Introduction
As the second most common cause of cancer-associated
mortality in the world, gastric cancer is particularly prevalent in
the Far East region (1). Despite
recent advances in treatments, gastric cancer is challenging to
cure unless it is found at an early stage of disease (2). In general, gastric cancer does not have
many typical symptoms, and patients may only display anorexia.
Therefore, early diagnosis is difficult unless the patient accepts
regular endoscopic examination. Gastric cancer requires
comprehensive treatment, including surgery, chemotherapy and
radiotherapy; however, once peritoneal metastasis occurs therapy
frequently becomes ineffective, although chemotherapy has been used
as a clinical treatment (3,4). Peritoneal dissemination is the most
frequent pattern of metastasis and recurrence in patients with
gastric cancer, and it has been previously reported that the
detection rate of free cancer cells in the peritoneal cavity was
44% in patients with serosal invasion (5). Peritoneal metastasis is an important
contributing factor in the mortality of patients with gastric
cancer, accounting for ~50% of such mortalities (6). Pertitoneal metastasis of gastric cancer
frequently causes no symptoms, and is commonly diagnosed via
ultrasound or computed tomography, though it is also possible to
diagnose during surgery (4).
Generally, the pathway for gastric cancer peritoneal implantation
is considered to be the following: Infiltration of the stomach
serosal layer, exfiltration from tumour tissue, transfer into the
peritoneal cavity, transformation into biologically active cancer
cells and proliferation in the peritoneum to form active, implanted
cancerous tissue (7). Cell adhesion
is an important step in this the process. Integrin β1 is an
important β-subunit of the integrin family, which are a family of
adhesion factors. Integrin β1 has a mediatory role in the
interaction between the extracellular matrix and the cell (8). The abnormal expression of integrin β1 is
associated with celiac implantation in cancer (9). Studies have shown that dextran sulphate
(DS) can prevent the implantation of B-16 melanoma cells in omental
milky spots and the peritoneum, and that it can prolong the
survival of mice with peritoneal-related carcinoma (10). The present study investigated the
mechanism of the DS-dependent inhibition of the peritoneal
metastasis of gastric cancer cells using in vitro and in
vivo assays, and was approved by the Institutional Ethics
Committee of Ningxia Medical University.
Materials and methods
In vitro experimental materials
Cells and cell culture
The human gastric cancer MKN1 cell line (Institute
of Cancer Research Repository of Japan, Tokyo, Japan) was cultured
in Roswell Park Memorial Institute (RPMI) 1640 medium with 10%
foetal calf serum (FCS) (both Hangzhou Sijiqing Biological
Engineering Company, Hangzhou, China) at 37°C under 5%
CO2.
Drug
DS (molecular weight, 500,000 Da; Sigma-Aldrich, St.
Louis, MO, USA) was dissolved in phosphate-buffered saline (PBS) at
a 2% concentration and sterilised using a 22-mm filter. The final
concentration of DS was 0.3% in the experimental group. The same
volume of PBS was used in the control group.
Antibodies
A rabbit anti-human anti-integrin β1 monoclonal
antibody (2 mg/ml; catalog no., BA0958-1; Wuhan Boster Biological
Engineering Co., Ltd., Wuhan, China). A goat anti-rabbit
biotin-conjugated immunoglobulin (Ig) G (2 mg/ml; catalog no.,
BA1003; Wuhan Boster Biological Engineering Co., Ltd.) was used to
stain for primary antibody. A fluorescein isothiocyanate
(FITC)-conjugated rabbit anti-human anti-integrin β1 antibody (2
mg/ml; catalog no., BA1114; dilution, 1:50; Wuhan Boster Biological
Engineering Co., Ltd.) was used to stain for integrin β1 in living
cells.
In vivo experimental materials
Cells
The gastric cancer BGC-823 cell line (Repository of
Beijing Jin Zijing Biological Pharmaceutical Technology, Ltd.,
Beijing, China) was cultured in RPMI 1640 medium with 10% FCS at
37°C under 5% CO2 and 70% humidity.
Animals
Male BALB/c nude mice at 5–6 weeks of age, weighing
18–22 g, were purchased from Beijing Weitonglihua Experimental
Animal Technical Co., Ltd. [animal license SCXX (Beijing),
2006–0009]. The mice were maintained under specific pathogen-free
conditions, and provided with aseptic food and water ad
libitum.
