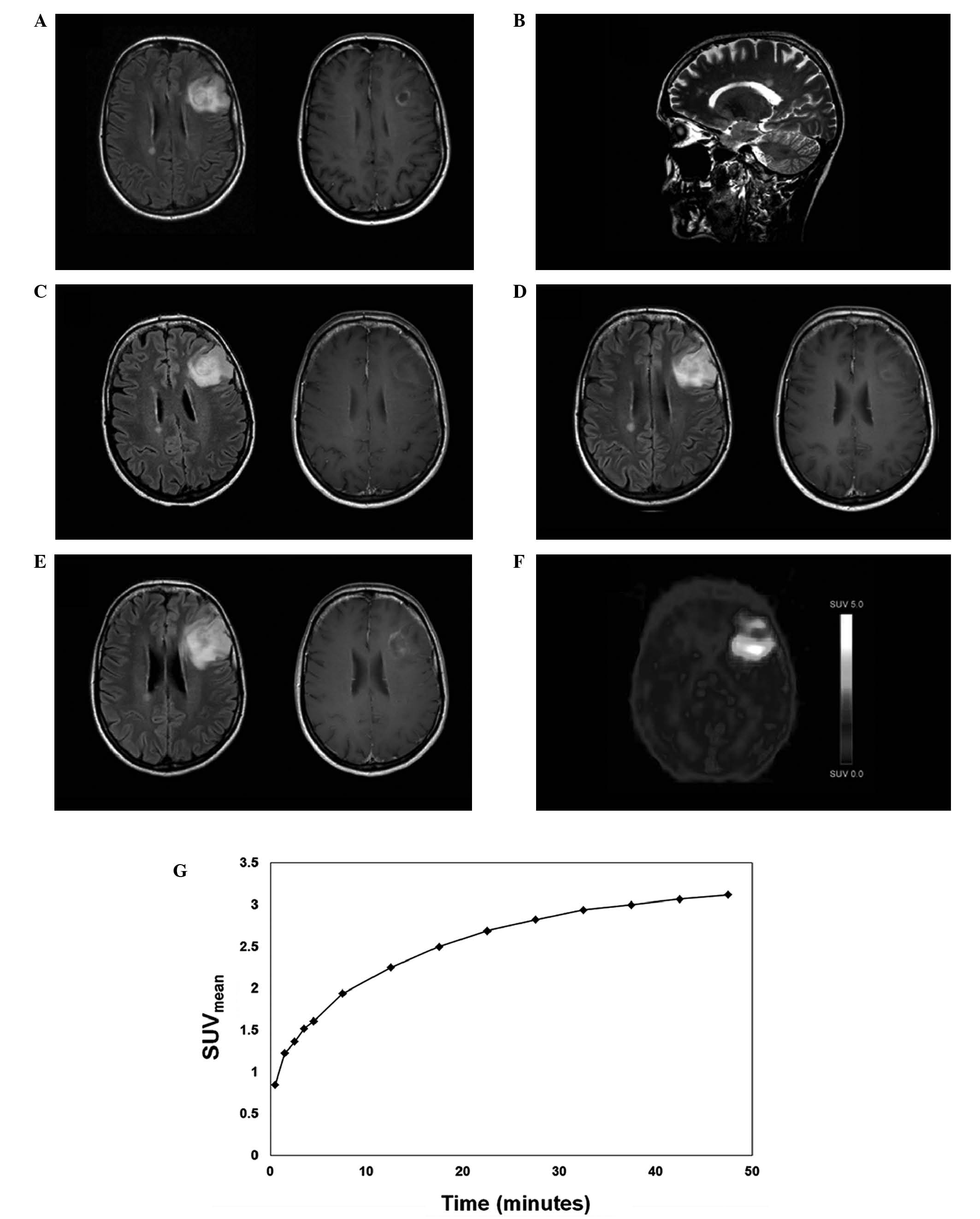
generalized seizure in December 2010. MRI revealed a left frontal
ring-enhancing lesion with additional non-enhancing periventricular
lesions and further lesions in the corpus callosum (Fig. 1A and B). As treatment, dexamethasone
(8 mg, intravenously, 3 times per day) was administered for several
days, and the patient commenced levetiracetam (1,000 mg,
intravenously, 3 times per day) as anti-epileptic medication.
