Introduction
Gallbladder carcinoma (GBC) is a rare disease among
the gastrointestinal cancers; however, GBC is a common malignant
tumor in the extrahepatic biliary tract system, accounting for
80–95% of biliary tract cancers (1,2). The
morbidity rate of GBC has markedly increased due to the advancing
age of patients, and the increased rate of cholelithiasis and
chronic inflammation (3). As GBC
lacks a specific clinical presentation, the disease is usually
diagnosed at the advanced stages, or is incidentally identified
during or following a cholecystectomy (3). Despite the lack of remarkable clinical
features, the unique anatomical features of the gallbladder,
including abundant blood and lymphatic vessels, mean that GBCs
easily invade the surrounding structures, and this is the most
common method of GBC metastasis (4).
The prognosis of GBC is poor, and the 5-year survival rate is
extremely low (~16%) (5).
The treatment of GBC includes surgery, chemotherapy
and palliative therapies (6).
However, the cause of GBC has not previously been clear. CD74
molecule (CD74), also known as Ii or the invariant chain, is a type
II transmembrane glycoprotein that has diverse immunological
functions. The basic function of CD74 is associated with major
histocompatibility complex (MHC) II, and CD74 is hypothesized to be
associated with the processing of class II MHC molecules on
antigen-presenting cells. In addition to antigen presentation, CD74
has also been identified as the receptor of macrophage migration
inhibitory factor (MIF), which activates inflammation, and the
expression of CD74 has been reported in numerous human malignant
tumors, including breast, pancreatic and colorectal cancer
(7–10). CD74 is important in carcinogenesis, as
it acts as an accessory signaling molecule for cell proliferation
(11). However, it is unknown whether
CD74 is associated with the occurrence and development of GBC. The
present study investigated the expression of CD74 in GBC tissues in
order to elucidate the availability of CD74 as a novel biomarker
for GBC. The associations between the level of CD74 expression the
clinicopathological parameters, and epithelial growth factor
receptor (EGFR), a known participant in numerous malignant
diseases, were analyzed. CD74 was indicated to potentially be a
core factor in the progression of GBC.
Materials and methods
Patients
A total of 54 patients who were diagnosed
histopathologically with GBC underwent tumor resection at the
Renmin Hospital of Wuhan University (Wuhan, China) between July
2009 and July 2013. The mean age of the patients was 61.2 years old
and none of the patients received pre-operative or intraoperative
chemotherapy or radiotherapy. The GBC tissues were divided into two
groups; one of which was used to create paraffin-embedded tissue
sections and the other of which was placed in liquid nitrogen for
western blot analysis. The clinicopathological data of the patients
were collected in order to analyze the association between the
expression levels of CD74 and the clinicopathological data. All
experiments using human tissues were reviewed and approved by the
Committee for Ethical Reviews of Research involving Human Subjects
of Remin Hospital of Wuhan University, and performed according to
the Declaration of Helsinki.
Immunohistochemistry (IHC)
The paraffin-embedded tissues were cut into 3-µm
thick tissue sections. To examine the expression of CD74 and EGFR,
immunohistochemical analysis was performed using a standard
streptavidin-peroxidase staining method. Briefly, the sections were
deparaffinized and dehydrated using a graded series of ethanol
solutions. The microscope slides were immersed in 10 mM citrate
buffer (pH 6.0; catalog no., G1202; Wuhan Goodbio Biotechnology
Co., Ltd., Wuhan, China) and boiled for 5 min at 121°C in the
pressure cooker (Supor YS20ED; Supor, Hangzhou, China) for the
antigen retrieval. The slides were allowed to cool at room
temperature. Hydrogen peroxide (0.3%; catalog no., H44023919;
Guangdong Heng Jian Pharmaceutical Co., Ltd., Jiangmen, China) was
used to halt the endogenous peroxidase activity for 15 min at room
temperature. Non-specific binding was blocked using goat serum (5%;
catalog no., C0265; Beyotime Institute of Biotechnology, Haimen,
China) for 10 min. The primary antibodies used were a mouse
anti-human anti-CD74 monoclonal antibody (dilution, 1:150; catalog
no., ab9514; Abcam, Cambridge, UK) and a rabbit anti-human
anti-EGFR monoclonal antibody (dilution, 1:100; catalog no.,
ZA0505; ZSGB-BIO, Beijing, China). Sections were incubated with the
primary antibodies overnight at 4°C, and then incubated with
secondary antibodies from the UltraSensitiveTM SP (Mouse/Rabbit)
IHC kit (catalog no., SP-9000, ZSGB-Bio). The staining results were
visualized using 3,5-diaminobenzidine (DAB; catalog no., G1211;
Wuhan Goodbio Biotechnology Co., Ltd.). For each
immunohistochemical analysis, phosphate-buffered saline (PBS) was
used instead of the primary antibody for the negative control.
