Introduction
Chromophobe renal cell carcinoma (chRCC) is the
third most common subtype of kidney cancer and accounts for ~5% of
all RCC cases. The 5-year disease-free survival rate of chRCC is
reported to be increased compared with that of other RCC subtypes,
including clear cell, sarcomatoid and papillary renal cell
carcinoma (pRCC) (1). Although the
outcomes of chRCC are typically more favorable compared with those
of other subtypes, the disease still demonstrates a 6–7%
probability of tumor progression and metastasis (2).
Histologically, chRCC consists of large polygonal
cells with a slightly reticulated cytoplasm, and with clear and/or
eosinophilic cells (3,4). The similarities between the histological
features of chRCC and oncocytoma, a benign tumor of the kidney, may
lead to the misdiagnosis of chRCC (5).
A cytogenetic analysis conducted in a previous study
revealed an association between chRCC and the loss of chromosomes
1, 2, 6, 10, 13, 17 and 21; therefore, such losses may be prominent
abnormalities useful for the diagnosis of the disease (6). In addition, differential gene expression
has been used to assist in the diagnosis of chRCC. For example, the
V-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT)
gene is indicated to be overexpressed in chRCC compared with pRCC
(7). Notably, Petit et al
(8) reported that the rate of
positive KIT expression on immunohistochemical staining was ~90% in
chRCC tissues and ~70% in oncocytoma tissues. Tan et al
(9) used high-resolution
single-nucleotide polymorphism profiling to distinguish between
chRCCs and oncocytomas, and pathway analyses emphasized the
involvement of Erb-B2 receptor tyrosine kinase 2 [human epidermal
growth factor receptor (HER) 2] signaling in chRCC. However, in the
study conducted by Tan et al, the immunohistochemical
analysis did not identify a significant difference in extracellular
HER2 expression between chRCCs and oncocytomas.
The roles of other HER family genes, including HER1,
HER3 and HER4, have not been well studied in chRCC. The present
study aimed to investigate the abnormalities of the HER family and
assess a potential association with chRCC.
Materials and methods
Tissue specimens
In total, 11 chRCC specimens from patients diagnosed
between 2005 and 2009 were included in the present study, with
approval from the Human Subject Research Ethics
Committee/Institutional Review Board at the Chang Gung Memorial
Hospital (Taoyuan, Taiwan) (IRB No.:101-3236B). The diagnosis and
classification of chRCC was confirmed using pathological analysis,
according to the histopathological evaluation of paraffin-embedded
sections using the pathological tumor-node-metastasis (TNM) staging
criteria. All cases were classified as stage I or II on a
four-stage scale, with the exception of cases 7 and 8, which were
classified as stage III. All tumors that were >4 cm in size were
selected (one case ≤4 cm in size). None of the patients exhibited
lymph node involvement or metastases. The majority of the patients
were diagnosed with low-grade malignancy, grade II tumors, with the
exception of cases 8 and 10 (Table
I).
 | Table I.Clinical features of 11 chromophobe
renal cell carcinoma patients. |
Table I.
Clinical features of 11 chromophobe
renal cell carcinoma patients.
|
| Patients |
|---|
|
|
|
|---|
| Variable | n | % |
|---|
| Age at diagnosis,
yearsa |
|
|
| ≤53 | 6 | 55 |
|
>53 | 5 | 45 |
| Gender |
|
|
|
Female | 3 | 27 |
| Male | 8 | 73 |
| Cell type |
|
|
|
Typical | 7 | 64 |
|
Eosinophilic | 4 | 36 |
| Metastasis |
|
|
|
Present | 0 |
0 |
|
Absent | 11 | 100 |
| TNM stage |
|
|
| I | 6 | 55 |
| II | 3 | 27 |
| III | 2 | 18 |
| Tumor size, cm |
|
|
| ≤4 | 1 |
9 |
|
>4–7 | 6 | 55 |
|
>7 | 4 | 36 |
| Grade |
|
|
| 1 | 0 |
0 |
| 2 | 9 | 82 |
| 3 | 1 |
9 |
|
Unknown | 1 |
9 |
| Necrosis |
|
|
|
Present | 3 | 27 |
|
Absent | 8 | 73 |
Fluorescence in situ hybridization
(FISH)
Fresh tissues were collected from 11 chRCC patients
for FISH analysis. Touch imprint cytology smears were performed on
all frozen tumor samples, following fixation in methanol-acetic
acid (dilution, 3:1). Dual color probes were prepared for the
target genes. To screen for the HER family genes, several bacterial
artificial chromosomes (BACs) were selected, according to the
National Center for Biotechnology Information or University of
California, Santa Cruz databases, and purchased from the Children's
Hospital Oakland (Oakland, CA, USA) (Table II). BACs DNA were isolated using the
High-Speed Plasmid Mini kit (Geneaid, Taipei, Taiwan), according to
the manufacturer's protocol. The DNA were labeled with fluorescent
dye by nick translation according to the protocol published by Weng
et al (10), in which HER1
(BAC clone no. RP11-14K11, RP11-339F13 and CTD-2199A14) and HER2
(BAC clone no. RP11-94L15) were labeled with Red-deoxyuridine
triphosphatase (dUTP) (Enzo Life Sciences, Inc., Farmingdale, NY,
USA), and HER3 (BAC clone no. RP11-973D8), HER4 (BAC clone no.