Physical examination at that time and on follow-up visits revealed
no pathological findings. A serum screen for anti-nuclear
antibodies, anti-neutrophil cytoplasmic antibodies,
anti-cardiolipin antibodies, angiotensin converting enzyme,
lysozyme and C-reactive protein yielded negative results or normal
values. An infection screen, including human immunodeficiency
virus, treponemal and borrelia serology tests yielded negative
results or results within the normal ranges. There was no elevated
cell count in the CSF, glucose and lactate values were within
normal range, and fluorescence-activated cell sorting analysis
revealed no atypical cells. However, the CSF was positive for
oligoclonal bands. Somatosensory and visual evoked potentials were
normal.
Three weeks later, a follow-up MRI revealed a slight
reduction of contrast-enhancement (Fig.
1C). Barkhof criteria (13) for
the MRI supported a diagnosis of MS. Owing to a decrease of
contrast-enhancement on follow-up MRI, a planned biopsy was
withdrawn, and the patient was further observed by clinical
examination and MRI.
This reduction of contrast-enhancement sustained for
>3 years (Fig. 1D), until it
reappeared along with an increase in lesion size (Fig. 1E). However, this finding was not
accompanied by any clinical deterioration of the patient.
Subsequently, methlyprednisolone pulse therapy (1,000 mg,
intravenously, 3 times per day) was administered for 5 days,
following which no effect on the contrast-enhancing lesion was
observed on a follow-up MRI. To explore the possibility of the
coexistence of a primary brain tumor, 18F-FET PET was
performed (213 MBq 18F-FET, intravenous; dynamic
acquisition over 50 min; reconstruction of a static frame from
20–40 min). Static 18F-FET PET revealed high
tumor-to-brain ratio (TNR) values, indicating a neoplastic lesion
(TNRmax, 3.8; Fig. 1F)
(14). Kinetic analysis revealed a
clear uptake pattern in the mean standardized uptake value, with a
rapid initial increase followed by a slow further tracer
accumulation (Fig. 1G), a pattern
which has been described in low-grade glioma (15). A stereotactic biopsy revealed a World
Health Organization (WHO) grade II glioma (16). Subsequent complete resection of the
tumor provided material that allowed the diagnosis of a WHO grade
III oligoastrocytoma. Following diagnosis, the patient was
irradiated and consequently treated with a combination of
procarbazine (100 mg daily, days 8–22) and lomustine (110 mg/kg,
day 1) for 6 eight-week cycles. Brain MRI examination performed at
the most recent follow-up in November 2015, revealed that the
patient exhibited stable disease.
This report was approved by the local ethics
committee, and the patient provided written informed consent for
its publication.
Discussion
The present case report outlines the clinical course
of a patient initially diagnosed with tumefactive MS, with white
matter lesions reminiscent of MS, positive unmatched oligoclonal
bands in the CSF, sustained response to corticosteroids and
clinical stability following corticosteroid therapy. However, as
proven by histology, the diagnosis was eventually determined to be
an anaplastic oligoastrocytoma, likely coexisting with MS, and was
established >3 years after initial clinical presentation.
The decision in favor of biopsy and subsequent
resection in this particularly eloquent brain area was supported by
18F-FET PET results. Static and dynamic analyses of
18F-FET uptake yielded results typical for glioma.
Several larger case series have analyzed 18F-FET uptake
in cerebral lesions of unknown significance, among which few cases
of demyelinating lesions have been reported (16,17).
