Introduction
Colorectal cancer (CRC) is the third most common
cancer and the fourth leading cause of cancer mortality according
to GLOBOCAN 2012 (1). High tumor
stage and histological grade, in addition to the number of resected
lymph nodes and intravascular invasion have all been identified as
prognostic factors (2). Thus,
investigation of the mechanisms underlying CRC etiology is of
clinical importance.
The association between inflammation and cancer was
identified in the 19th century by Rudolf Virchow (3). Since then, studies have confirmed the
broad and significant effect of inflammation on tumor development,
progression and response to therapy (4–6). The
presence of inflammatory cells, growth factors, activated stroma
and DNA-damage-promoting agents in the inflammatory environment
function to sustain cell proliferation and increase neoplastic risk
(5). Meanwhile, the tumor increases
inflammatory process and promotes tumor proliferation and
metastases development by decreasing apoptosis and increasing
angiogenesis and DNA damage (4,5). Notably,
the scope and effects of an inflammatory reaction have always been
assessed according to a number of biochemical markers, including
the neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio
(PLR), which are inexpensive markers of systemic inflammation
(7). Previous studies have indicated
that elevated NLR or PLR suggested a poor prognosis for various
types of cancer, including ovarian (8), breast (9,10),
non-small cell lung (11), esophageal
(12,13), gastric (7,14),
hepatocellular (15) and CRC
(2,16–18).
However, the current literature leaves certain questions
unanswered. Firstly, it is unknown as to which parameter is
superior for predicting the outcome of CRC. Secondly, to the best
of our knowledge, only 2 studies have compared the prognostic value
of NLR and PLR in patients with CRC (17,18). One
study demonstrated that elevated NLR and PLR were identified as
significant poor prognostic factors in metastatic CRC, and that NLR
led to improved prognostic predictors (17). On the contrary, the other study
indicated that PLR was a superior prognostic marker (18). However, the grouping of NLR and PLR in
these studies was based on a cut-off value that was calculated in
other studies, which did not accurately reflect the data.
Therefore, the aim of the present study was to explore the clinical
significance of NLR and PLR as independent prognostic factors in
patients with CRC and to identify the factor that is more effective
in this role.
Patients and methods
Patients
A retrospective analysis was performed on data from
216 patients with CRC that underwent radical surgery at Chinese
People's Liberation Army (PLA) General Hospital (Beijing, China)
between July 2006 and June 2012. Patients were selected for the
present study according to the following inclusion criteria: CRC
confirmed by histopathology; radical resection with microscopically
tumor-free resection margins; and complete blood count,
clinicopathological and follow-up data. The exclusion criteria
excluded patients for the following reasons: Underwent palliative
surgery; complications of intestinal obstruction, hemorrhage or
enterobrosis resulting in emergency surgery; clinical evidence of
infection, systemic inflammation or autoimmune disorder; underwent
or accepted neoadjuvant chemotherapy or radiotherapy; succumbed to
CRC or other causes within 30 days of surgery; and a history of
other malignancies. All enrolled CRC patients were staged according
to the American Joint Committee on Cancer tumor-node-metastasis
(TNM) classification system (19) and
were treated according to the National Comprehensive Cancer Network
clinical practice guidelines in oncology for colon (20) and rectal cancer (21). All the patients provided written
informed consent prior to the present study, which was approved by
the Ethics Committee of Chinese PLA General Hospital.
Follow-up of patients
Letters and telephone interviews were used to
follow-up each patient. The last follow-up date was July 1, 2013.
The overall survival (OS) time was defined as the time between
surgery and mortality from any cause or to the last date of
follow-up.
NLR and PLR grouping
All blood samples were taken 1 week prior to
surgery. NLR was defined as the absolute neutrophil count divided
by the absolute lymphocyte count. The patients were divided into
two groups based on the NLR value, the NLR <4.98 and NLR ≥4.98
groups. PLR was defined as the absolute platelet count divided by
the absolute lymphocyte count. The patients were divided into two
groups based on the PLR value, the PLR <246.36 and PLR ≥246.36
groups.
Statistical analysis
Statistical analysis was performed with IBM SPSS
software version 20 (IBM SPSS, Armonk, NY, USA). Receiver operating
characteristic (ROC) curves were plotted to calculate the area
under the ROC curve (AUC). The Youden index (YI) was calculated to
determine the optimal cutoff value for NLR and PLR. Linear
regression was performed to evaluate the association between NLR
and PLR. Continuous variables are presented as the mean ± standard
error and categorical variables are presented as frequencies and
percentages. The differences between clinicopathological
characteristics grouped by NLR or PLR were compared using the
Pearson χ2 test or Fisher's exact test for categorical
variables and Student's t-test for continuous variables.
Survival time curves and 5-year OS rates were calculated using the
Kaplan-Meier method of univariate analysis, and the differences
were compared using the log-rank test. Independent prognostic
factors were evaluated using the Cox proportional hazard model of
multivariate analysis. Variables with a univariate analysis value
of P<0.05 were entered into multivariate analysis. Each test was
two-tailed. P<0.05 was considered to indicate a statistically
significant difference.
