Introduction
Phyllodes tumor (PT) is a rare type of biphasic
fibroepithelial neoplasm that accounts for <1% of all breast
tumors and represents 2–3% of fibroepithelial neoplasms (1,2) with a
peak age of incidence of 45–49 years (3,4). According
to the standards set by the World Health Organization (WHO), PTs
may be classified as benign, borderline or malignant based on the
degree of stromal cell atypia, mitotic status, degree of stromal
overgrowth, tumor necrosis and appearance of tumor margins
(5). PTs are predominantly benign
with only ~10% identified as malignant. The majority of malignant
transformation of PTs typically occurs in the stromal compartment
and rarely in the epithelial compartment. Breast carcinoma within
PT accounts for 1–2% of all PTs (6).
Surgery is considered the standard treatment for PT (7). Invasive ductal carcinomas (IDC) of the
breast accounts for 80% of all breast cancers, and these tumors
demonstrate a worse survival rate than invasive lobular carcinoma
(8), with overall 5-year survival
rates of 84.1 and 85.6%, respectively (9). An IDC that is incidentally found within
a borderline PT has been reported only once before in the
literature (10). The current study
presents a case of IDC within a borderline PT, and reviews 32 cases
of breast carcinoma within a PT that have been reported in the
literature.
Case report
In July 2012, a 52-year-old female presented to the
Department of Breast Surgery, First Hospital of Jilin University
(Changchun, China) with a firm, palpable, irregularly-shaped lump
with an ill-defined margin in the outer upper quadrant of the left
breast. The lump, which was originally identified by the patient 6
months previously, had increased in size from 1.5×1.0 cm at
presentation to 2.5×2.0 cm after 3 months. Physical examination
revealed that the tumor did not adhere to or invade the overlying
skin or the thoracic wall. Enlarged axillary lymph nodes were not
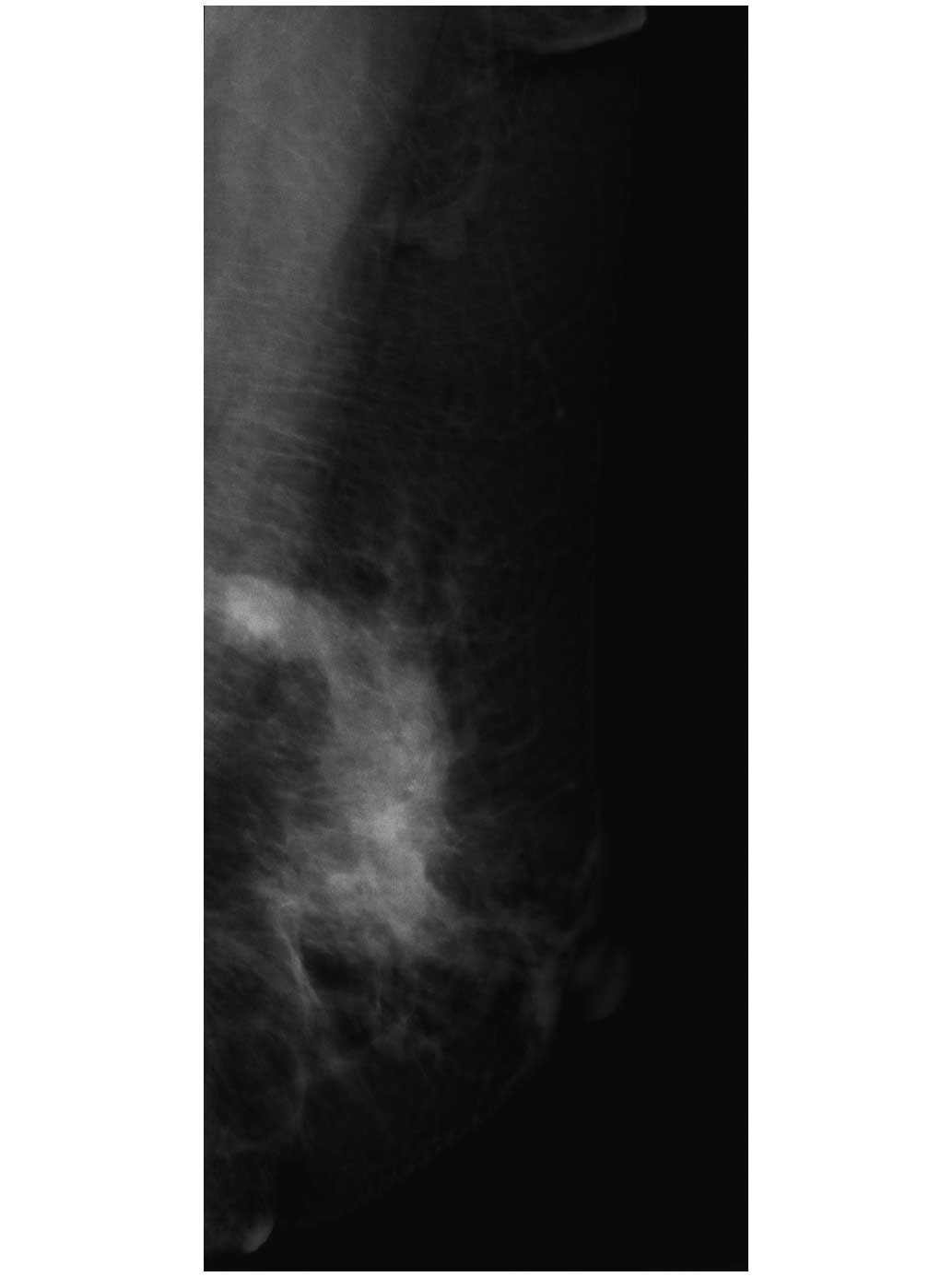
identified upon physical examination. Mammography imaging revealed
a high-density mass with a diameter of 2.5 cm and an irregular
margin (Fig. 1), and sonographic
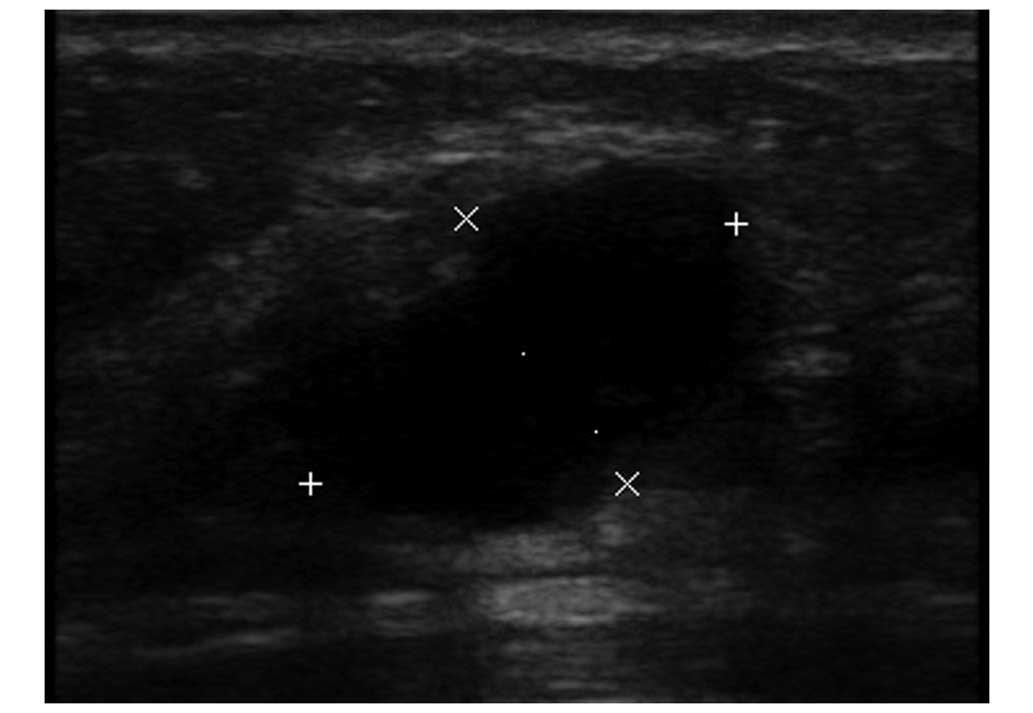
examination demonstrated a partially ill-defined hypoechoic mass
with a diameter of 2.1 cm (Fig. 2). A
core needle biopsy revealed borderline or malignant PT with a
breast carcinoma component.