RP11-384K20) and chromosome 17q11.2–12 (BAC clone no. RP11-79O4),
which is adjacent to chromosome 17 centromere (CEN17) and used as a
HER2 control, were labeled with Green-dUTP (Enzo Life Sciences,
Inc.) (Table II). Hybridization was
performed at 37°C for 8–10 h. All probes were homemade and the
accuracy and specificity of all probes was confirmed via
hybridization onto commercially available CGH Metaphase Target
Slides (Abbott Laboratories Inc., Chicago, IL, USA). All images
were captured using a Leica DM2500 microscope (Leica Microsystems
GmbH, Wetzlar, Germany) with an ASI CCD camera (CCD-1300DS; Applied
Spectral Imaging, Ltd., Migdal HaEmtek, Israel), and subsequently
analyzed with FISHView EXPO version 5.5 software (Applied Spectral
Imaging, Ltd.). In each experiment a minimum of 150 interphase
nuclei were analyzed.
 | Table II.The tested genes, associated BACs and
labeled fluorescent dyes. |
Table II.
The tested genes, associated BACs and
labeled fluorescent dyes.
| Gene name | BAC | Labeled dye |
|---|
| HER1 | RP11–14K11,
RP11–339F13 and CTD-2199A14 | Red-dUTP |
| HER2 | RP11–94L15 | Red-dUTP |
| HER3 | RP11–973D8 | Green-dUTP |
| HER4 | RP11–384K20 | Green-dUTP |
| CEN17 | RP11–79O4 | Green-dUTP |
Statistical analysis
Statistical analyses were performed using the SPSS
statistical package (version 17.0; SPSS, Inc., Chicago, IL, USA).
The Spearman's rank correlation coefficient was calculated in order