Results
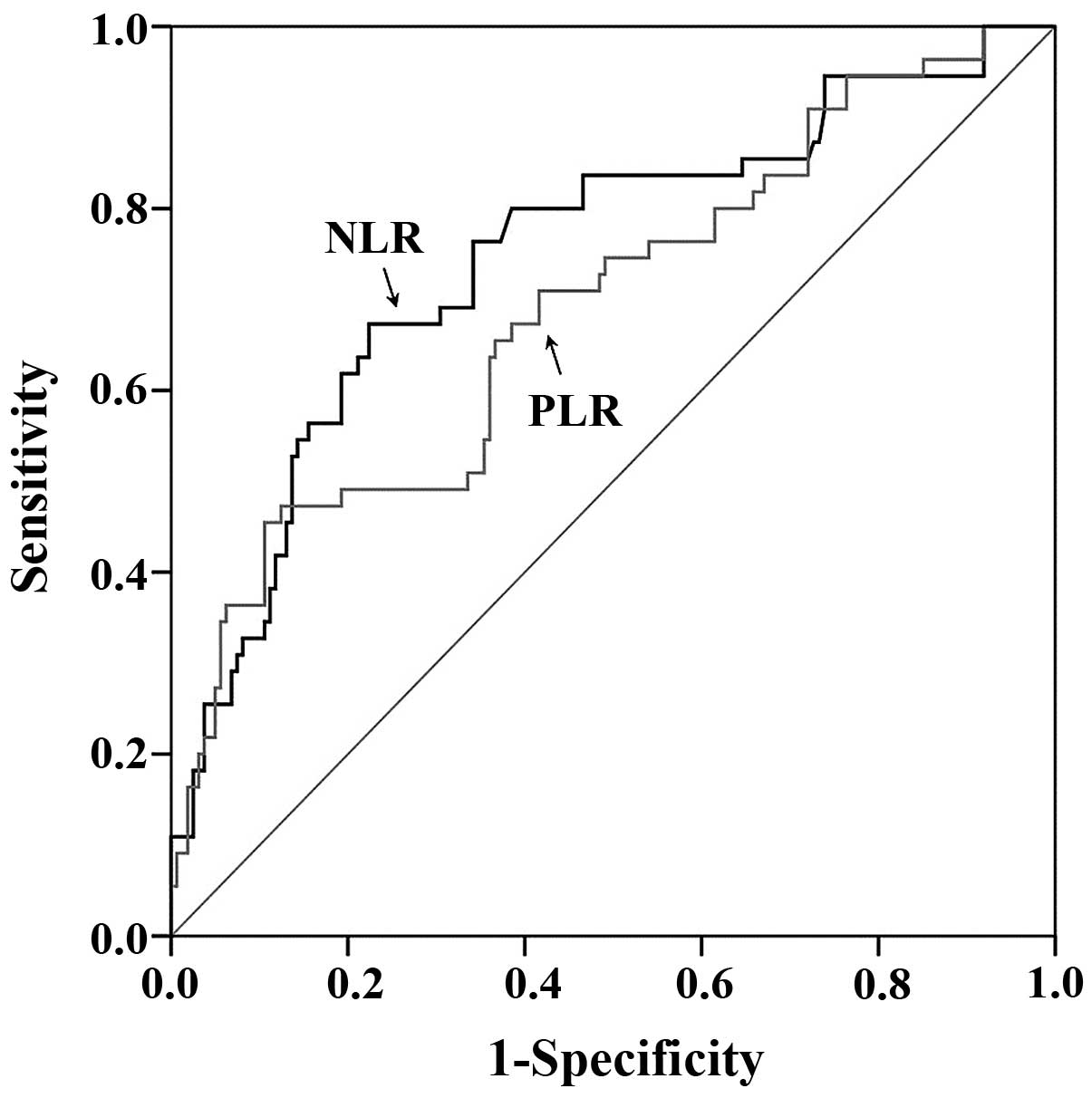
The ROC curve for optimal cutoff value
and AUC
When the NLR was 4.98, YI was at its maximum
(YI=0.4491), demonstrating that 4.98 was the optimal cutoff value
for NLR. Therefore, patients were divided into low NLR (<4.98)
and high NLR (≥4.98) groups. When the PLR was 246.36, YI was at its
maximum (YI=0.3490). Therefore, patients were divided into low PLR
(<246.36) and high PLR (≥246.36) groups.
The AUC for NLR was 0.748 (95% CI, 0.684–0.804;
P<0.0001) and PLR was 0.690 (95% CI, 0.623–0.751; P<0.0001;
Fig. 1).
Association of NLR and PLR with
clinicopathological characteristics
In total, 216 patients were enrolled in the present
study. The median follow-up time was 38 months, with a range of
3–85 months. At the final follow-up date, 161 patients (74.5%) were
alive. A comparison between the clinicopathological characteristics
and the NLR and PLR are exhibited in Table I. High NLR and PLR were associated
with poor tumor differentiation and a larger tumor diameter,
respectively (P<0.05). A high PLR was also associated with a
poor primary tumor classification (T classification) (P=0.006). In
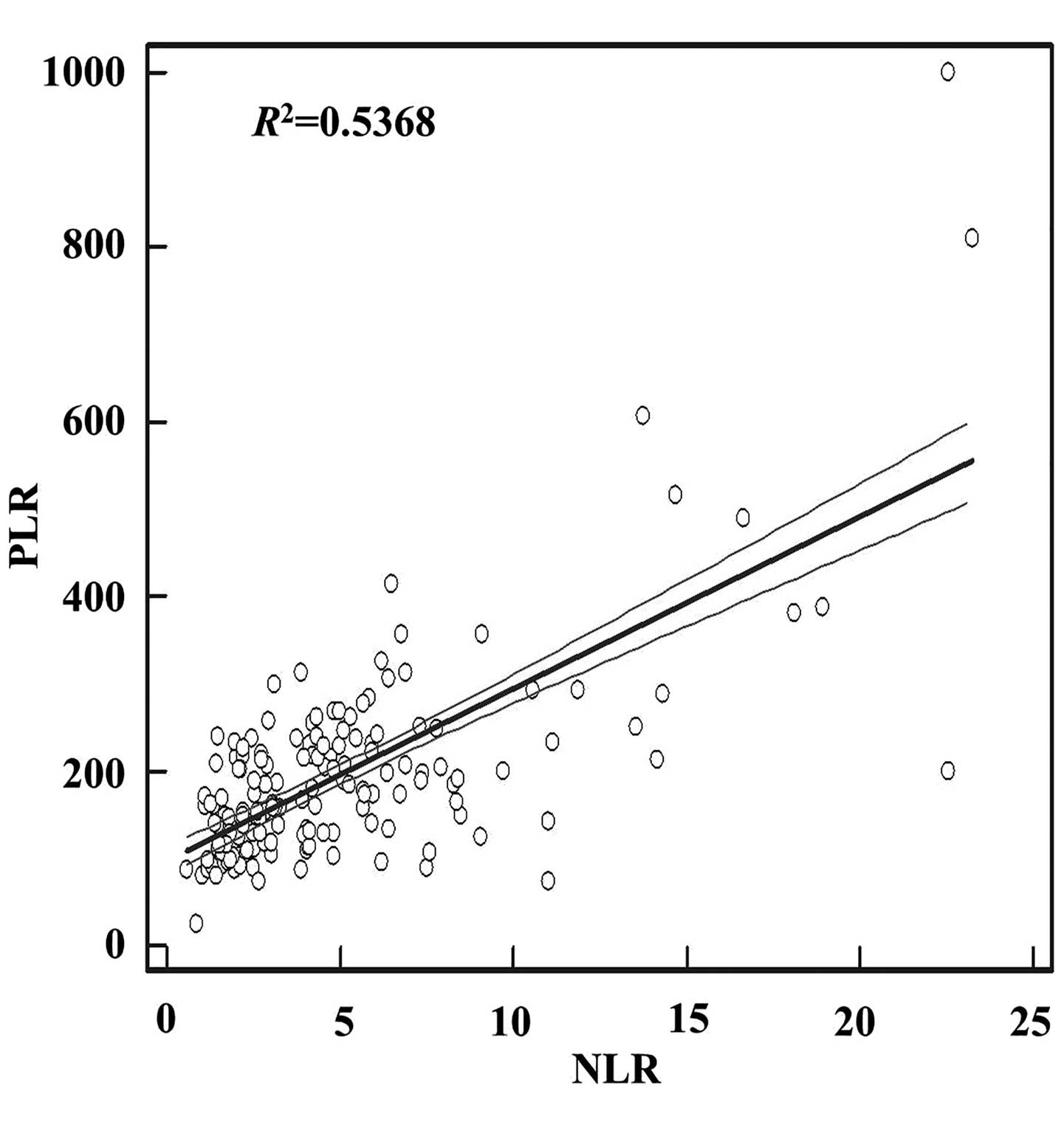
addition, there was a positive association between NLR and PLR. The
regression equation was as follows: y=97.3626+19.7032×
(R2=0.5368; P<0.0001; Fig.