The diagnosis was determined by analysis of the core
needle biopsy, as follows. The tumor was well-circumscribed and
3.0×2.5×1.2 cm in size, according to macroscopic examination. The
mitotic count in the most active area was 2–4 mitoses per 10
high-powered fields. Based on an increase in the number of mitotic
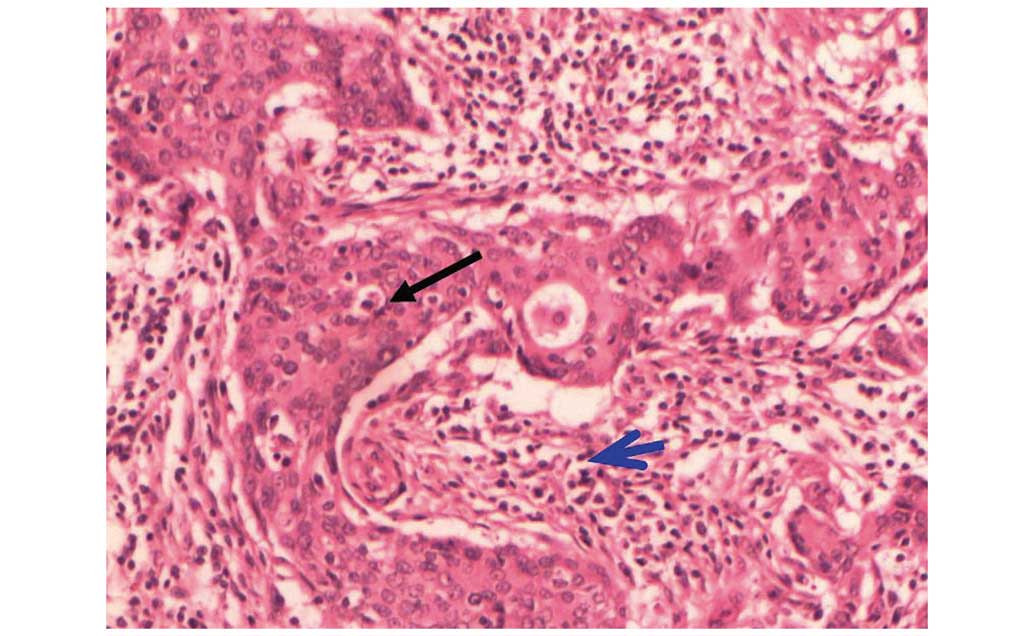
figures and according to the WHO 2003 grading system (11), the tumor was classified as a
borderline PT. IDC was also observed in a focal area of spindle
cells (Fig. 3). The results of
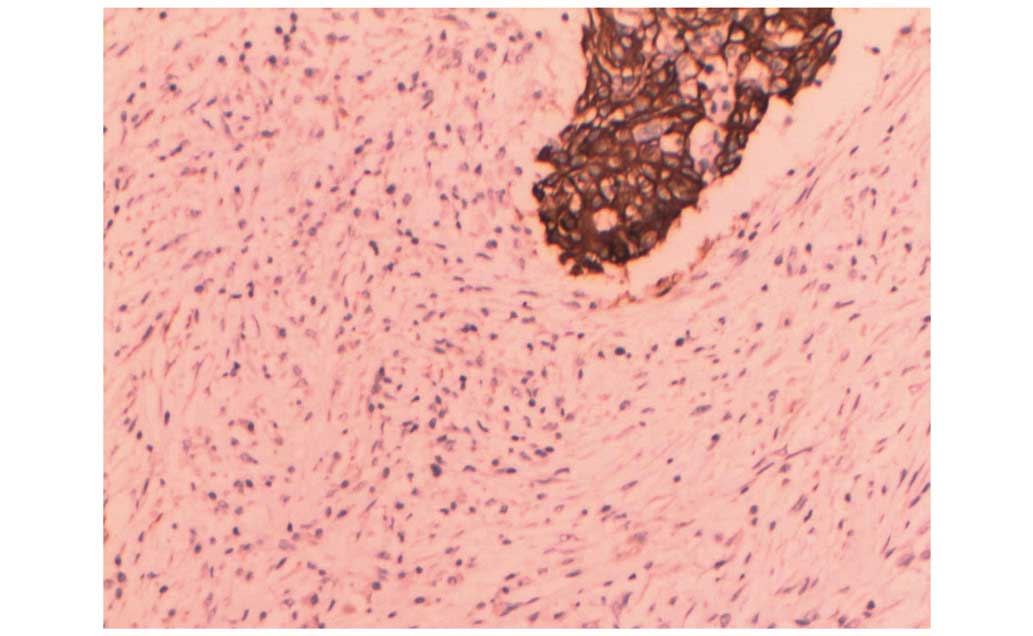
MaxVision™ immunohistochemical staining (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) of the IDC
cells were as follows: Estrogen receptor negative; progesterone
receptor negative; HER-2 negative; Ki-67 index, 30%; cytokeratin
(CK) 5/6 positive; vimentin positive; and pan-CK positive (Fig. 4).