to evaluate the association between the loss of HER2 and the copy
number variation or gene structure alterations of other members of
the HER family. P<0.05 was considered to indicate a
statistically significant difference.
Results
FISH analysis
At least 150 nuclei of fluorescent in situ
signals were counted for each sample. The abnormalities, including
copy number variations and gene structure alterations, were
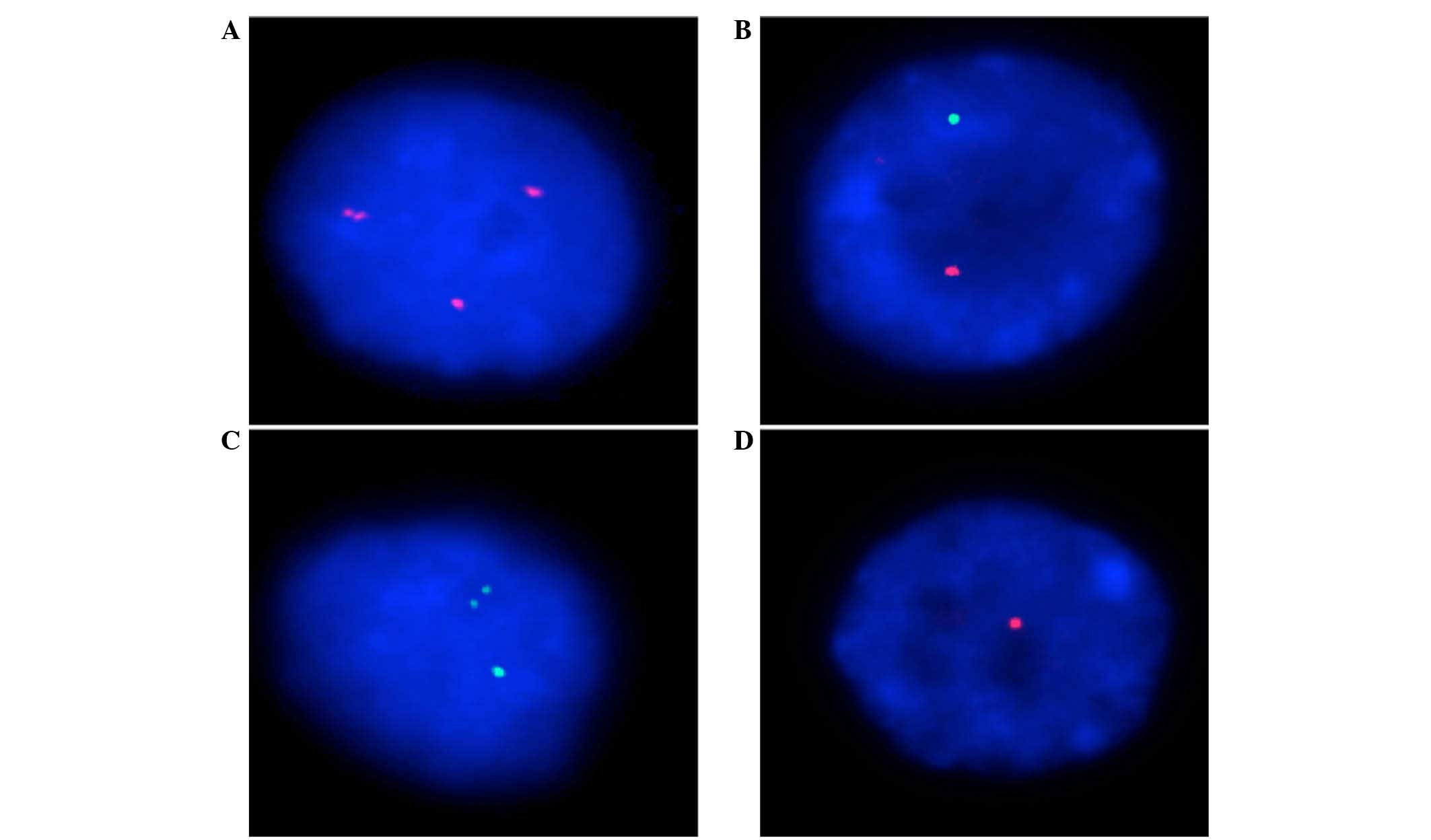
identified for each gene (Fig. 1). In
general, the loss of one copy of HER2 (confirmed using CEN17) and
HER4 was considered to be a major alteration in all cases, and
occurred in 26–97% and 60–89%, respectively (Table III; Fig.
1A and B). However, amplifications of the HER1 gene appeared to
occur more commonly in chRCC cases, at 12–35%, with the exception
of cases 1 and 2, which were 6%. In addition, a high percentage of
gene structure alterations, which were demonstrated by the
break-apart alteration of probes, were also observed in the HER1
and HER3 genes, at 7–41% and 19–30%, respectively (Table III; Fig.
1C and D).
 | Table III.Percentage of all HER gene
alterations in 11 chromophobe renal cell carcinoma clinical samples
using fluorescence in situ hybridization analysis. |
Table III.
Percentage of all HER gene
alterations in 11 chromophobe renal cell carcinoma clinical samples
using fluorescence in situ hybridization analysis.