2).
 | Table I.Clinicopathological characteristics
of 216 patients with colorectal cancer, grouped by NLR and PLR. |
Table I.
Clinicopathological characteristics
of 216 patients with colorectal cancer, grouped by NLR and PLR.
|
| NLR | PLR |
|---|
|
|
|
|
|---|
| Characteristic | NLR <4.98, n
(%) | NLR ≥4.98, n
(%) | P-value | PLR <246.36, n
(%) | PLR ≥246.36, n
(%) | P-value |
|---|
| Total | 141 (100.0) | 75
(100.0) |
| 172
(100.0) | 44
(100.0) |
|
| Gender |
|
|
0.659 |
|
|
0.114 |
|
Male | 91
(64.5) | 46 (61.3) |
| 114 (66.3) | 23 (52.3) |
|
|
Female | 50
(35.5) | 29 (38.7) |
| 58
(33.7) | 21 (47.7) |
|
| Age, years |
53.90±12.1 | 54.49±10.8 |
0.239 | 53.81±11.3 |
55.27±13.0 |
0.145 |
| PLR | 154.94±57.1 | 278.89±162.4 | <0.001 |
|
|
|
| NLR |
|
|
| 3.81±2.8 | 10.19±6.0 | <0.001 |
| Tumor location |
|
| 0.887 |
|
|
0.398 |
|
Colon | 73
(51.8) | 40 (53.3) |
| 87
(50.6) | 26 (59.1) |
|
|
Rectum | 68
(48.2) | 35 (46.7) |
| 85
(49.4) | 18 (40.9) |
|
|
Differentiation |
|
|
0.019 |
|
|
0.019 |
|
Well | 26
(18.4) | 5 (6.7) |
| 28
(16.3) | 3 (6.8) |
|
|
Moderate | 83
(58.9) | 43 (57.3) |
| 104 (60.5) | 22 (50.0) |
|
|
Poor | 32
(22.7) | 27 (36.0) |
| 40
(23.3) | 19 (43.2) |
|
| Tumor diameter,
cm |
4.54±1.9 | 5.73±3.1 |
0.007 | 4.58±2.0 |
6.40±3.4 |
0.001 |
| T
classification |
|
|
0.187 |
|
|
0.006 |
|
T1+T2 | 23
(16.3) | 7 (9.3) |
| 28
(16.3) | 2 (4.5) |
|
| T3 | 81
(57.4) | 41 (54.7) |
| 101 (58.7) | 21 (47.7) |
|
| T4 | 37
(26.2) | 27 (36.0) |
| 43
(25.0) | 21 (47.7) |
|
| LN metastasis |
|
|
0.070 |
|
|
0.058 |
| N0 | 77
(54.6) | 36 (48.0) |
| 93
(54.1) | 20 (45.5) |
|
| N1 | 50
(35.5) | 23 (30.7) |
| 60
(34.9) | 13 (29.5) |
|
| N2 | 14 (9.9) | 16 (21.3) |
| 19
(11.0) | 11 (25.0) |
|
| Distant
metastasis |
|
|
0.053 |
|
|
0.122 |
| M0 | 128 (90.8) | 61 (81.3) |
| 154 (89.5) | 35 (79.5) |
|
| M1 | 13 (9.2) | 14 (18.7) |
| 18
(10.5) | 9
(20.5) |
|
| TNM staging |
|
|
0.062 |
|
|
0.082 |
| I | 16
(11.3) | 4 (5.3) |
| 19
(11.0) | 1 (2.3) |
|
| II | 61
(43.3) | 28 (37.3) |
| 71
(41.3) | 18 (40.9) |
|
|
III | 53
(37.6) | 29 (38.7) |
| 66
(38.4) | 16 (36.4) |
|
| IV | 11 (7.8) | 14 (18.7) |
| 16 (9.3) | 9
(20.5) |
|
Association of NLR and PLR with the
5-year OS rate
The 1, 3 and 5-year OS rates were 95.8, 76.0 and
70.2%, respectively. Univariate analyses demonstrated that the NLR,
PLR, tumor location (P=0.003), tumor differentiation (P=0.014), T
classification (P<0.001), lymph node (LN) metastasis