Evaluation of the immunohistochemical
findings
Two independent observers with IHC experience
blindly evaluated the results. The BX53 upright microscope (Olympus
Corporation, Tokyo, Japan) was used to capture images of the
immunohistochemical staining results. Image-Pro Plus version 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA) was used to
judge the area and density of the dyed region, and the integrated
optical density (IOD) value of the IHC section. According to the
staining intensity, the results of IHC were assigned a score as
follows: No dye, 0; pale yellow dye, 1; yellow dye, 2; and brown
dye, 3. The percentage of tumor cells was determined by taking an
average score of at least 5 regions under ×200 magnification. The
mean percentage was then divided into four scored categories:
<5% tumor cells, 0; 5–25% tumor cells, 1; 26–50% tumor cells, 2;
and 51–100% tumor cells, 3. Finally the score for staining
intensity and the tumor cell percentage were added together as
follows: An overall score between 0–2 was defined as negative
expression; and an overall score between 3–6 was defined as
positive expression (12).
Western blot analysis
The fresh GBC tissues were homogenized in ice-cold
lysis buffer (Wuhan Goodbio Biotechnology Co., Ltd.) in the
presence of protease inhibitor cocktail (catalog no., G2006; Wuhan
Goodbio Biotechnology Co., Ltd.). The concentrations of protein in
the samples were determined using the Bradford method (13) with bovine serum albumin (Wuhan Goodbio
Biotechnology Co., Ltd.) as a standard. In brief, equal amounts of
protein sample were separated on 10% sodium dodecyl sulfate
polyacrylamide gels and then transferred to a polyvinylidene
difluoride membrane. The membrane was blocked with 5% skimmed milk
in TBST (Tris-buffered saline containing 0.1% Tween-20) at room
temperature for 2 h and then incubated with the rabbit anti-human
anti-CD74 polyclonal antibody (1:1,000; Abcam) 4°C overnight.
Subsequent to extensive rinsing with TBST (3 washes for 10 min
each), the blots were incubated with IRDye 800CW goat anti-rabbit
polyclonal secondary antibody (dilution, 1:10,000; catalog no.,
926–32211; LI-COR Biosciences, Lincoln, NE, USA) at room
temperature for 1.5 h, and then the expression of CD74 was detected