Drugs and reagents
DS was prepared for the in vivo experiment at
a concentration of 0.3%, as aforementioned. FCS and RPMI 1640
medium were used for the cell culture. A polymerase chain reaction
(PCR) kit (Advantage for RT-for PCR kit; catalog no., 639505;
Takara Biotechnology Co., Ltd., Dalian, China), total RNA kit
(RNA-Solv Reagent; catalog no., R6834-01; Omega Bio-Tek, Inc.,
Norcross, GA, USA) and RT reagent kit [EXONUCLEASE III; catalog
no., 9037-44-9; Fermentas, Inc.; Thermo Fisher Scientific (China)
Inc., Beijing, China] were used for reverse transcription (RT)-PCR.
Rabbit anti-human anti-integrin β1 monoclonal antibody was used to
detect integrin β1 (dilution, 1:100) and goat anti-rabbit
biotin-conjugated IgG (dilution, 1:1,000) was used to stain for the
primary antibody.
Examination by phase-contrast microscopy
The MKN1 cells (5×104 cells/well) were cultured in a
75-ml plate, with DS added to the experimental group. The DS was
diluted in PBS at a concentration of 0.3%. The total volume of DS
solution was 1 ml. The same volume of PBS was used in the control
group. The cells were observed under a phase-contrast microscope
after 4 h of culture. In each group, the survival rate was >90%.
The cells in the two groups were then collected for the
determination of gene expression.
Immunofluorescence staining
The MKN1 cells were cultured in 35-mm glass-bottom
culture dishes coated with poly-L-lactic acid (PLL) in culture
medium containing DS or PBS. After 2 h of culture, the cells were
rinsed with PBS, fixed with 4% formalin/PBS at 37°C for 15 min, and
then stained with an anti-integrin β1 monoclonal antibody (1:100
dilution) at 4°C for 12 h. The cells were then stained with the
goat anti-rabbit biotin-conjugated IgG secondary antibody (1:1,000
dilution) for 2 h in the dark after being rinsed repeatedly with
PBS. The stained cells were observed using a confocal microscope
(LSM 510; Leica Microsystems GmbH, Wetzlar, Germany).
Integrin β1 fluorescent immunostaining of living
cells
The cells were cultured in a 0.2% bovine serum
albumin solution containing the FITC-conjugated anti-integrin β1
antibody for 90 min. The cells were cultured in a 35-mm
glass-bottom culture dish coated with PLL and media containing 10%
FCS after 3 washes in PBS, and then examined in a culture tray with
a confocal microscope at 37°C under 5% CO2. At 2 h after
plating, the cells were treated with DS at a final concentration of
0.3%. The cells were then examined 30 and 60 min after the addition
of DS.
Expression levels of integrin β1 mRNA
The expression levels of integrin β1 mRNA were
determined using a semi-quantitative RT-PCR method. Total RNA was
isolated according to the manufacturer's protocol using the RNeasy
total RNA kit (RNA-Solv Reagent). Additional purification was
achieved by DNaseI digestion (Qiagen China Co., Ltd., Shanghai,
China). For RT-PCR analysis, 10 µl total RNA was used for
first-strand cDNA synthesis using oligo-dT primers (0.5 µl) and the
SuperScript III First-Strand synthesis system (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The PCR assay was
performed using a Biosafer 9703 PCR machine (Safer China Co., Ltd.,
Nanjing, China) and AmpliTaq DNA polymerase (Roche Diagnostics,
Basel, Switzerland). cDNA quality was checked by PCR of the
housekeeping gene β-actin. Primer pairs for the integrin β1 chains
were selected according to the sequences published in the GenBank
database (National Center for Biotechnology Information, ML, USA).
The forward primer for integrin β1 was
5′-GCAGTAAGCATCCATTGGACCCGTGGGACACTCTGGATT-3′, complementary to bp
2284–2261 of integrin β1. The reverse primer was
3′-CGTCATTCGTAGGTATAG-5′. The forward and reverse primers for
β-actin were 5′-GTGGGGCGCCCCAGGCACCA-3′ and
3′-CTTAGCACGCACTGTTATTTCCTC-5′, respectively. Primers were
purchased from Beijing SBS Genetech Co., Ltd. (Beijing, China), and
1 µl of each primer was used. Following the denaturation of cDNA (2
µl) at 95°C for 5 min, temperature cycling (35 cycles) was
performed as follows: A denaturation step at 95°C for 45 sec, a
40-sec annealing step at 56°C and a 60-sec elongation step at 72°C.
Temperature cycling was concluded with a final elongation step for
10 min at 72°C. Products (10 µl) were analyzed by agarose gel
electrophoresis and visualized by ethidium bromide staining under
ultraviolet light (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The experiment was repeated three times. The relative
integrin β1 mRNA expression level was calculated in non-adherent
and adherent cells from the experimental and control groups.