(P<0.001), distant metastasis (P<0.001), TNM staging
(P<0.001) and administration of post-operative adjuvant
chemotherapy (P=0.047) were associated with the 5-year OS rate
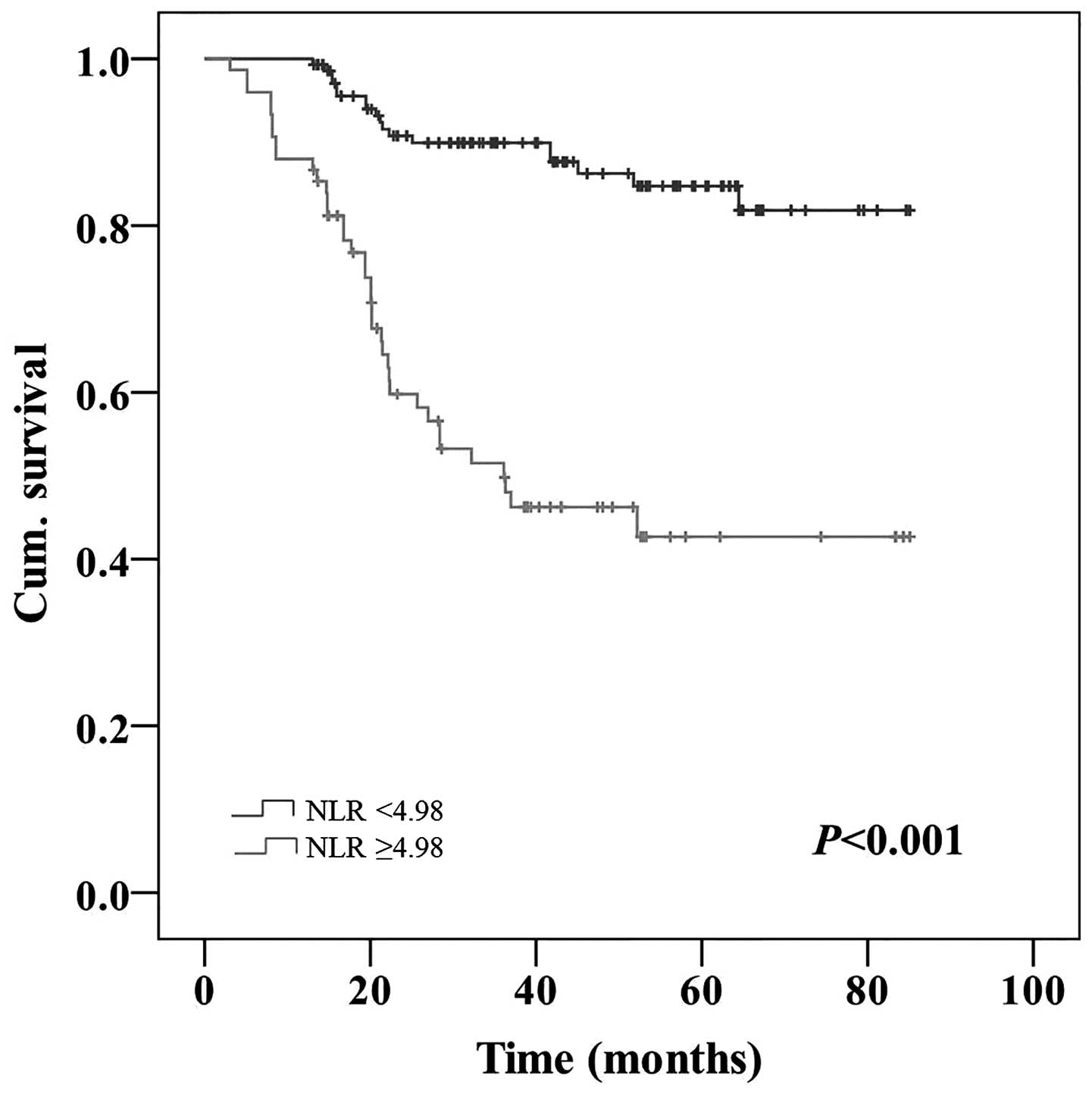
(Table II). Patients with a high NLR
possessed a significantly poorer 5-year OS rate compared with
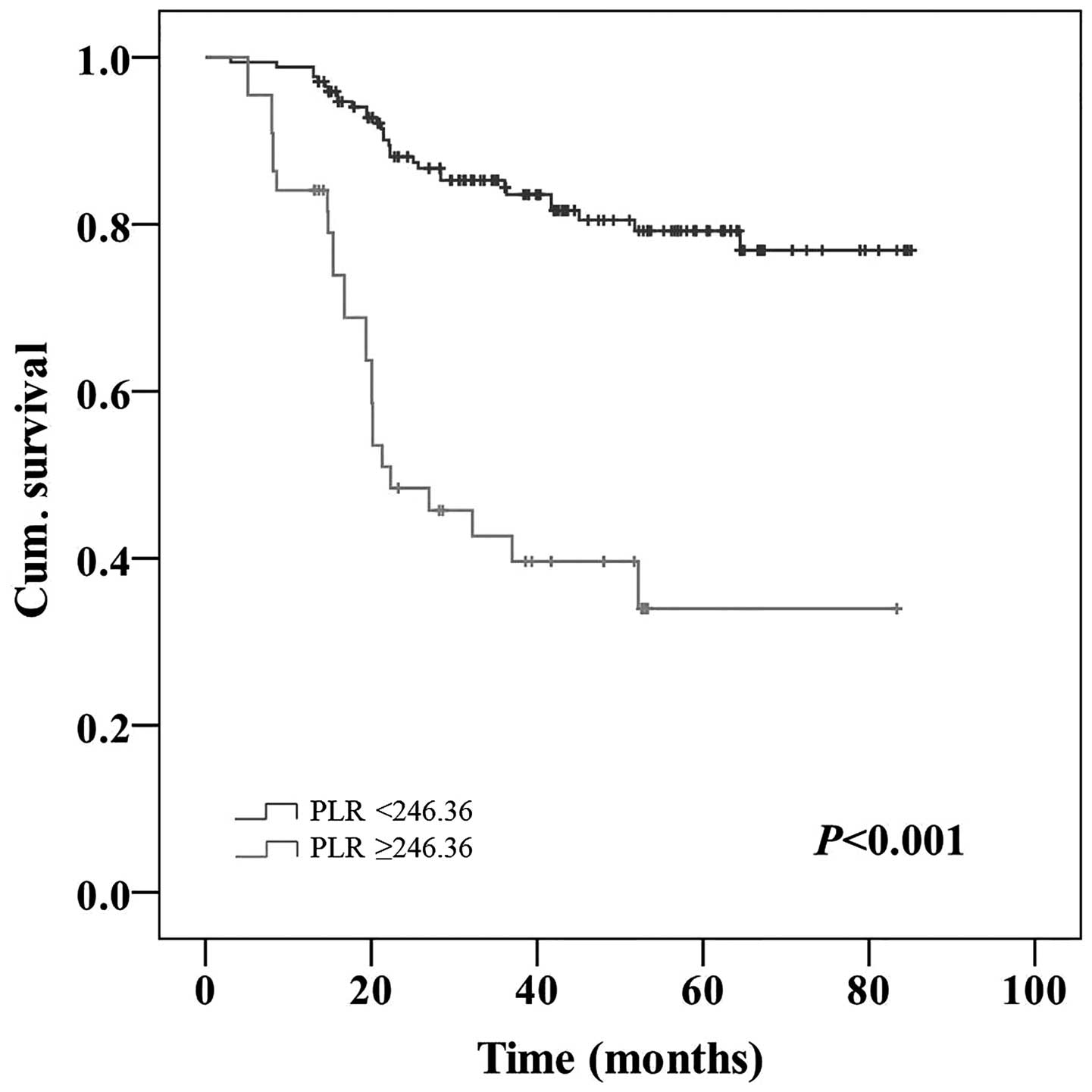
patients with a low NLR (42.7 vs. 84.7%; P<0.001; Fig. 3). Patients with a high PLR possessed a
significantly poorer 5-year OS rate compared with patients with a
low PLR (34.0 vs. 79.2%; P<0.001; Fig.
4).
 | Table II.Univariate analyses in 216 patients
with colorectal cancer. |
Table II.
Univariate analyses in 216 patients
with colorectal cancer.
| Characteristic | n | 5-year OS rate,
% | P-value |
|---|
| Gender |
|
|
0.724 |
|
Male | 137 | 72.4 |
|
|
Female | 79 | 65.4 |
|
| Age, years |
|
|
0.662 |
|
<50 | 75 | 71.1 |
|
|
≥50 | 141 | 69.0 |
|
| NLR |
|
| <0.001 |
|
<4.98 | 141 | 84.7 |
|
|
≥4.98 | 75 | 42.7 |
| PLR |
|
| <0.001 |
|
<246.36 | 172 | 79.2 |
|
|
≥246.36 | 44 | 34.0 |
| Tumor location |
|
|
0.003 |
|
Colon | 113 | 62.9 |
|
|
Rectum | 103 | 77.4 |
|
|
Differentiation |
|
|
0.014 |
|
Well | 31 | 71.0 |
|
|
Moderate | 126 | 76.8 |
|
|
Poor | 59 | 55.0 |
|
| Tumor diameter,
cm |
|
|
0.967 |
|
<5 | 104 | 68.9 |
|
| ≥5 | 112 | 71.0 |
|
| T
classification |
|
| <0.001 |
|
T1+T2 | 30 | 87.7 |
|
| T3 | 122 | 82.6 |
|
| T4 | 64 | 36.1 |
|
| LN metastasis |
|
| <0.001 |
| N0 | 113 | 86.3 |
|
| N1 | 73 | 63.5 |
|
| N2 | 30 | 22.2 |
|
| Distant
metastasis |
|
| <0.001 |
| M0 | 189 | 76.2 |
|
| M1 | 27 |
9.6 |
|
| TNM staging |
|
| <0.001 |
| I | 20 | 88.9 |
|
| II | 89 | 88.2 |
|
|
III | 82 | 57.7 |
|
| IV | 25 |
0.0 |
|
| Chemotherapy |
|
|
0.047 |
|
Yes | 149 | 73.6 |
|
| No | 67 | 62.7 |
|
| Score |
|
| <0.001 |
| 0 | 133 | 85.5 |
|
| 1 | 47 | 60.0 |
|
| 2 | 36 | 27.2 |
|
Patients with high values for NLR and PLR were
allocated a score of 2, patients with a high NLR or PLR value were
allocated a score of 1, and patients that did not possess high NLR
or PLR values were allocated a score of 0. Univariate analysis
revealed that patients with a score of 2 possessed a significantly
poorer 5-year OS rate (27.2%) compared with patients with a score
of 1 (60.0%) or 0 (85.5%; P<0.001). Patients with a score of 1
had a significantly poorer 5-year OS rate compared with patients
with a score of 0 (P<0.001; Table
II).