using the Odyssey CLx infrared imaging system (LI-COR
Biosciences).
Statistical analysis
Data were analyzed with SPSS statistical software
version 19.0 (IBM SPSS, Armonk, NY, USA). The χ2 test
was used to assess the association between the expression of CD74
and the clinicopathological characters. The correlation between
CD74 and EGFR was evaluated using the Spearman's rank correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunostaining in normal and cancerous
tissues, as determined by IHC
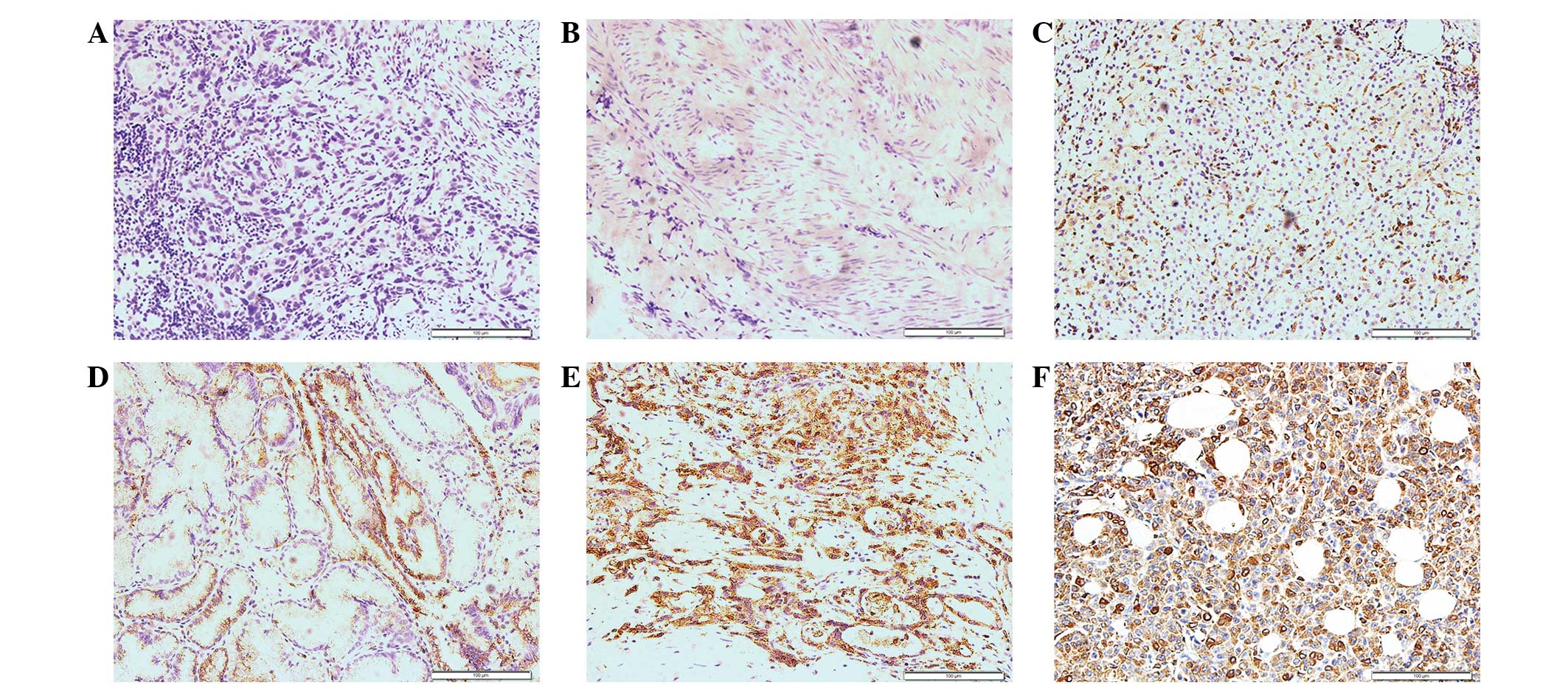
The positive expression of CD74 was indicated by
yellow or brown staining in the cytoplasm. In the normal
gallbladder tissues, the expression of CD74 was absent. In the GBC
tissues, CD74 was more strongly stained in the
poorly-differentiated carcinoma compared with the
well-differentiated tissues (Fig.
1).
Expression of CD74 in GBC tissues, as
determined by western blot analysis
The expression of CD74 was detected in tissue
samples taken immediately following resection of GBC tumors, and
the western blotting results revealed that in the
poorly-differentiated GBC tissues, CD74 was upregulated compared
with the moderately- and well-differentiated GBC tissues. The
outcome of the western blot analysis was consistent with the
immunohistochemical findings (Fig.
2).
Correlation between CD74 expression
and clinicopathological characteristics
The correlation between CD74 expression and the
clinicopathological characteristics of GBC are shown in Table I (14).
In the GBC tissues, the poorly-differentiated carcinomas exhibited
increased CD74(+) expression compared with the well- and
moderately-differentiated carcinomas, the tumor-node-metastasis
(TNM) stage II–IV tissues exhibited increased CD74(+) expression
compared with the TNM stage 0-II tissues, and the group of tissues
with increased depth of tumor infiltration,
T3-T4, exhibited increased CD74(+) expression
compared with the Tis-T2 group. There were no
significant differences in the expression of CD74 for certain
clinical features, including gender, age and lymph node
metastasis.
 | Table I.Correlation between CD74 expression
and the clinicopathological characteristics of gallbladder
carcinoma. |
Table I.