|
| HER1, % | HER2, % | CEN17, % | HER3, % | HER4, % |
|---|
|
|
|
|
|
|
|
|---|
| Case no. | L | A | B | L | A | B | L | A | L | A | B | L | A | B |
|---|
| 1 | 2 | 6 | 25 | 78 | 0 | 13 | 94 | 0 | 8 | 4 | 21 | 75 | 0 | 9 |
| 2 | 0 | 6 | 41 | 91 | 1 | 6 | 73 | 2 | 15 | 3 | 30 | 89 | 0 | 6 |
| 3 | 3 | 35 | 18 | 83 | 0 | 9 | 87 | 1 | 10 | 18 | 22 | 69 | 1 | 7 |
| 4 | 5 | 22 | 22 | 76 | 0 | 5 | 74 | 2 | 14 | 7 | 21 | 70 | 1 | 5 |
| 5 | 2 | 34 | 11 | 72 | 0 | 7 | 91 | 0 | 8 | 8 | 24 | 64 | 1 | 7 |
| 6 | 3 | 14 | 28 | 75 | 0 | 7 | 74 | 2 | 4 | 13 | 25 | 79 | 0 | 11 |
| 7 | 5 | 20 | 7 | 88 | 0 | 2 | 86 | 0 | 10 | 3 | 19 | 86 | 0 | 3 |
| 8 | 1 | 16 | 38 | 71 | 1 | 6 | 72 | 1 | 14 | 6 | 26 | 74 | 0 | 10 |
| 9 | 2 | 12 | 21 | 97 | 0 | 2 | 88 | 0 | 15 | 1 | 22 | 77 | 0 | 11 |
| 10 | 7 | 19 | 11 | 83 | 0 | 2 | 86 | 0 | 12 | 9 | 24 | 75 | 0 | 10 |
| 11 | 7 | 21 | 12 | 26 | 9 | 7 | 41 | 6 | 13 | 10 | 28 | 60 | 5 | 5 |
Statistical analysis
The Spearman's rank correlation coefficient
indicated that the amplification of the HER2 gene was associated
with the break-apart alteration of the HER3 gene (P=0.005). The
loss of the HER2 gene was strongly correlated with the loss of HER4
(P=0.019). In addition, the amplification of the HER1 gene was
strongly negatively associated with HER4 gene loss (P=0.013), and
positively correlated with the amplification of HER4 (P=0.004)
(Table IV). The amplification of
HER3 was not significantly associated with the loss of HER2 and
HER4 (P=0.075 and 0.067, respectively), and the amplification of
HER1 (P=0.058) (Table IV).
 | Table IV.P-values indicating the correlations
between genetic alterations as determined using Spearman's rank
correlation coefficient. |
Table IV.
P-values indicating the correlations
between genetic alterations as determined using Spearman's rank
correlation coefficient.
|
| HER2 | HER3 | HER4 |
|---|
|
|
|
|
|
|---|
| Gene | Loss | Amplification | Break-apart | Loss | Amplification | Break-apart | Loss | Amplification | Break-apart |
|---|
| HER1 |
|
|
|
|
|
|
|
|
|
|
Loss | 0.605 | 0.584 | 0.531 | 0.513 | 0.136 | 0.427 | 0.357 | 0.229 | 0.346 |
|
Amplification | 0.276 | 0.620 | 0.780 | 0.514 | 0.058 | 0.547 |
0.013a |
0.004a | 0.179 |
|
Break-apart | 0.910 | 0.260 | 0.431 | 0.376 | 0.639 | 0.180 | 0.349 | 0.312 | 0.299 |
| HER2 |
|
|
|
|
|
|
|
|
|
|
Loss | – | – | – | 0.309 | 0.075 | 0.293 |
0.019a | 0.111 | 0.872 |
|
Amplification | – | – | – | 0.130 | 0.919 |
0.005a | 0.670 | 0.617 | 0.483 |
|
Break-apart | – | – | – | 0.078 | 0.113 | 0.651 | 0.195 | 0.263 | 0.940 |
| HER3 |
|
|
|
|
|
|
|
|
|
|
Loss | – | – | – | – | – | – | 0.691 | 0.863 | 0.771 |
|
Amplification | – | – | – | – | – | – | 0.067 | 0.083 | 0.851 |
|
Break-apart | – | – | – | – | – | – | 0.851 | 0.863 | 0.563 |
Discussion
The HER family of genes consists of four members:
HER1, HER2, HER3 and HER4. The amplification of the HER1 and HER2
genes is commonly involved in a number of tumor types, including
lung, colorectal and breast cancers, and may result in tumor
progression, invasion and migration, and a poor prognosis (11–13). A
fundamental aspect of signaling transduction in HER gene family
members, with the exception of HER3, is the formation of hetero- or
homodimers, and the transphosphorylation of their intracellular
regions to trigger the initial signal that leads to downstream
signaling activities (14). The
significance of overexpression of HER2 as a predictor of breast
cancer progression and prognosis has been previously established.