Multivariate analyses of independent
prognostic factors
Multivariate analyses using the Cox proportional
hazards model identified NLR, PLR, T classification (P<0.001;
95% CI, 2.008–6.156), LN metastasis (P=0.030; 95% CI, 1.059–3.161)
and post-operative adjuvant chemotherapy (P=0.021; 95% CI,
1.110–3.625) as independent prognostic factors (Table III). The risk of succumbing to CRC
for patients with a high NLR was >4 times higher than patients
with a low NLR (relative risk (RR)=4.074; P<0.001; 95% CI,
1.975–8.405). The risk of succumbing to CRC for patients with a
high PLR was >2 times higher than patients with a low PLR
(RR=2.029; P=0.029; 95% CI, 1.077–3.821).
 | Table III.Multivariate analyses in 216 patients
with colorectal cancer. |
Table III.
Multivariate analyses in 216 patients
with colorectal cancer.
| Feature | P-value | RR (95% CI) |
|---|
| NLR | <0.001 | 4.074
(1.975–8.405) |
| PLR |
0.029 | 2.029
(1.077–3.821) |
| Tumor location |
0.362 | 0.741
(0.389–1.411) |
|
Differentiation |
0.755 | 0.918
(0.538–1.567) |
| T
classification | <0.001 | 3.516
(2.008–6.156) |
| LN metastasis |
0.030 | 1.830
(1.059–3.161) |
| Distant
metastasis |
0.389 | 1.840
(0.459–7.375) |
| TNM staging |
0.576 | 1.322
(0.497–3.516) |
| Chemotherapy |
0.021 | 2.006
(1.110–3.625) |
Association between the NLR and PLR
and 5-year OS rate stratified by TNM staging
Patients in the NLR and PLR groups were stratified
according to TNM staging. The results indicated that patients with
TNM stage II or III disease and a high NLR or PLR possessed a
significantly poorer 5-year OS rate compared with patients with a
low NLR (stage II, P=0.002; stage III, P<0.001) or PLR (stage
II, P<0.001; stage III, P<0.001; Table IV).
 | Table IV.The 5-year OS rate for NLR and PLR
stratified by TNM staging. |
Table IV.
The 5-year OS rate for NLR and PLR
stratified by TNM staging.
|
| TNM stage I | TNM stage II | TNM stage III | TNM stage IV |
|---|
|
|
|
|
|
|
|---|
| Ratio | n | 5-year OS rate,
% | n | 5-year OS rate,
% | n | 5-year OS rate,
% | n | 5-year OS rate,
% |
|---|
| NLR |
|
|
|
|
|
|
|
|
|
<4.98 | 16 |
86.7 | 61 | 95.0 | 53 | 82.0 | 11 |