Correlation between CD74 expression
and the clinicopathological characteristics of gallbladder
carcinoma.
|
|
| CD74 expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
features | No. of patients
(n=54) | + | − | χ2
value | P-value |
|---|
| Gender |
|
|
| 0.087 | 0.768 |
| Male | 17 | 12 | 5 |
|
|
|
Female | 37 | 23 | 14 |
|
|
| Age, years |
|
|
| 0.055 | 0.814 |
|
<61 | 23 | 14 | 9 |
|
|
| ≥61 | 31 | 21 | 10 |
|
|
| Differentiation |
|
|
| 8.277 | 0.016 |
| Well | 8 | 2 | 6 |
|
|
|
Moderate | 28 | 18 | 10 |
|
|
| Poor | 18 | 15 | 3 |
|
|
| Depth of tumor
infiltration |
|
|
| 8.406 | 0.004 |
|
TIS-T2 | 24 | 10 | 14 |
|
|
|
T3-T4 | 30 | 25 | 5 |
|
|
| Lymph-node
metastasis |
|
|
| 0.549 | 0.459 |
| No | 29 | 17 | 11 |
|
|
| Yes | 25 | 12 | 13 |
|
|
| TNM stage |
|
|
| 4.853 | 0.028 |
|
0-I | 5 | 1 | 4 |
|
|
|
II–IV | 49 | 34 | 15 |
|
|
Correlation between CD74 expression
and EGFR expression in GBC
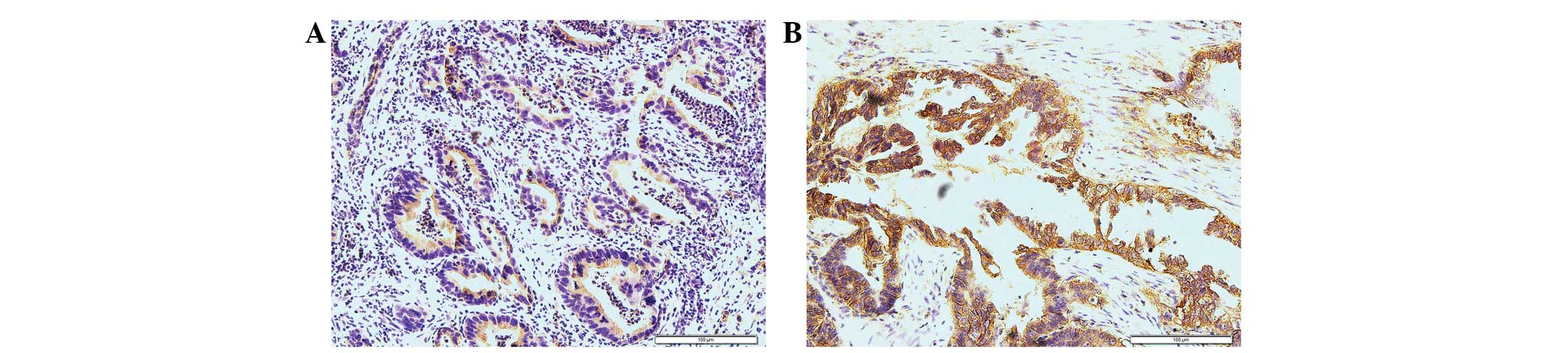
The expression and IOD values of EGFR were examined
in the GBC tissues. EGFR expression in GBC tissues may be observed
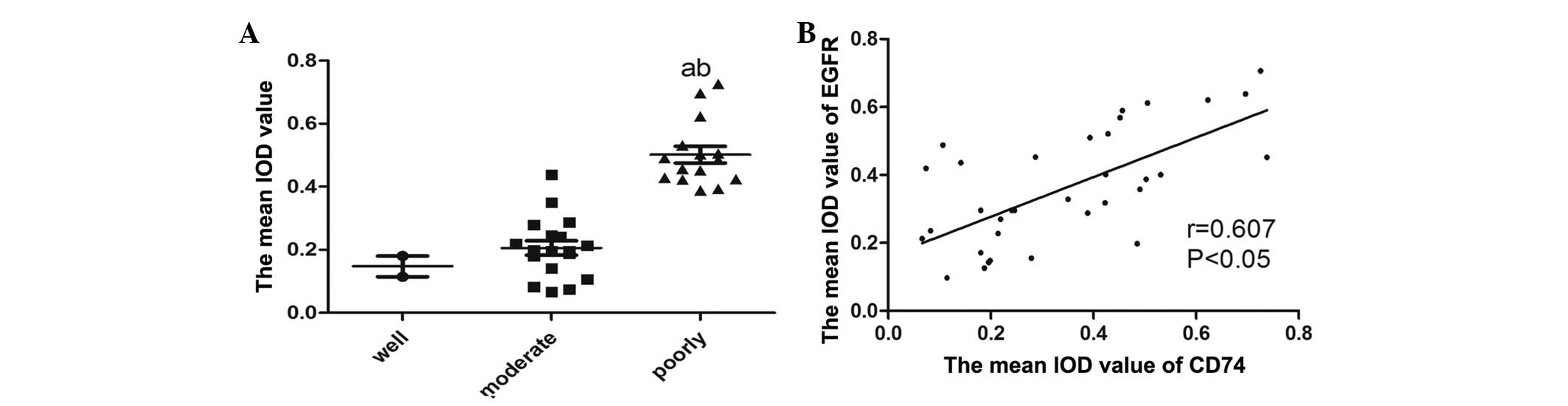
in Fig. 3. The IOD values of EGFR are
presented in Fig. 4A. Using the
Spearman's rank correlation coefficient, the IOD values of CD74
were shown to be positively correlated with the levels of EGFR
(Fig. 4B). The tissue sections that
exhibited the expression of CD74 strongly tended to be associated
with the increased expression of EGFR.
Discussion
GBC is the seventh most common cancer of the
digestive system (15). The early
diagnosis of GBC occurs infrequently due to the lack of specific
clinical characteristics associated with the disease, and as GBC is
challenging to distinguish from common digestive tract diseases,
including cholelithiasis and chronic cholecystitis. Therefore, the
majority of GBC patients are in the advanced stages of the disease
by the time they arrive at hospital for treatment. The advanced
stages of GBC are associated with a poor prognosis and high