Therefore, anti-HER2 antibodies and dimerization inhibitors of
other HER family receptors have been used effectively to treat
breast cancer (15,16).
The findings of the present study revealed that the
majority of the cell populations demonstrated the loss of one copy
of the HER2 and HER4 genes, and analysis of CEN17 provided
additional support for the HER2 results (Table III; Fig.
1A and B). Therefore, the findings of the present study were
consistent with those of previous studies, which indicate that the
downregulation of HER2 is commonly observed in RCC cells (Table V) (17–19). The
various expression patterns of HER2 have been previously discussed
and, in a comparison of the subtypes of RCC, the expression of HER2
was associated with the pRCC and chRCC tumor types, but not
associated with tumor grade and stage (20). These findings are similar to the
statistical results of the present study, which indicated that none
of the clinical characteristics correlated with each other in the
FISH analysis.
 | Table V.Percentage of the cell population
that demonstrated chromosome 2 or 17 losses, analyzed using
fluorescence in situ hybridization. In total, 28 cases were
assessed from two previous publications and the present study. |
Table V.
Percentage of the cell population
that demonstrated chromosome 2 or 17 losses, analyzed using
fluorescence in situ hybridization. In total, 28 cases were
assessed from two previous publications and the present study.
|
|
|
| % chromosome
lossa, mean (range) |
|
|---|
|
|
|
|
|
|
|---|
| First author | Year published | No. of cases
Ch2/CEN17 | Chromosome 2 | CEN17 | Ref. |
|---|
| Brunelli et
al | 2010 | 11/11 | 43 (4–84) | 55 (10–76) | (21) |
| Iqbal et
al | 2000 | 6/4b | 60
(15–89) | 65 (40–82) | (22) |
| Weng et
al | Present study | 11/11 | 74
(60–89) | 79 (41–94) | – |
| Total | – |
| 59 (4–89) | 67 (10–94) |
|
Despite the clinical data, the statistical analysis
and interpretation of the FISH data regarding the various types of
genetic alterations in the HER genes indicated that the
amplification of the HER2 gene was strongly correlated with the
structural rearrangement of the HER3 gene (P=0.005). By contrast,
the loss of one copy of HER2 was significantly correlated with the
loss of one allele of the HER4 gene (P=0.019). In addition, the
amplification of the HER1 gene was strongly positively correlated
with the amplification of HER4 (P=0.004), and negatively associated
with the loss of a HER4 allele (P=0.013) (Table IV). According to previous studies,
the loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 are considered
to be cytogenetic features of chRCC (6). As HER2 is known to be located on the
chromosome 17q12, and HER4 on chromosome 2q33.3–34, the loss of the
HER2 and HER4 genes in the present chRCC tissue samples may be
explained (Table III; Fig. 1A and B). However, due to the limited
sample size of the present study, cases from previous studies were
combined and reviewed with the present data for additional
analysis. Similar phenomena, including the monosomy of chromosomes
2 (mean % of cell population, 59%; range, 4–89%) and 17 (mean % of
cell population, 67%; range, 10–94%) in chRCC cases were observed
(Table V) (21,22). At
present, the strong correlation between the loss of the HER2 and
HER4 genes (P=0.019) has only been observed in chRCC, and not in
other subtypes of RCC; and, although the underlying reasons for
chRCC cell proliferation or tumoral development remain unknown,
this finding may be considered to be a valuable diagnostic or
treatment marker to distinguish chRCC from other RCC subtypes.
The rearrangement of the genetic structure of the
HER1 and HER3 genes, confirmed by detecting the break-apart of
probes, was observed in every tumor sample in the present study
(Table III; Fig. 1C and D). Notably, the limited distance
between the break-apart probe signals was observed in all cases, in
various percentages of the population of cells. This finding
strongly implies that the genes may harbor unknown sequences that
insert into one or two alleles of the HER1 and HER3 genes;
therefore, the nucleotide sequence recombination may create
chimeric fusion oncogenes, and alter the gene expression resulting
in tumor induction or progression. Previous studies reported that
the expression of HER1 and HER3 may act as a predictive factor for
the distant metastasis of rectal cancer (23). The overexpression of HER1 has been
observed in numerous studies; however, there are no definite
significant findings regarding HER1 in RCC (17,24,25).