0.0 |
|
≥4.98 | 4 | 100.0 | 28 | 74.0 | 29 | 15.2 | 14 |
0.0 |
|
P-value |
| 0.514 |
|
0.002 |
| <0.001 |
| 0.338 |
| PLR |
|
|
|
|
|
|
|
|
|
<246.36 | 19 |
88.9 | 71 | 94.1 | 66 | 74.2 | 16 |
0.0 |
|
≥246.36 | 1 | NA | 18 | 65.5 | 16 |
7.5 | 9 | 16.7 |
|
P-value |
| NA |
| <0.001 |
| <0.001 |
| 0.256 |
Association between patients grouped
by post-operative adjuvant chemotherapy and the 5-year OS rate
stratified by NLR or PLR
The patients were divided into two groups according
to post-operative adjuvant chemotherapy. Univariate analyses
stratified by the NLR or PLR revealed that adjuvant chemotherapy
did not affect the 5-year OS rate in patients with a low NLR
(P=0.255) or PLR (P=0.259). However, adjuvant chemotherapy
increased the 5-year OS rate from 29.2 to 49.1% in patients with a
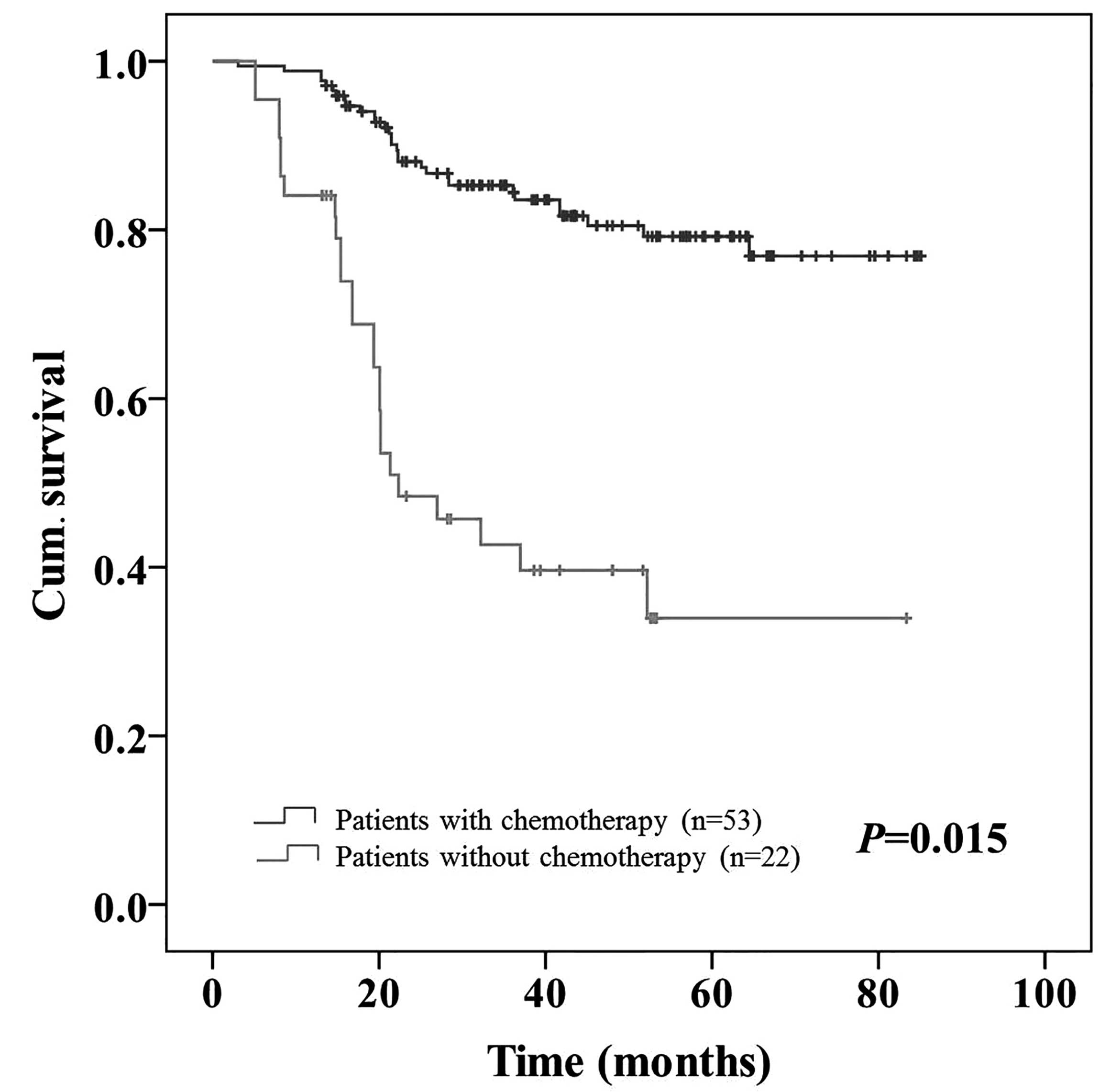
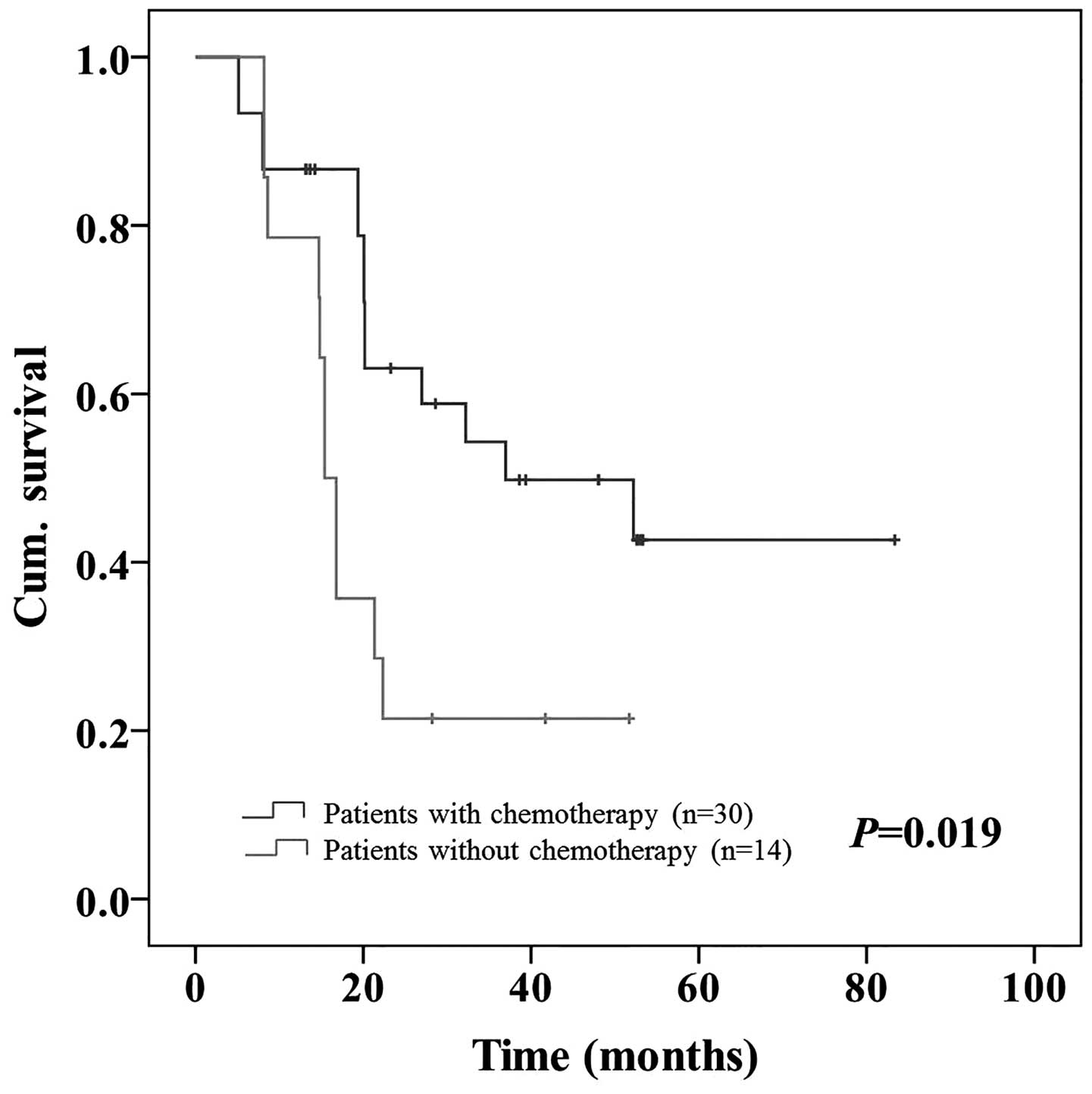
high NLR (P=0.015; Fig. 5) and from
21.4 to 42.7% in patients with a high PLR (P=0.019; Fig. 6; Table
V).
 | Table V.The 5-year OS rate for adjuvant
chemotherapy stratified by NLR or PLR. |
Table V.
The 5-year OS rate for adjuvant
chemotherapy stratified by NLR or PLR.
|
| NLR <4.98 | NLR ≥4.98 | PLR <246.36 | PLR ≥246.36 |
|---|
|
|
|
|
|
|
|---|
| Chemotherapy | n | 5-year OS rate,
% | n | 5-year OS rate,
% | n | 5-year OS rate,
% | n | 5-year OS rate,
% |
|---|
| Yes | 96 | 87.2 | 53 | 49.1 | 119 | 81.5 | 30 | 42.7 |
| No | 45 | 79.7 | 22 | 29.2 | 53 | 74.1 | 14 | 21.4 |
| P-value |
| 0.255 |
| 0.015 |
| 0.259 |
| 0.019 |
Discussion
CRC is the third most common cancer and the fourth
leading cause of cancer-associated mortality, according to GLOBOCAN
2012 (1). In China, an increase in
CRC risk factors, including an aging population and changes in
eating habits (increased meat intake and reduced fiber intake), has
rendered CRC the fifth most common cancer in the country, following
lung, stomach, liver and esophageal cancers (22).
In 1863, Rudolf Virchow hypothesized that the origin
of cancer was at sites of chronic inflammation, and this causal
association between inflammation, innate immunity and cancer is
more widely accepted at present (3,5).