Relative mRNA expression was calculated using the 2−ΔΔCq
method (11) and levels were
normalized to the internal control, β-actin.
In vivo experimental methods
Establishment of nude mice with celiac
implantation of gastric cancer cells
In total, 90 BALB/c nude mice were randomly divided
into the control group (n=40) and the experimental group (n=50),
and treated with 0.5% pentobarbital sodium (50 mg/kg). A gastric
cancer BGC-823 cell suspension was intraperitoneally injected at a
concentration of 1×107 cells/ml in a volume of 0.2 ml, and 0.3% DS
(1 ml) was intraperitoneally injected into the experimental group,
whereas 0.9% saline (1 ml) was intraperitoneally injected into the
control group. The entire process strictly adhered to sterile
techniques. The control and experimental groups were randomly
divided into five groups (control, n=8; experimental, n=10). The
mice were sacrificed at 1, 3, 7 and 14 days post-injection, and a
natural death group of 5 mice was established in the control and
experimental groups. In the natural death group, the mice were
sacrificed when the tumor grew to a size that inhibited the ability
of the mice to eat. Then the tumor nodules in the peritoneal cavity
were taken to be counted and for use in the other experiments. The
greater omentum tissue was divided into two sections, one for
hematoxylin and eosin (H&E) and immunohistochemical staining,
and another for RT-PCR analysis.
H&E and immunohistochemical staining
The specimens were processed by formalin fixation,
paraffin embedding, slicing, H&E staining and
immunohistochemical staining (streptavidin peroxidase conjugated
method). Integrin β1 was localized in the cell membrane and
cytoplasm. Five representative fields of view were selected. An
Image-Pro Plus image automatic analysis system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to determinate the
optical density value and calculate the average optical density
(OD) value of the selected field.
RT-PCR
The total RNA of the tissue was extracted using a
total RNA kit. The primers and the RT-PCR procedure was the same as
in the in vitro experiment described previously. The PCR
products were separated using 2% agarose gel electrophoresis with
ethidium bromide staining, and images were obtained using a Bio-Rad
gel imaging system (Gel Doc™ XR+, Bio-Rad Laboratories, Inc.). The
images were imported into the Quantity One® analysis software
(Bio-Rad Laboratories, Inc.) for analysis. The gene expression
abundance was represented as the relative OD. The average OD value
was calculated as integrin β1 mRNA abundance / glyceraldehyde-3
phosphate dehydrogenase mRNA abundance. Relative mRNA expression
was calculated using the 2−ΔΔCq method (11) and levels were normalized to the
internal control, β-actin.
Statistical analysis
SPSS version 21.0 (IBM SPSS, Armonk. NY, USA) was
used to perform the statistical analyses. The χ2 test
was used for the analysis of categorical variables, which were
expressed as numbers and percentages or frequencies. Continuous
variables were expressed as the mean ± standard deviation and were
analyzed using the Student's or paired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
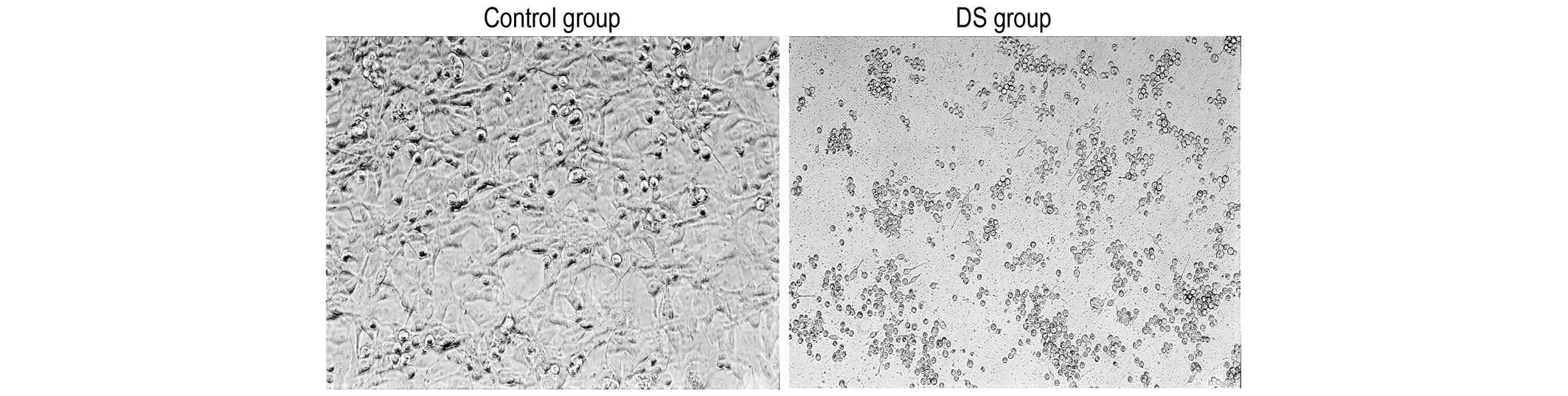
DS causes MKN1 cells to lose
connection
In the control group, the MKN1 cells exhibited a
spindle or flat morphology, forming a monolayer via mutual contact
between the cells after plating for 4 h. In the DS group, a number
of the cells were non-adherent. The majority of the adherent cells
retained a round shape, and contact between the cells was rarely
observed (Fig. 1).