Considering the diagnosis of IDC within a borderline
PT, a simple mastectomy and sentinel lymph node biopsy (SLNB) were
performed on July 10, 2012. Intraoperative frozen pathological
analysis of 3 of the sentinel lymph nodes (SLNs) identified one
micrometastasis. An axillary lymph node dissection and subsequent
pathological examination did not reveal metastasis in any of the 18
nodes tested. The patient underwent six cycles of chemotherapy
cycled every 21 days, consisting of 75 mg/m2 paclitaxel,
75 mg/m2 pirarubicin and 500 mg/m2
cyclophosphamide, all administered on day 1. In addition, the
patient underwent seven weeks of radiotherapy (25 cycles at 5,000
cGy; 200 cGy, each treatment). The tumor did not recur and no
metastasis was observed during the first 23 months subsequent to
treatment.
Discussion
PT may coexist with breast cancer in three
situations. It may coexist in the bilateral breast, for example
with IDC in one breast and a malignant PT in the other breast
(12). PT has also been detected in
the ipsilateral breast, such as IDC in the upper outer quadrant of
the left breast and malignant PT in the lower outer quadrant of the
left breast (13). Finally, PT may
coexist with breast cancer in the same mass, as occurred in the
current case. Breast carcinoma arising within PT is extremely rare.
A literature search of the PubMed database (www.pubmed.com) was performed using the following
search terms: ‘breast cancer with phyllodes tumor’ and ‘coexistence
of breast cancer and phyllodes tumor’. A total of 1,593 studies
were retrieved. Using the following criteria, it was determined
that <40 cases of breast carcinoma arising within PT have
previously been reported in the literature (Table I) (1,2,7,8,10,11,14–38).
Inclusion criteria: i) published between 1974 and 2013; ii) English
language; and iii) PT coexisting with breast cancer in the same
tumor. Exclusion criteria: i) PT and breast cancer coexisting in
the bilateral breast; ii) PT and breast cancer coexisting in the
ipsilateral breast in different tumors; iii) no detailed
pathological results; and iv) only the abstract available in
English, full-text in a different language. The age of the patients
with a coexistent breast carcinoma and PT ranged between 26 and 80
years, with a median age of 52 years. The reported breast carcinoma
subtypes included in situ and invasive lobular and ductal
(no specific type) carcinoma, invasive tubular carcinoma, squamous
cell carcinoma and invasive cribriform carcinoma. Malignant
epithelial elements were reported in all types of PT. Breast
carcinoma was most commonly reported in malignant (n=14) and benign
(n=15) PTs, but rarely in borderline PTs (n=4, including the
present case). Of the three cases of borderline PTs reported
(excluding the present case), Kuo et al presented the case
of a patient with a painless mass in the left breast, which had
been present for 4 years. Following rapid growth of the tumor, the
patient was diagnosed with invasive ductal carcinoma arising within
a phyllodes tumor with isolated tumor cells identified in the
sentinel lymph node (19). Mastectomy
and sentinel lymph node biopsy were performed followed by hormonal
therapy (goserelin acetate and tamoxifen), adjuvant chemotherapy
(5-fluorouracil, epirubicin and cyclophosphamide) and
reconstructive surgery. No tumor recurrence was reported during the
15 month follow-up period. Quinlan-Davidson et al (18) reported the case of a patient with a
painless mass in the right breast that had been present for several
years. Following two years of rapid growth of the mass the patient
was diagnosed with borderline phyllodes tumor with an incidental
invasive tubular carcinoma and lobular carcinoma in situ
component. An excisional biopsy was performed and subsequently the
patient underwent a re-excision for margin safety and a sentinel
lymph node biopsy, which revealed that all three sentinel lymph
nodes were negative for malignancy. In addition, Deodhar et
al (32) reported a case of
borderline phyllodes tumor with a ductal carcinoma in situ
(DCIS) component. However, the outcome of the patient was not
reported. Coexisting breast carcinoma within PTs more commonly
demonstrated a ductal phenotype (IDC, n=7; DCIS, n=15) compared
with a lobular phenotype (ILC, n=1; lobular carcinoma in
situ, n=7). A pure carcinoma in situ element was
identified in 17 cases and was determined to be invasive in the
other 16 cases. In addition, 6 cases were found to possess two
types of malignant epithelial elements (6,14–18). The PT size was not described in one
case and the mean diameter of the tumor was 7.9±5.3 cm. Yamaguchi
et al (1) reported 7 cases of
DCIS in PTs with a mean tumor size of 11.9 cm (15). Nio et al (23) reported that the mean diameter of
breast carcinoma within PTs was 8.0 cm (14). The carcinoma size of the present case
could not be measured.