In line with these reports, the presented case
highlights the potential value of 18F-FET PET as a tool
to distinguish between MS and primary brain tumors. Both static and
dynamic parameters (as demonstrated for the first time in the
present case) are important to make this distinction. In
particular, the present case demonstrates that these parameters
allow the detection of a glioma on a background of an unequivocal
diagnosis of MS, where larger lesions may be highly suggestive of
tumefactive MS. In such cases, 18F-FET PET should be
added early to the portfolio of diagnostic procedures. Overall,
further systematic evaluation is warranted to explore the value of
18F-FET PET imaging in the workup of unclear, putatively
inflammatory cerebral lesions.
References
|
1
|
Nilsson P, Larsson EM, Kahlon B, Nordström
CH and Norrving B: Tumefactive demyelinating disease treated with
decompressive craniectomy. Eur J Neurol. 16:639–642. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucchinetti CF, Gavrilova RH, Metz I, et
al: Clinical and radiographic spectrum of pathologically confirmed
tumefactive multiple sclerosis. Brain. 131:1759–1775. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green AJ, Bollen AW, Berger MS, et al:
Multiple sclerosis and oligodendroglioma. Mult Scler. 7:269–273.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SH, Buwembo JE and Li Q: Concurrence
of glioma and multiple sclerosis. Can J Neurol Sci. 32:349–351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Given CA 2nd, Stevens BS and Lee C: The
MRI appearance of tumefactive demyelinating lesions. AJR Am J
Roentgenol. 182:195–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butteriss DJ, Ismail A, Ellison DW and
Birchall D: Use of serial proton magnetic resonance spectroscopy to
differentiate low grade glioma from tumefactive plaque in a patient
with multiple sclerosis. Br J Radiol. 76:662–665. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hardy TA and Chataway J: Tumefactive
demyelination: An approach to diagnosis and management. J Neurol
Neurosurg Psychiatry. 84:1047–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takenaka S, Shinoda J, Asano Y, et al:
Metabolic assessment of monofocal acute inflammatory demyelination
using MR spectroscopy and (11)C-methionine-, (11)C-choline-, and
(18)F-fluorodeoxyglucose-PET. Brain Tumor Pathol. 28:229–238. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gulyás B and Halldin C: New PET
radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl
Med Mol Imaging. 56:173–190. 2012.PubMed/NCBI
|
|
10
|
Floeth FW, Sabel M, Stoffels G, Pauleit D,
Hamacher K, Steiger HJ and Langen KJ: Prognostic value of
18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific
incidental brain lesions. J Nucl Med. 49:730–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langen KJ, Hamacher K, Weckesser M, et al:
O-(2-[18F]Fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical
applications. Nucl Med Biol. 33:287–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piroth MD, Pinkawa M, Holy R, et al:
Prognostic value of early [18F]Fluoroethyltyrosine positron
emission tomography after radiochemotherapy in glioblastoma
multiforme. Int J Radiat Oncol Biol Phys. 80:176–184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rovaris M, Barkhof F, Calabrese M, De
Stefano N, Fazekas F, Miller DH, Montalban X, Polman C, Rocca MA,
Thompson AJ, et al: MRI features of benign multiple sclerosis:
toward a new definition of this disease phenotype. Neurology.
72:1693–1701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rapp M, Heinzel A, Galldiks N, et al:
Diagnostic performance of 18F-FET PET in newly diagnosed cerebral
lesions suggestive of glioma. J Nucl Med. 54:229–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pöpperl G, Kreth FW, Mehrkens JH, et al:
FET PET for the evaluation of untreated gliomas: Correlation of FET
uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol
Imaging. 34:1933–1942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hutterer M, Nowosielski M, Putzer D,
Jansen NL, Seiz M, Schocke M, McCoy M, Göbel G, la Fougère C,
Virgolini IJ, Trinka E, Jacobs AH and Stockhammer G:
[18F]-fluoro-ethyl-L-tyrosine PET: A valuable diagnostic tool in
neuro-oncology, but not all that glitters is glioma. Neuro Oncol.
15:341–351. 2013. View Article : Google Scholar : PubMed/NCBI
|