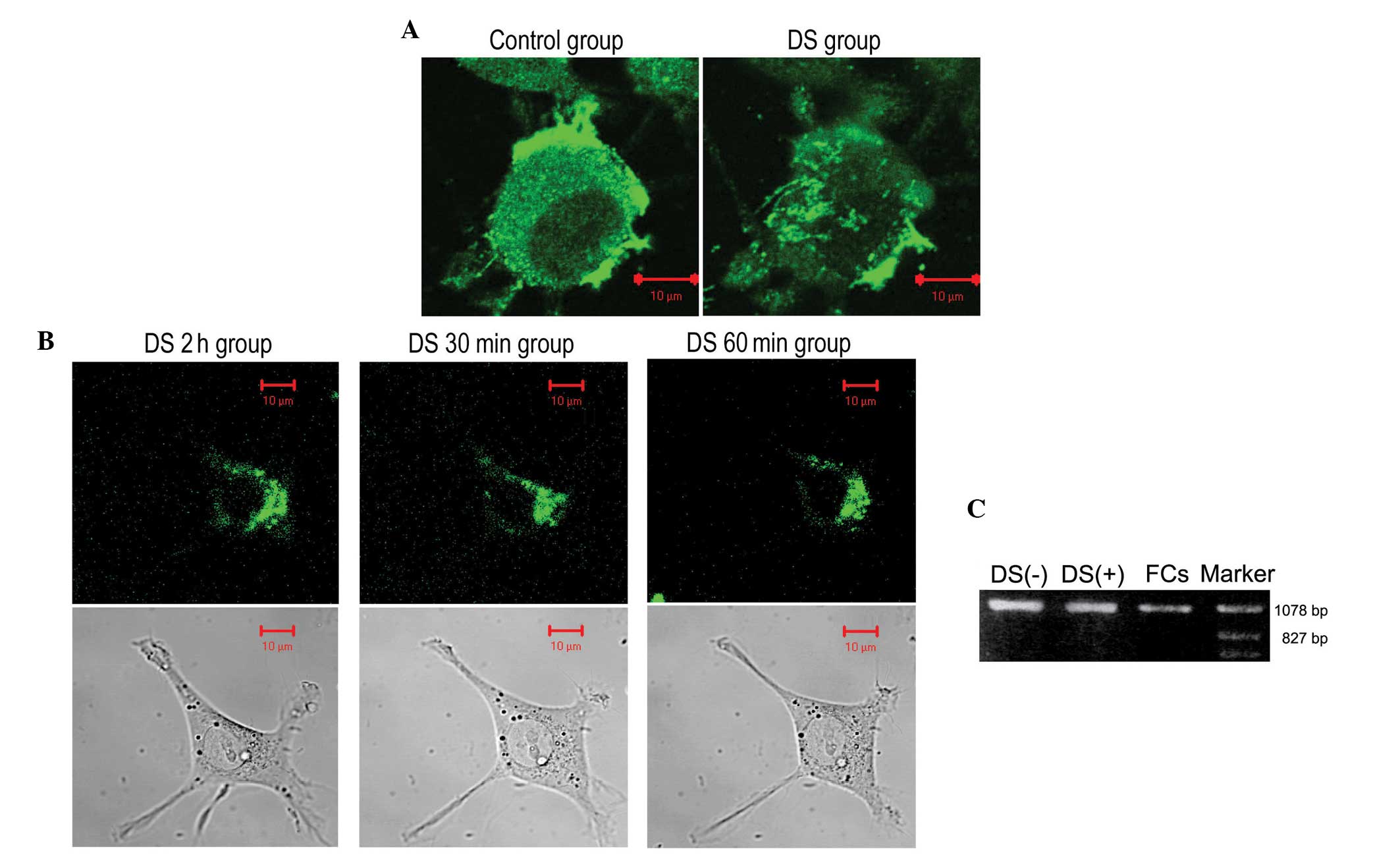
DS inhibits the expression of integrin
β1 in MKN1 cells
In the control group, following 2 h of culture, the
cell membrane of the MKN1 cells contained integrin β1 that formed
clusters on the underside of the cells. The cells were connected by
pseudopodia that strongly expressed integrin β1. In the DS group,
the cell membrane contained integrin β1, but no clusters were
observed (Fig. 2A). The MKN1 cells
formed pseudopodia and attached to the culture dish after
treatment. After 2 h of culture, large integrin β1 clusters had
formed on the bottom of the cells. The cells became flat and formed
multiple large pseudopodia. After treatment with DS for 30 and 60
min, the size of the integrin β1 clusters decreased, and the
pseudopodia became shorter and then disappeared over time (Fig. 2B). Adherent cells in the control and
DS groups, and non-adherent cells in the DS group expressed
integrin β1. The integrin β1 mRNA expression levels of the adherent
and free cells in the DS group were reduced to 74 and 38% compared
with the control group (Fig. 2C).