mortality rate; the mean survival rate of patients with advanced
GBC is ~6 months and the 5-year survival rate is <32% (7,16).
Therefore, finding a novel tumor biomarker that may be used for the
early diagnosis of GBC may have a positive effect on the 5-year
survival rate of GBC. Numerous clinical studies have suggested that
CD74 is important in the pathogenesis of hematological malignancies
and various solid carcinomas (17).
The present study reported the expression of CD74 and EGFR in GBC
by immunohistochemical staining, and used Spearman's rank
correlation coefficient in order to study the association between
the expression of CD74 and EGFR in GBC. The results revealed that
CD74 was overexpressed in the GBC tissues, and that the mean
staining intensity of CD74 in the poorly-differentiated GBC tissues
was increased compared with that in the well- and
moderately-differentiated tissues. The results of the western blot
analysis also showed that the expression of CD74 was upregulated in
the poorly-differentiated GBC tissues. The precise role of CD74 in
GBC has not been previously elucidated. The study by Roche and
Cresswell (18) reported that CD74 is
the invariant chain of MHC-II and a type of transmembrane
glycoprotein. CD74 is expressed at high levels in
antigen-presenting cells, including B-cells, monocytes and
macrophages, so it is extremely important in antigen presentation.
D-related (DR)-CD74 complexes have a suppressive effect on the host
immune response, which results in the promotion of tumor
proliferation (19,20). Certain studies have suggested that the
overexpression of CD74 in malignancies may block endogenous tumor
antigen presentation by MHC-II, resulting in immune escape in
vivo (21). In B-cell lymphoma
and multiple myeloma, CD74 has been reported as a novel and
promising therapeutic target (22,23). In
non-hematological malignancies, including gastric carcinoma, renal
cancer and non-small cell lung cancer, CD74 has also been reported
as a novel biomarker for its value in predicting prognoses
(24–26).