 | Table I.Occurrence of breast carcinoma within
PT. |
Table I.
Occurrence of breast carcinoma within
PT.
|
| PT | Breast carcinoma |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| No. | First author
(Ref.) | Year | Age, years | Surgery | AxDs | Type | Diameter, cm | Type | Diameter, cm | LNI, n | Survival status | Follow-up time,
months |
|---|
| 1 | Leong et al
(26) | 1980 | 49 | LoEx | (−) | Benign | 6.0 | LCIS | NA | NA | NA | NA |
| 2 | Leong et al
(26) | 1980 | 51 | MX | (+) | Benign | 4.0 | ITC | NA | (−) | Alive | 21 |
| 3 | Cole-Beuglet et
al (14) | 1983 | 55 | LoEx | (−) | Benign | 3.5 | DCIS + LCIS | NA | NA | NA | NA |
| 4 | Cole-Beuglet et
al (14) | 1983 | 60 | LoEx | (−) | Benign |
3.0 | IDC | NA | NA | NA | NA |
| 5 | Grove et al
(27) | 1986 | 71 | MX | (+) | Benign | 19.0 | DCIS | 2.0 | (−) | Alive | 4 |
| 6 | Ishida et al
(10) | 1984 | 41 | MX | (−) | Benign |
5.6 | IDC | Focal | NA | Alive | 30 |
| 7 | Ward et al
(28) | 1986 | 55 | MX | NA | Benign |
4.0 | LCIS | Focal | NA | NA | NA |
| 8 | Knudsen et
al (15) | 1987 | 71 | MX | (+) | Benign |
7.0 | DCIS + LCIS | Multi-focal | (−) | Alive | 6 |
| 9 | Yasumura et
al (29) | 1988 | 47 | MX | (+) | Benign | 13.0 | IDC | NA | (−) | Alive | 66 |
| 10 | Kodama et al
(30) | 2003 | 47 | MX | (−) | Benign | 17.0 | LCIS | Focal | NA | Alive | 108 |
| 11 | Parfitt et
al (16) | 2004 | 26 | LoEx | (+) | Benign |
3.3 | DCIS + IDC | NA | (+)4/13 | Alive | 36 |
| 12 | Ramdass et
al (31) | 2006 | 69 | NA | NA | Benign | NA | SCC | NA | NA | NA | NA |
| 13 | Yamaguchi et
al (1) | 2008 | 54 | MX | (−) | Benign | 15.0 | DCIS | Focal | NA | Alive | 12 |
| 14 | Nio et al
(23) | 2011 | 53 | LoEx | (−) | Benign |
3.5 | DCIS | 0.5 | NA | Alive | 24 |
| 15 | Shirah et al
(17) | 2011 | 49 | LoEx |
| Benign |
4.8 | LCIS + ILC | 0.2 | NA | NA | NA |
| 16 | Deodhar et
al (32) | 1997 | 51 | LoEx | (−) | Borderline | 14.0 | DCIS | Focal | NA | NA | NA |
| 17 | Kuo et al
(19) | 2010 | 26 | MX | SLNB | Borderline | 10.0 | IDC | 2.5 | ITC | Alive | 15 |
| 18 | Quinlan-Davidson
et al (18) | 2011 | 53 | LoEx | SLNB | Borderline |
6.5 | ITC + LCIS | 2.4 | (−) | NA | NA |
| 19 | Present case | 2014 | 52 | MX | (+) | Borderline |
3.0 | IDC | Focal | (+)1/21 | Alive | 23 |
| 20 | Seemayer et
al (33) | 1975 | 27 | MX | (−) | Malignant |
6.0 | DCIS | Focal | NA | NA | NA |
| 21 | Klausner et
al (34) | 1983 | 60 | MX | (+) | Malignant |
4.0 | IDC | Focal | (−) | NA | NA |
| 22 | Hunger et al
(35) | 1984 | 57 | MX | (+) | Malignant | 15.5 | SCC | NA | (−) | NA | NA |
| 23 | Schwickerath et
al (7) | 1992 | 47 | MX | (+) | Malignant |
2.0 | DCIS | NA | (−) | NA | NA |
| 24 | Padmanabhan et
al (2) | 1997 | 47 | MX | (+) | Malignant |
7.5 | LCIS | Focal | (−) | Alive | 6 |
| 25 | Nishimura et
al (24) | 1998 | 80 | LoEx | (−) | Malignant | 10.5 | DCIS | NA | NA | Deceased | 3 |
| 26 | Alò et al
(36) | 2001 | 39 | MX | NA | Malignant |
9.0 | DCIS | NA | NA | NA | NA |
| 27 | Lim et al