DS inhibits the growth of
celiac-implanted tumour nodules and the expression of integrin β1
in in vivo experiments
The celiac-implanted tumour nodules in the DS and
control groups were assessed. The number of tumour nodules on the
greater omentum, abdominal wall and superior mesenteric increased
over time following injection of the DS and cells. In the control
group, the tumour nodules on the greater omentum appeared the
earliest, and the omentum presented the greatest number of nodules.
There was a significant decrease (P<0.010) in the number of
celiac-implanted tumour nodules at each time point from day 3 to 14
in the DS group compared to the control group (Table I; Fig.
3A). The integrin β1 protein was positively expressed in the
cytoplasm as a brown colour in the tumour cells. The amount of
positive immunoreactivity was higher in the control group than in
the DS group on the day 14 (P<0.001; Table II; Fig.
3B). The expression of integrin β1 mRNA had significantly
decreased in the DS group at each time point from day 3 to 14 when
compared with the control group at the corresponding time points
(P<0.010; Table III; Fig. 3C).
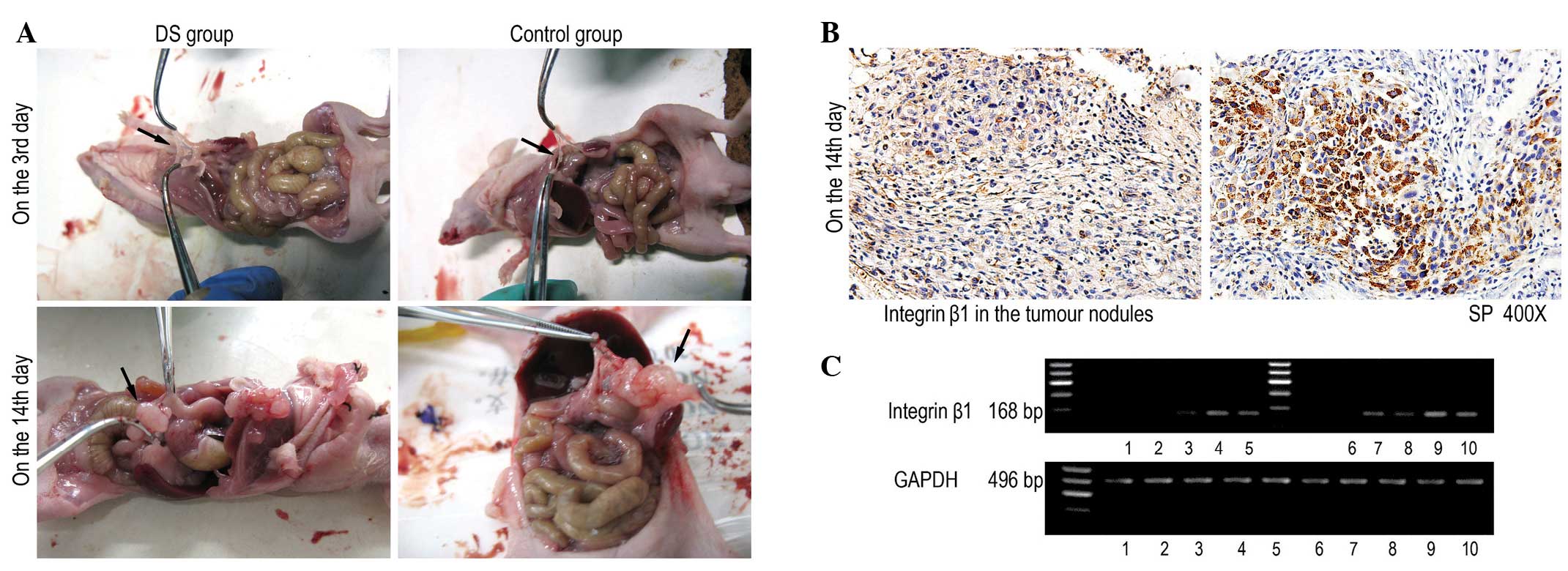 | Figure 3.DS inhibits the growth of
celiac-implanted tumour nodules and the expression of integrin β1
in in vivo experiments. (A) A significant decrease in the
number of celiac-implanted tumour nodules was observed from days 3
and 14 in the DS group. (B) 3,3′-Diaminobenzidine was used as a
staining agent (brown). Positive staining for integrin β1
expression was observed in the cytoplasm. Cells negative for
integrin β1 were stained by haematoxylin. (magnification, x400).