In the present study, the mean IOD value of CD74 in
the poorly-differentiated GBC tissues was increased compared with
the well- and moderately-differentiated tissues, and the western
blotting results were consistent with this finding. These results
indicated that CD74 may take part in the process of differentiation
in GBC tumor cells. The role of CD74 in carcinogenesis may be
associated with MIF, which is an important inflammatory cytokine
and has a strong association with carcinogenesis (27). CD74 is the receptor of MIF, and the
MIF and CD74 ligand/receptor combination may be a key factor in
determining the difference between carcinogenesis and chronic
inflammation. The ligand/receptor combination may increase the
proliferation of epithelial cells through the tumor protein p53
(p53) pathway (28). p53 is a tumor
suppressor, and when p53 is blocked from the cytomembrane to the
nucleus, the function of the apoptotic pathway is decreased and
proliferation is increased (29). The
MIF and CD74 combination may also increase the proliferation of
epithelial cells by activating EGFR, which has been established as
a biomarker and therapeutic target in various solid tumors and is
important in the proliferation, migration and invasion of tumor
cells (30). The combination may also
upregulate the pro-inflammatory cytokine interleukin-8, which then
binds to a receptor on the surface of the epithelial cell. This
novel ligand/receptor combination may regulate the level of EGFR
(31,32). The present study detected the
expression of EGFR and CD74 by IHC within the same section of GBC
tissue, and the mean IOD value of CD74 and EGFR was tested using
the Spearman's rank correlation coefficient. The result revealed
that the IHC staining of CD74 positively correlated with EGFR in
the GBC tissues. The samples that exhibited an increased expression
level of CD74 were associated with an increased expression level of
EGFR.
In conclusion, the IHC and western blotting results
of CD74 revealed that CD74 was closely associated with the degree
of differentiation in the GBC tissues, and that the correlation
between CD74 and EGFR, as determined by Spearman's rank correlation
coefficient, was positive. As the present study did not examine the
expression of CD74 in GBC at the RNA and cellular levels,
additional studies are required to determine the precise role of
CD74 in GBC. The present results elucidate the potential role of
CD74 as a key participator in the progression of GBC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370562) and the
Projects of Medical and Health Technology Development Program in
Shandong Province (grant no. 2013GGB14096). The authors would like
to thank Dr. Yongfei Tang of the Department of Pathology, Renmin
Hospital of Wuhan University (Wuhan, China) for assistance with the
evaluation of immunohistochemical findings.
References
|
1
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
2
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, de Ruiz Alonso P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaushik SP: Current perspectives in
gallbladder carcinoma. J Gastroenterol Hepatol. 16:848–854. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gore RM and Shelhamer RP: Biliary tract
neoplasms: Diagnosis and staging. Cancer Imaging. 7:S15–S23. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carriaga MT and Henson DE: Liver,
gallbladder, extrahepatic bile ducts and pancreas. Cancer. 75(Suppl
1): S171–S190. 1995. View Article : Google Scholar
|
|
6
|
Wernberg JA and Lucarelli DD: Gallbladder
cancer. Surg Clin North Am. 94:343–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mastoraki A, Papanikolaou IS,
Konstandiadou I, Sakorafas G and Safioleas M: Facing the challenge
of treating gallbladder carcinoma. Review of the literature.
Hepatogastroenterology. 57:215–219. 2010.PubMed/NCBI
|
|
8
|
Tassi E, Braga M, Longhi R, Gavazzi F,
Parmiani G, Di Carlo V and Protti MP: Non-redundant role for IL-12
and IL-27 in modulating Th2 polarization of carcinoembryonic
antigen specific CD4 T cells from pancreatic cancer patients. PloS
One. 4:e72342009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JF, Hua R, Liu DJ, Liu W, Huo YM and
Sun YW: Effect of CD74 on the prognosis of patients with resectable
pancreatic cancer. Hepatobiliary Pancreat Dis Int. 13:81–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kindt N, Lechien JR, Nonclercq D, Laurent
G and Saussez S: Involvement of CD74 in head and neck squamous cell
carcinomas. J Cancer Res Clin Oncol. 140:937–947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenwood C, Metodieva G, Al-Janabi K,
Lausen B, Alldridge L, Leng L, Bucala R, Fernandez N and Metodiev
MV: Stat1 and CD74 overexpression is co-dependent and linked to
increased invasion and lymph node metastasis in triple-negative
breast cancer. J Proteomics. 75:3031–3040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pinheiro C, Longatto-Filho A, Scapulatempo
C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA,
Schmitt F and Baltazar F: Increased expression of monocarboxylate
transporters 1, 2 and 4 in colorectal carcinomas. Virchows Arch.
452:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Liu W, Qu Q, Hong T, Xu X, Li B,
Wang Y and He X: Clinical assessment using TNM staging for 151
patients with gallbladder carcinoma. Chinese Journal of
Hepatobiliary Surgery. 20:507–510. 2014.(In Chinese and
English).
|
|
15
|
Reddy SK and Clary BM: Surgical management
of gallbladder cancer. Surg Oncol Clin N Am. 18:307–324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stinton LM and Shaffer EA: Epidemiology of
gallbladder disease: Cholelithiasis and cancer. Gut Liver.
6:172–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagata S, Jin YF, Yoshizato K, Nagata S,
Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, Kitamura M,
Takahashi H, Eguchi H, Ohigashi H, et al: CD74 is a novel
prognostic factor for patients with pancreatic cancer receiving
multimodal therapy. Ann Surg Oncol. 16:2531–2538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roche PA and Cresswell P: Invariant chain
association with HLA-DR molecules inhibits immunogenic peptide
binding. Nature. 345:615–618. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hippo Y, Yashiro M, Ishii M, Taniguchi H,
Tsutsumi S, Hirakawa K, Kodama T and Aburatani H: Differential gene
expression profiles of scirrhous gastric cancer cells with high
metastatic potential to peritoneum or lymph nodes. Cancer Res.
61:889–895. 2001.PubMed/NCBI
|
|
20
|
Jiang Z, Xu M, Savas L, LeClair P and
Banner BF: Invariant chain expression in colon neoplasms. Virchows
Arch. 435:32–36. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M, Qiu G, Jiang Z, von Hofe E and
Humphreys RE: Genetic modulation of tumor antigen presentation.
Trends Biotechnol. 18:167–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Griffiths GL, Mattes MJ, Stein R, Govindan
SV, Horak ID, Hansen HJ and Goldenberg DM: Cure of SCID mice
bearing human B-lymphoma xenografts by an anti-CD74
antibody-anthracycline drug conjugate. Clin Cancer Res.
9:6567–6571. 2003.PubMed/NCBI
|
|
23
|
Burton JD, Ely S, Reddy PK, Stein R, Gold
DV, Cardillo TM and Goldenberg DM: CD74 is expressed by multiple
myeloma and is a promising target for therapy. Clin Cancer Res.
10:6606–6611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ioachim HL, Pambuccian SE, Hekimgil M,
Giancotti FR and Dorsett BH: Lymphoid monoclonal antibodies
reactive with lung tumors. Diagnostic applications. Am J Surg
Pathol. 20:64–71. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lazova R, Moynes R, May D and Scott G:
LN-2 (CD74). A marker to distinguish atypical fibroxanthoma from
malignant fibrous histiocytoma. Cancer. 79:2115–2124. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Young AN, Amin MB, Moreno CS, Lim SD,
Cohen C, Petros JA, Marshall FF and Neish AS: Expression profiling
of renal epithelial neoplasms: A method for tumor classification
and discovery of diagnostic molecular markers. Am J Pathol.
158:1639–1651. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez-Niño MD, Sanz AB, Ruiz-Andres O,
Poveda J, Izquierdo MC, Selgas R, Egido J and Ortiz A: MIF, CD74
and other partners in kidney disease: Tales of a promiscuous
couple. Cytokine Growth Factor Rev. 24:23–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beswick EJ, Pinchuk IV, Suarez G, Sierra
JC and Reyes VE: Helicobacter pylori CagA-dependent macrophage
migration inhibitory factor produced by gastric epithelial cells
binds to CD74 and stimulates procarcinogenic events. J Immunol.
176:6794–6801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung H, Seong HA and Ha H: Critical role
of cysteine residue 81 of macrophage migration inhibitory factor
(MIF) in MIF-induced inhibition of p53 activity. J Biol Chem.
283:20383–20396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maharshak N, Cohen S, Lantner F, Hart G,
Leng L, Bucala R and Shachar I: CD74 is a survival receptor on
colon epithelial cells. World J Gastroenterol. 16:3258–3266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beswick EJ and Reyes VE: Macrophage
migration inhibitory factor and interleukin-8 produced by gastric
epithelial cells during Helicobacter pylori exposure induce
expression and activation of the epidermal growth factor receptor.
Infect Immun. 76:3233–3240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka
H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, et al: IL-8
promotes cell proliferation and migration through
metalloproteinase-cleavage proHB-EGF in human colon carcinoma
cells. Cytokine. 29:275–282. 2005.PubMed/NCBI
|