Certain reports suggested that the polyploidy and overexpression of
HER1 may be associated with RCC progression (17,24,25). HER3
is known to be distinct from other HER genetic family members, as
it lacks crucial amino acid residues of the kinase domain for
catalytic activity; however, the overexpression of HER3 in the cell
membrane was previously associated with a poor prognosis and
decreased survival time in patients with head and neck squamous
cell carcinoma (26). In addition,
numerous studies have investigated signal transduction via
HER2/HER3 dimerization, which is often described to be the most
active signaling dimer. Therefore, analyzing the activities of the
dimers rather than isolated markers may aid the understanding of
the interactions and transduction mechanisms of the HER family.
In conclusion, one or two copies of the HER1 and
HER3 genes were indicated to be inserted with an unknown gene,
which may alter gene function, and result in various transduction
activities. In addition, a strong correlation between the loss of
the HER2 and HER4 genes may be used as a valuable marker for
distinguishing the differential treatments or diagnoses of chRCC
and other RCC subtypes.
Acknowledgements
The authors would like to thank the support of the
National Taipei University of Technology and Mackay Memorial
Hospital (grant no. NTUT-MMH-105-05), the Chang Gung Memorial
Hospital (grant no. CMRPG3B1531, CMRPG380601 and CMRPG380602) and
the National Science Council (grant nos. 100-2314-B-027-001 and
101–2314-B-182A-019), and Mr. Jhou Cheng-Han (National Taipei
University of Technology, Taiwan, R.O.C) for formatting the
paper.
References
|
1
|
Ornellas AA, Andrade DM, Ornellas P,
Wisnescky A and Schwindt AB: Prognostic factors in renal cell
carcinoma: Analysis of 227 patients treated at the Brazilian
National Cancer Institute. Int Braz J Urol. 38:185–194. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vera-Badillo FE, Conde E and Duran I:
Chromophobe renal cell carcinoma: A review of an uncommon entity.
Int J Urol. 19:894–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thoenes W, Störkel S and Rumpelt HJ: Human
chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl
Mol Pathol. 48:207–217. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peyromaure M, Misrai V, Thiounn N,
Vieillefond A, Zerbib M, Flam TA and Debré B: Chromophobe renal
cell carcinoma: Analysis of 61 cases. Cancer. 100:1406–1410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yusenko MV, Zubakov D and Kovacs G: Gene
expression profiling of chromophobe renal cell carcinomas and renal
oncocytomas by Affymetrix GeneChip using pooled and individual
tumours. Int J Biol Sci. 5:517–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Speicher MR, Schoell B, du Manoir S,
Schröck E, Ried T, Cremer T, Störkel S, Kovacs A and Kovacs G:
Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in
chromophobe renal cell carcinomas revealed by comparative genomic
hybridization. Am J Pathol. 145:356–364. 1994.PubMed/NCBI
|
|
7
|
Yamazaki K, Sakamoto M, Ohta T, Kanai Y,
Ohki M and Hirohashi S: Overexpression of KIT in chromophobe renal
cell carcinoma. Oncogene. 22:847–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petit A, Castillo M, Santos M, Mellado B,
Alcover JB and Mallofré C: KIT expression in chromophobe renal cell
carcinoma: Comparative immunohistochemical analysis of KIT
expression in different renal cell neoplasms. Am J Surg Pathol.