(C) The expression of integrin β1 mRNA had significantly decreased
in the DS group at each time point from day 3 to 14 when compared
with the control group at the corresponding time points. Lanes 1–5
represent days 1, 3, 7 and 14, and the time of natural death,
respectively, in the control group. Lanes 6–10 represent days 1, 3,
7 and 14, and the time of natural death, respectively, in the DS
group. DS, dextran sulphate; GAPDH, glyceraldehyde-3 phosphate
dehydrogenase. |
 | Table I.Comparison of the number of
celiac-implanted metastatic nodules in the groups of nude mice. |
Table I.
Comparison of the number of
celiac-implanted metastatic nodules in the groups of nude mice.
| Group | n | day 1 | day 3 | day 7 | day 14 | Natural death
group | F-value | P-value |
|---|
| Control | 8 | 0.00±0.00 | 2.33±1.20 | 15.00±1.53 | 27.00±1.73 | 31.67±2.40 | 55.21 | <0.001 |
| Experimental | 10 | 0.00±0.00 | 0.67±0.33 |
4.33±1.33 | 13.83±1.76 | 16.33±1.41 |
|
|
| t-value |
| – | −1.34 | −5.26 | −5.33 | −5.51 |
|
|
| P-value |
| – | 0.30 | 0.00 | 0.00 | 0.01 |
|
|
 | Table II.Comparison of integrin β1
immunoreactivity in the experimental and control groups at
different time points. |
Table II.
Comparison of integrin β1
immunoreactivity in the experimental and control groups at
different time points.
| Group | n | day 1 | day 3 | day 7 | day 14 | Natural death
group | F-value | P-value |
|---|
| Control | 8 | 114.73±14.38 | 108.81±14.95 | 151.96±16.74 | 219.68±16.24 | 184.78±16.63 | 95.99 | <0.001 |
| Experimental | 10 | 52.43±4.93 | 52.20±9.26 |
7.22±1.25 | 175.87±13.31 | 128.20±14.10 |
|
|
| t-value |
| ﹣4.10 | ﹣3.22 | ﹣8.62 | −2.09 | ﹣2.60 |
|
|
| P-value |
| 0.00 | 0.01 | 0.00 | 0.05 | 0.02 |
|
|
 | Table III.Comparison of integrin β1 mRNA
expression between the experimental and control groups at different
time points. |
Table III.
Comparison of integrin β1 mRNA
expression between the experimental and control groups at different
time points.
| Group | n | day 1 | day 3 | day 7 | day 14 | Natural death
group | F-value | P-value |
|---|
| Control | 8 | 1.17±0.05 | 1.30±0.10 | 2.44±0.02 | 2.67±0.05 | 2.06±0.01 | 186.13 | <0.001 |
| Experimental | 10 | 0.68±0.03 | 0.89±0.03 | 1.26±0.04 | 1.69±0.02 | 1.87±0.06 |
|
|
| t-value | – | ﹣7.83 | ﹣3.95 | ﹣25.91 | ﹣17.06 | ﹣3.39 |
|
|
| P-value | – | 0.00 | 0.04 | 0.00 | 0.00 | 0.02 |
|
|
Discussion
The diagnosis rate of advanced gastric cancer is
37–39%, and >50% of patients with stage I–III gastric cancer
experience post-operative recurrence with subsequent development to
an advanced stage (12). Peritoneal
metastasis is the main cause of mortality from gastric cancer, and
can even be caused by surgery (12).