Inflammation is significant in tumor development, progression and
response to therapy (4). The presence
of inflammatory cells, growth factors, activated stroma and
DNA-damage-promoting agents in the inflammatory environment act to
sustain cell proliferation and increase neoplastic risk (5). In turn, the tumor increases the
inflammatory process and promotes tumor proliferation and
metastases development by decreasing apoptosis and increasing
angiogenesis and DNA damage (4,5). The
strongest association of chronic inflammation with malignancy has
been reported in CRC arising from inflammatory bowel diseases
(5,23). Therefore, the prognosis of CRC is not
only associated with the biological behavior of the tumor, but also
with the inflammatory reaction of the host.
Inflammatory reactions have always been assessed by
a number of biochemical markers, in particular traditional
hematological markers, including C-reactive protein (CRP) and
differential leukocyte and platelet counts. CRP is an index of
systemic inflammation and has been identified as a prognostic
factor in patients with CRC and gastric cancer (24,25).
However, serum CRP levels are not routinely assessed in the
pre-operative assessment (12,14,18).
Instead, NLR and PLR, calculated from leukocyte differential counts
and platelet counts, respectively, are more readily available and
inexpensive compared to CRP (7).
Notably, numerous previous studies have already indicated that an
elevated NLR and PLR is associated with a poorer survival in
patients with ovarian (8), breast
(9,10), non-small cell lung (11), esophageal (12,13),
gastric (7,14) and hepatocellular cancers (15) and CRC (2,16–18).
NLR is the ratio of the absolute neutrophil count to
the absolute lymphocyte count, and therefore the association
between a high NLR and a poor prognosis, as revealed in the present
study, is possibly indicative of the tumor-promoting activity
associated with neutrophilia in the tumor environment. Tumors are
known to produce myeloid growth factors, including granulocyte
colony-stimulating factor, tumor necrosis factor-α, interleukin
(IL)-1and IL-6, which may increase the number of neutrophilic
granulocytes at the site of the tumor (13,18).
Neutrophilia promotes tumor growth and metastasis by remodeling the
extracellular matrix and releasing reactive oxygen species, nitric
oxide and arginase, which suppress the T cell response and
increases the rate of mutagenesis (10). Additionally, neutrophilia suppresses
lymphocyte activity, therefore counteracting the antitumor immune
response (7). An elevated NLR has
been associated with a poor survival rate in breast (10), esophageal (12,13)and
gastric cancers (7,14) and CRC (26). Chiang et al demonstrated that
patients with an elevated NLR (>3) in colon cancer appeared to
possess larger tumors and a more advanced tumor stage, and patients
with stages I–III CRC possessed a poorer 5-year disease-free
survival rate (26).
PLR is the ratio of the absolute platelet count to
absolute lymphocyte count, and therefore, the association of a high
PLR with a poor prognosis, as revealed in the present study, is
possibly indicative of the tumor-promoting activity associated with
platelets. Platelets are known to be important in hemostasis and
thrombosis (27). In addition,
platelets mediate tumor cell growth, dissemination and angiogenesis
(28). In turn, tumor cells induce
platelet aggregation, which is known to be the trigger for the
development of cancer-associated thrombosis (28). Platelets recruited to the tumor
microenvironment consequently release platelet-derived growth
factor and transforming growth factor to promote tumor growth
(7,29). However, platelets also regulate
angiogenesis by releasing numerous proangiogenic proteins,
including vascular endothelial growth factor, and aid the
maintenance of vascular integrity, therefore facilitating tumor
cell survival and growth (10,29). In
addition, platelets shield tumor cells from host immune
surveillance and direct cellular contact with natural killer cells
by inducing platelet mimicry and constructing a mesh with fibrin
that surrounds tumor cells within the vasculature during
hematogenous dissemination (29).
Tumor cells possess the ability to manipulate platelet activity to
optimize tumor growth, proliferation, survival and metastasis
(29). Several studies have
identified the association between a poor survival rate and
elevated PLR in solid tumors (2,8,9,11,16,30).
Szkandera et al revealed that an elevated PLR was
significantly associated with a decreased time to recurrence and
demonstrated a trend towards a decreased OS time in patients with
stage II and III colon cancer that underwent curative resection
(2). Liu et al reported that
patients with CRC and a higher PLR possessed a significantly lower
5-year OS rate compared with patients with a low PLR, and
identified pre-operative PLR as a clinically significant factor for
the assessment of the prognosis of resectable CRC (16).
An elevated NLR or PLR is always accompanied by
lymphopenia, which is caused by systemic inflammation and leads to
the release of a number of inhibitory immunological mediators,
particularly IL-10 and transforming growth factor-β. These
inhibitory immunological mediators may exert an immunosuppressive
effect with an impaired lymphocyte function (31).
The present study demonstrated that CRC patients
with a high NLR or PLR tended to possess more clinicopathological
factors associated with advanced disease, including poor tumor
differentiation, the presence of a large tumor and a higher T
classification. Regarding post-operative outcomes, in the present
study, CRC patients with a high NLR or PLR possessed a
significantly poorer 5-year OS rate compared with patients with a
low NLR or PLR, particularly in patients with TNM stage II and III
disease, indicating that NLR and PLR were effective independent
prognostic factors. In addition, the present study aimed to
identify which prognostic factor was the more effective. In this
respect, 2 previous studies presented notable data. Kwon et
al analyzed 200 patients that underwent curative resection and
revealed that NLR and PLR were good prognostic biomarkers of OS
rate in a univariate analysis, but only PLR was an independent
prognostic factor in multivariate Cox proportional hazards analysis
(18). He et al demonstrated
that an elevated NLR, PLR and carcinoembryonic antigen (CEA) level
were significant predictors of a poorer OS rate and
progression-free survival time following first-line chemotherapy in
patients with metastatic CRC, but only NLR and CEA were validated
as independent factors (17). These
two studies selected cutoff values for NLR and PLR from previous
studies (17,18). By contrast, the present study set the
optimal cutoff value according to the maximal YI calculated from
the ROC curve and grouped the patients accordingly, which is more
suitable to the clinical data and more accurate for the specific
study group. Additionally, the present study compared the
predictive value of each factor using two methods.
Firstly, the present study compared NLR and PLR
using their respective AUC values. According to the present
results, the AUC for NLR was 0.748, which was greater compared with
PLR (AUC=0.690). Secondly, the present study compared the risk of
patients with a high NLR and PLR succumbing to CRC. The present
results demonstrated that the risk of patients succumbing to CRC
was increased in patients with a high NLR compared with patients
with a high PLR (RR, 4.074 vs. 2.029). The AUC and RR data indicate
that NLR is superior to PLR as a predictive factor for patients
with CRC. Notably, Ishizuka et al investigated the
prediction of cancer-specific survival time in patients with CRC
using a parameter based on a combination of platelet count (COP)
and NLR, and concluded that COP-NLR was a useful predictor of
survival (32). Consequently, the
present study also considered the combined effect of NLR and PLR on
the prognostic significance. Accordingly, the present study
allocated a score of 2, 1 or 0, according to the NLR and PLR, and
performed a univariate analysis. The present results revealed that
the combination of NLR and PLR was a valid prognostic factor.