(25) | 2005 | 45 | MX | (−) | Malignant | 12.0 | DCIS | 0.6 | NA | Deceased | 108 |
| 28 | Nomura et al
(37) | 2006 | 75 | MX | (−) | Malignant |
3.5 | DCIS | NA | NA | Alive | 32 |
| 29 | Sugie et al
(20) | 2007 | 54 | MX | (+) | Malignant |
8.0 | SCC | NA | (−) | Deceased | 40 |
| 30 | Korula et al
(21) | 2008 | 51 | MX | (+) | Malignant | 21.0 | DCIS | NA | (+)2/12 | Alive | 11 |
| 31 | Macher-Goeppinger
et al (38) | 2010 | 70 | MX | (+) | Malignant |
6.0 | IDC | 2.5 | (−) | NA | NA |
| 32 | Abdul Aziz et
al (6) | 2010 | 43 | LoEx | (−) | Malignant |
3.5 | ITC + DCIS | 0.2 | NA | Alive | 12 |
| 33 | Choi et al
(22) | 2012 | 62 | MX | (+) | Malignant | 10.0 | ICC | 6.0 | (−) | Alive | 24 |
In the previously reported literature, 10 cases were
treated with local excision and 23 cases were treated with
mastectomy. Of the 16 cases that received axillary surgery, three
cases exhibited axillary lymph node metastasis and one possessed an
isolated tumor cell in the SLN. As there is no standard adjuvant
treatment strategy for this type of disease, a variety of systemic
therapies were applied to the various cases. In total, 6 cases
received chemotherapy with various regimens (16,19–23), 5
cases received radiotherapy (13,16,18,20),
and 4 cases received endocrine therapy, 3 of which received
tamoxifen (1,16,21) and 1
received tamoxifen and goserelin (19). Patient outcomes were described in 19
cases. The follow-up time was between 3 and 108 months. In total,
16 patients were alive at the end of last follow-up. Distance
metastasis occurred in the lung in 2 cases at 3 and 32 months
subsequent to surgery, respectively (20,24).
Similar to the current case, Kuo et al (19) and Parfitt et al (16) reported the combination of surgery,
chemotherapy and radiotherapy for patients with lymph node
metastasis who present breast carcinoma within PT. In addition, 3
cases succumbed to the disease, 3, 40 and 108 months subsequent to
surgery, respectively (20,24,25)
(Table I).
In summary, the present study reports a rare case of
IDC within a borderline PT. The imaging experiments performed
lacked specificity. Instead, histology and immunohistochemistry are
the golden standard for diagnosing this type of disease. The
combination treatment of surgery, chemotherapy and radiotherapy was
effective in the current case. Various types of breast carcinoma
have been identified to coexist with PT in different masses;
however, no standard therapeutic regimen has been established for
the coexistence of PT and breast cancer in the same mass. The
determination of an appropriate treatment strategy predominantly
depends on the characteristics of the individual breast tumor, such
as the hormone receptor status, HER-2 status and axillary lymph
node metastasis status. Thus, future cases should undergo detailed
analysis of tumor characteristics with reference to the molecular
subtype and clinical pathological characteristics in order to
select the optimal treatment strategy for breast cancer within
phyllodes tumors.