Presently, chemotherapy is the main comprehensive treatment;
however, due to the lack of specificity, the effect of
chemotherapeutics is insufficient, even with multiple therapies
(12). Patients with early-stage
gastric cancer usually do not present with symptoms; therefore, the
disease has often developed to an advanced stage when the patients
are diagnosed, and ~50% of patients with advanced-stage cancer
develop peritoneal metastases (12).
Thus, there is an urgent requirement to develop therapies to
prevent the peritoneal metastasis of gastric cancer, and DS may be
such a potential medicine.
The metastasis of gastric cancer cells is a complex
multistep process, involving invasion, adhesion, migration and
other types of malignant biological behaviours (13). In particular, it involves three
successive steps: i) Tumour invasion such that tumour cells leave
the mucosa of the stomach; ii) migration of the non-adherent cancer
cells into the abdominal cavity, where they adhere to the
peritoneum; and iii) invasion and novel capillary formation with
proliferation around the vasculature, with eventual formation of
nodules of metastatic carcinoma (14). Among the numerous factors that affect
tumour invasion and metastasis, integrins are gaining increasing
attention due to their unique structure and function. Integrins
comprise a transmembrane protein family composed of dimers of two
subunits, α and β, and integrin β1 dimerises with different α
subunits to constitute the vast majority of integrin complexes in
the extracellular matrix (14).
Integrins mainly function in two ways in the occurrence and
development of tumours: i) Mediating the adhesion of tumour cells
to the extracellular matrix by changing the composition of the
substrate and adjusting the biological function of the matrix to
promote the invasion and metastasis of tumour cells; and ii)
mediating messages from the extracellular matrix to cells, as this
information often affects cell growth and differentiation (14). The transfer of abnormal information by
integrin promotes tumour cell growth, dedifferentiation and distant
metastasis (15).
Cell migration is necessary for tumour invasion and
metastasis, and can be divided into several steps: the polarisation
of cells, the budding of the cell membrane, actin contraction,
movement of cells at adhesion areas, and the separation of the tail
and base of cells (16). The ligands
of integrins include extracellular matrix components, such as
fibronectin, fibrinogen, collagen, vitronectin and laminin, which
mediate interactions between cells and the extracellular matrix,
and promote cell adhesion and the formation of novel tissues
(16). Integrin activation triggers
cell signalling that leads to the reorganisation of the
cytoskeleton and causes cell morphology changes (17). Studies on the formation of integrin
clusters and the migration of cells indicate that integrin is
transferred from the trailing edge of the cell to the front of the
cell, initiating the formation of local adhesion areas at the tip
of the pseudopodia (18).
Studies have suggested that the abnormal expression
of integrin on the surface of tumour cells may be directly
associated with the invasion and metastasis of the tumour. Integrin
mediates adhesion between cells, induces angiogenesis, and plays a
significant role in the metastasis and infiltrating growth of the
malignant tumour (19,20). Integrin β1 is an important element in
the integrin family and plays a key role in gastric cancer cell
adhesion to the peritoneum by mediating the adhesion between cancer
cells and various fibres of the peritoneal base layer, allowing
cancer cells to adhere to the peritoneal layer (21). Intraperitoneal injection of a
monoclonal antibody against integrin β1 can significantly reduce
adhesion, inhibit the peritoneal implantation of cancer cells and
reduce the number of peritoneally implanted nodules (22).
The present in vitro experiment demonstrated
that the expression of integrin β1 mRNA in adherent cells in the
experimental group was reduced to 74% of the level in the control
group, and that the expression level of integrin β1 cDNA in
free-floating cells in the experimental group was reduced to 38% of
that in the control group. This result showed that DS inhibited the
expression of integrin β1 quantitatively. Fluorescence
immunostaining showed that DS inhibited the expression of integrin
β1 and reduced integrin clustering, thus indicating that DS
inhibited the expression of integrin β1 qualitatively.
Additionally, DS prevented MKN1 cells from forming pseudopodia and
changing shape to become morphologically stable. When the cells
were treated with DS prior to adherence, a number were unable to
adhere, and the majority of the adherent cells maintained a round
shape. When the cells were treated with DS once they had adhered,
their pseudopodia became shorter or smaller. In this experiment,
the integrin β1 clustering also decreased. Thus, DS may inhibit
integrin β1 activity, preventing cytoskeleton reorganisation and
inhibiting the adhesion of MKN1 cells to the culture dish.