28:676–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan MH, Wong CF, Tan HL, Yang XJ, Ditlev
J, Matsuda D, Khoo SK, Sugimura J, Fujioka T, Furge KA, et al:
Genomic expression and single-nucleotide polymorphism profiling
discriminates chromophobe renal cell carcinoma and oncocytoma. BMC
Cancer. 10:1962010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weng WH, Ahlén J, Lui WO, Brosjö O, Pang
ST, Von Rosen A, Auer G, Larsson O and Larsson C: Gain of 17q in
malignant fibrous histiocytoma is associated with a longer
disease-free survival and a low risk of developing distant
metastasis. Br J Cancer. 79:720–726. 2003. View Article : Google Scholar
|
|
11
|
Ross JS, Fletcher JA, Bloom KJ, Linette
GP, Stec J, Symmans WF, Pusztai L and Hortobagyi GN: Targeted
therapy in breast cancer: The HER-2/neu gene and protein. Mol Cell
Proteomics. 3:379–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shia J, Klimstra DS, Li AR, Qin J, Saltz
L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, et al:
Epidermal growth factor receptor expression and gene amplification
in colorectal carcinoma: An immunohistochemical and chromogenic
in situ hybridization study. Mod Pathol. 18:1350–1356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grob TJ, Kannengiesser I, Tsourlakis MC,
Atanackovic D, Koenig AM, Vashist YK, Klose H, Marx AH, Koops S,
Simon R, et al: Heterogeneity of ERBB2 amplification in
adenocarcinoma, squamous cell carcinoma and large cell
undifferentiated carcinoma of the lung. Mod Pathol. 25:1566–1573.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sergina NV and Moasser MM: The HER family
and cancer: Emerging molecular mechanisms and therapeutic targets.
Trends Mol Med. 13:527–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross JS and Fletcher JA: The HER-2/neu
oncogene in breast cancer: Prognostic factor, predictive factor,
and target for therapy. Stem Cells. 16:413–428. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nahta R and Esteva FJ: HER2 therapy:
Molecular mechanisms of trastuzumab resistance. Breast Cancer Res.
8:2152006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weidner U, Peter S, Strohmeyer T,
Hussnätter R, Ackermann R and Sies H: Inverse relationship of
epidermal growth factor receptor and HER2/neu gene expression in
human renal cell carcinoma. Cancer Res. 50:4504–4509.
1990.PubMed/NCBI
|
|
18
|
Latif Z, Watters AD, Bartlett JM,
Underwood MA and Aitchison M: Gene amplification and overexpression
of HER2 in renal cell carcinoma. BJU Int. 89:5–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomasson M, Hedman H, Junttila TT,
Elenius K, Ljungberg B and Henriksson R: ErbB4 is downregulated in
renal cell carcinoma - a quantitative RT-PCR and
immunohistochemical analysis of the epidermal growth factor
receptor family. Acta Oncol. 43:453–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seliger B, Rongcun Y, Atkins D, Hammers S,
Huber C, Störkel S and Kiessling R: HER-2/neu is expressed in human
renal cell carcinoma at heterogeneous levels independently of tumor
grading and staging and can be recognized by HLA-A2.1-restricted
cytotoxic T lymphocytes. Int J Cancer. 87:349–359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunelli M, Delahunt B, Gobbo S, Tardanico
R, Eccher A, Bersani S, Cossu-Rocca P, Parolini C, Balzarini P,
Menestrina F, et al: Diagnostic usefulness of fluorescent
cytogenetics in differentiating chromophobe renal cell carcinoma
from renal oncocytoma: A validation study combining metaphase and
interphase analyses. Am J Clin Pathol. 133:116–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iqbal MA, Akhtar M, Ulmer C, Al-Dayel F
and Paterson MC: FISH analysis in chromophobe renal-cell carcinoma.
Diagn Cytopathol. 22:3–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho-Pun-Cheung A, Assenat E,
Bascoul-Mollevi C, Bibeau F, Boissière-Michot F, Cellier D, Azria
D, Rouanet P, Senesse P, Ychou M and Lopez-Crapez E: EGFR and HER3
mRNA expression levels predict distant metastases in locally
advanced rectal cancer. Int J Cancer. 128:2938–2946. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao M, Shuin T, Misaki H and Kubota Y:
Enhanced expression of c-myc and epidermal growth factor receptor
(C-erbB-1) genes in primary human renal cancer. Cancer Res.
48:6753–6757. 1988.PubMed/NCBI
|
|
25
|
Moch H, Sauter G, Gasser TC, Bubendorf L,
Richter J, Presti JC Jr, Waldman FM and Mihatsch MJ: EGF-r gene
copy number changes in renal cell carcinoma detected by
fluorescence in situ hybridization. J Pathol. 184:424–429.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takikita M, Xie R, Chung JY, Cho H, Ylaya
K, Hong SM, Moskaluk CA and Hewitt SM: Membranous expression of
Her3 is associated with a decreased survival in head and neck
squamous cell carcinoma. J Transl Med. 9:1262011. View Article : Google Scholar : PubMed/NCBI
|