The treatment history of the patients was another
clinicopathological factor the present study investigated, although
previous studies by Kwon et al and He et al did not
consider the effect of an elevated NLR or PLR on adjuvant
chemotherapy (17,18). According to the present results,
patients with a high NLR or PLR that accepted adjuvant chemotherapy
possessed a significantly improved 5-year OS rate compared with
patients that did not possess an elevated NLR or PLR and did not
accept adjuvant chemotherapy (P=0.015 and P=0.019, respectively).
This difference was not observed in patients with a low NLR or PLR.
Therefore, adjuvant chemotherapy appeared to be more effective in
CRC patients with a high NLR or PLR.
In conclusion, the present study validates the use
of pre-operative NLR and PLR as independent prognostic factors for
CRC patients. Notably, NLR was observed to be more effective than
PLR for predicting CRC. In addition, the present data suggests that
neutrophils and platelets are important in promoting CRC
progression, but neutrophils are more crucial. Furthermore,
adjuvant chemotherapy appeared to be more effective in CRC patients
with a high NLR or PLR. However, as an observational, single
hospital, small-scale study, the present study is limited. Larger
prospective studies are required to confirm these preliminary
results.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 61170123) and Natural
Science Foundation of Hainan Province (grant no. 813226).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szkandera J, Pichler M, Absenger G, Stotz
M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H,
Kornprat P, Stojakovic T, et al: The elevated preoperative platelet
to lymphocyte ratio predicts decreased time to recurrence in colon
cancer patients. Am J Surg. 208:210–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9:2122007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim
KH and Kim HJ: Prognostic significance of neutrophil lymphocyte
ratio and platelet lymphocyte ratio in advanced gastric cancer
patients treated with FOLFOX chemotherapy. BMC Cancer. 13:3502013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krenn-Pilko S, Langsenlehner U, Thurner
EM, Stojakovic T, Pichler M, Gerger A, Kapp KS and Langsenlehner T:
The elevated preoperative platelet-to-lymphocyte ratio predicts
poor prognosis in breast cancer patients. Br J Cancer.
110:2524–2530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azab B, Shah N, Radbel J, Tan P, Bhatt V,
Vonfrolio S, Habeshy A, Picon A and Bloom S: Pretreatment
neutrophil/lymphocyte ratio is superior to platelet/lymphocyte
ratio as a predictor of long-term mortality in breast cancer
patients. Med Oncol. 30:4322013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Wu Y, Wang Z, Yao Y, Chen F, Zhang
H, Wang Y and Song Y: Pretreatment platelet-to-lymphocyte ratio
(PLR) as a predictor of response to first-line platinum-based
chemotherapy and prognosis for patients with non-small cell lung
cancer. J Thorac Dis. 5:783–789. 2013.PubMed/NCBI
|
|
12
|
Feng JF, Huang Y and Chen QX: Preoperative
platelet lymphocyte ratio (PLR) is superior to neutrophil
lymphocyte ratio (NLR) as a predictive factor in patients with
esophageal squamous cell carcinoma. World J Surg Oncol. 12:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng JF, Huang Y, Zhao Q and Chen QX:
Clinical significance of preoperative neutrophil lymphocyte ratio
versus platelet lymphocyte ratio in patients with small cell
carcinoma of the esophagus. Scientific World Journal.
2013:5043652013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang N, Deng JY, Liu Y, Ke B, Liu HG and
Liang H: The role of preoperative neutrophil-lymphocyte and
platelet-lymphocyte ratio in patients after radical resection for
gastric cancer. Biomarkers. 19:444–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai Q, Castro Santa E, Rico Juri JM,
Pinheiro RS and Lerut J: Neutrophil and platelet-to-lymphocyte
ratio as new predictors of dropout and recurrence after liver
transplantation for hepatocellular cancer. Transpl Int. 27:32–41.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Du X, Sun P, Xiao C, Xu Y and Li R:
Preoperative platelet-lymphocyte ratio is an independent prognostic
factor for resectable colorectal cancer. Nan Fang Yi Ke Da Xue Xue
Bao. 33:70–73. 2013.(In Chinese). PubMed/NCBI
|
|
17
|
He W, Yin C, Guo G, Jiang C, Wang F, Qiu
H, Chen X, Rong R, Zhang B and Xia L: Initial neutrophil lymphocyte
ratio is superior to platelet lymphocyte ratio as an adverse
prognostic and predictive factor in metastatic colorectal cancer.
Med Oncol. 30:4392013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon HC, Kim SH, Oh SY, Lee S and Lee JH,
Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ and Lee JH: Clinical
significance of preoperative neutrophil-lymphocyte versus
platelet-lymphocyte ratio in patients with operable colorectal
cancer. Biomarkers. 17:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). New York,
NY: Springer. 143–164. 2010.
|
|
20
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology: Colon Cancer.
Version 1. National Comprehensive Cancer Network (Washington).
2015.
|
|
21
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer.
Version 1. National Comprehensive Cancer Network (Washington).
2015.
|
|
22
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waldner MJ and Neurath MF:
Colitis-associated cancer: The role of T cells in tumor
development. Semin Immunopathol. 31:249–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim
KH, Goh RY, Choi HJ, Park KJ, Roh MS, et al: Clinical significance
of preoperative serum vascular endothelial growth factor,
interleukin-6, and C-reactive protein level in colorectal cancer.
BMC Cancer. 10:2032010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA,
Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, et al: Clinical
significances of preoperative serum interleukin-6 and C-reactive
protein level in operable gastric cancer. BMC Cancer. 9:1552009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiang SF, Hung HY, Tang R, Changchien CR,
Chen JS, You YT, Chiang JM and Lin JR: Can neutrophil-to-lymphocyte
ratio predict the survival of colorectal cancer patients who have
received curative surgery electively? Int J Colorectal Dis.
27:1347–1357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nieswandt B, Aktas B, Moers A and Sachs
UJ: Platelets in atherothrombosis: Lessons from mouse models. J
Thromb Haemost. 3:1725–1736. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goubran HA, Stakiw J, Radosevic M and
Burnouf T: Platelets effects on tumor growth. Semin Oncol.
41:359–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma D, Brummel-Ziedins KE, Bouchard BA
and Holmes CE: Platelets in tumor progression: A host factor that
offers multiple potential targets in the treatment of cancer. J
Cell Physiol. 229:1005–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Templeton AJ, Ace O, McNamara MG,
Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A,
Tannock IF and Amir E: Prognostic role of platelet to lymphocyte
ratio in solid tumors: A systematic review and meta-analysis.
Cancer Epidemiol Biomarkers Prev. 23:1204–1212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salazar-Onfray F, López MN and
Mendoza-Naranjo A: Paradoxical effects of cytokines in tumor immune
surveillance and tumor immune escape. Cytokine Growth Factor Rev.
18:171–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y
and Kubota K: Combination of platelet count and neutrophil to
lymphocyte ratio is a useful predictor of postoperative survival in
patients with colorectal cancer. Br J Cancer. 109:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|