The in vivo experiment showed that the number
of celiac-implanted tumour nodules increased significantly with the
duration of cancer cell injection. At the same time point, the
number of carcinoma nodules on the greater omentum in the
experimental group was decreased compared with that in the control
group. Integrin β1 immunoreactivity in the experimental group was
also decreased compared with that in the control group. Thus, DS
may inhibit integrin β1 expression to reduce the abdominal
metastasis of the gastric cancer cells.
In summary, the in vivo and in vitro
experiments showed that DS inhibits integrin β1 expression together
with the adhesion and celiac metastasis of cancer cells.
Acknowledgements
This study was supported by Ningxia Science and
Technology Support Project (grant no., NXKZ20130068).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Yu JC, Kang WM and Ma ZQ:
Treatment strategy for early gastric cancer. Surg Oncol.
21:119–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y: Gastric cancer. Surgery. Wang X and
Wang J: 8:People's Health Publication. (Beijing). 360–365.
2014.
|
|
4
|
Wang Z and Chen L: Present status of
treatment in peritoneal metastasis of gastric cancer. Zhonghua Wei
Chang Wai Ke Za Zhi. 18:194–197. 2015.(In Chinese). PubMed/NCBI
|
|
5
|
Broll R, Weschta M, Windhoevel U, Berndt
S, Schwandner O, Roblick U, Schiedeck TH, Schimmelpenning H, Bruch
HP and Duchrow M: Prognostic significance of free gastrointestinal
tumor cells in peritoneal lavage detected by immunocytochemistry
and polymerase chain reaction. Langenbecks Arch Surg. 386:285–292.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maehara Y, Hasuda S, Koga T, Tokunaga E,
Kakeji Y and Sugimache K: Postoperative outcome and sites of
recurrence in patients following curative resection of gastric
cancer. Br J Surg. 87:353–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yonemura Y, Bandou E, Kawamura T, Endou Y
and Sasaki T: Quantitative prognostic indicators of peritoneal
dissemination of gastric cancer. Eur J Surg Oncol. 32:602–606.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H
and Schwartz MA: Matrix-specific suppression of integrin activation
in shear stress signalling. Mol Biol Cell. 17:4686–4697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu G and Chen X: Why integrin as a
primary target for imaging and therapy. Theranostics. 1:30–47.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hagiwara A, Sawai K, Sakakura C, Shirasu
M, Ohgaki M, Imanishi T, Yamasaki J, Togawa T and Takahashi T:
Prevention of peritoneal metastasis of cancer with dextran
sulfate-an experimental study in mice. Anticancer Drugs. 8:894–897.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chervoneva I, Li Y, Schulz S, Croker S,
Wilson C, Waldman SA and Hyslop T: Selection of optimal reference
genes for normalization in quantitative RT-PCR. BMC Bioinformatics.
11:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasechnikov V, Chukov S, Fedorov E,
Kikuste I and Leja M: Gastric cancer: Prevention, screening and
early diagnosis. World J Gastroenterol. 20:13842–13862. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SR: Gastric cancer stem cells: A
novel therapeutic target. Cancer Lett. 338:110–119. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trepet X, Chen Z and Jacobson K: Cell
Migration. Compr Physiol. 2:2369–2392. 2012.PubMed/NCBI
|
|
17
|
Giancotti FG and Ruoslahli E: Integrin
signalling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zamir E, Katz M, Posen Y, Erez N, Yamada
KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z and Geiger B:
Dynamics and segregation of cell matrix adhesions in cultured
fibroblasts. Nat Cell Biol. 2:191–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yano H, Mazaki Y, Kurokawa K, Hanks SK,
Matsuda M and Sabe H: Roles played by a subset of integrin
signaling molecules in cadherin-based cell-cell adhesion. J Cell
Biol. 166:283–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janssen M, Frielink C, Dijkgraf I, Oyen W,
Edwards DS, Liu S, Rajopadhye M, Massuger L, Corstens F and Boerman
O: Improved tumor targeting of radiolabeled RGD peptides using
rapid does fractionation. Cancer Biother Radiopharm. 19:399–404.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lynch L, Vodyanik PI, Boettiger D and
Guvakova MA: Insulin-like growth factor I controls adhesion
strength mediated by alpha5 beta l integrins in motile carcinoma
cells. Mol Biol Cell. 16:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Howe GA and Addison C: β1 integrin: An
emerging player in the modulation of tumorigenesis and response to
therapy. Cell Adh Migr. 6:71–77. 2012. View Article : Google Scholar : PubMed/NCBI
|