References
|
1
|
Yamaguchi R, Tanaka M, Kishimoto Y, Ohkuma
K, Ishida M and Kojiro M: Ductal carcinoma in situ arising in a
benign phyllodes tumor: Report of a case. Surg Today. 38:42–45.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Padmanabhan V, Dahlstrom JE, Chong GC and
Bennett G: Phyllodes tumor with lobular carcinoma in situ and
liposarcomatous stroma. Pathology. 29:224–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvadori B, Cusumano F, Del Bo R,
Delledonne V, Grassi M, Rovini D, Saccozzi R, Andreola S and
Clemente C: Surgical treatment of phyllodes tumors of the breast.
Cancer. 63:2532–2536. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernstein L, Deapen D and Ross RK: The
descriptive epidemiology of malignant cystosarcoma phyllodes tumors
of the breast. Cancer. 71:3020–3024. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of Tumours of the Breast
(4th). IARC Press. Lyon: 2012.
|
|
6
|
Aziz Abdul M, Sullivan F, Kerin MJ and
Callagy G: Malignant phyllodes tumour with liposarcomatous
differentiation, invasive tubular carcinoma and ductal and lobular
carcinoma in situ: Case report and review of the literature.
Patholog Res Int. 2010:5012742010.PubMed/NCBI
|
|
7
|
Schwickerath J, Blessing MH and Wolff F: A
rare clinical manifestation of a combination tumor of cystosarcoma
phylloides malignum and an intraductal cancer. Geburtshilfe
Frauenheilkd. 52:557–559. 1992.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stacher E, Boldt V, Leibl S, Halbwedl I,
Popper HH, Ullmann R, Tavassoli FA and Moinfar F: Chromosomal
aberrations as detected by array comparative genomic hybridization
in early low-grade intraepithelial neoplasias of the breast.
Histopathology. 59:549–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arpino G, Bardou VJ, Clark GM and Elledge
RM: Infiltrating lobular carcinoma of the breast: Tumor
characteristics and clinical outcome. Breast Cancer Res. 6:149–156.
2004. View
Article : Google Scholar
|
|
10
|
Ishida T, Izuo M and Kawai T: Breast
carcinoma arising in cystosarcoma phyllodes: Report of a case with
a review of the literature. Jpn J Clin Oncol. 14:99–106.
1984.PubMed/NCBI
|
|
11
|
Tavassoli FA and Devilee P: World Health
Organization classification of tumours: Pathology and genetics of
tumours of the breast and female genital organs. IARC Press. Lyon:
99–103. 2003.
|
|
12
|
Merck B, Cansado Martínez P, Pérez Ramos
M, Martinez Banaclocha N, Lacueva Gómez FJ and Calpena R:
Infiltrating ductal carcinoma and synchronous malignant phyllodes
tumour. Diagnostic and therapeutic approaches. Clin Transl Oncol.
8:830–832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kefeli M, Yildiz L, Akpolat I, Balci P and
Ozen N: The coexistence of invasive ductal carcinoma and malignant
phyllodes tumor with liposarcomatous and chondrosarcomatous
differentiation in the same breast in a post-osteosarcoma case.
Pathol Res Pract. 204:919–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cole-Beuglet C, Soriano R, Kurtz AB, Meyer
JE, Kopans DB and Goldberg BB: Ultrasound, x-ray mammography and
histopathology of cystosarcoma phylloides. Radiology. 146:481–486.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knudsen PJ and Ostergaard J: Cystosarcoma
phylloides with lobular and ductal carcinoma in situ. Arch Pathol
Lab Med. 111:873–875. 1987.PubMed/NCBI
|
|
16
|
Parfitt JR, Armstrong C, O'Malley F, Ross
J and Tuck AB: In-situ and invasive carcinoma within a phyllodes
tumor associated with lymph node metastases. World J Surg Oncol.
2:462004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirah GR, Lau SK, Jayaram L, Bouton ME,
Patel PN and Komenaka IK: Invasive lobular carcinoma and lobular
carcinoma in situ in a phyllodes tumor. Breast J. 17:307–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quinlan-Davidson S, Hodgson N, Elavathil L
and Shangguo T: Borderline phyllodes tumor with an incidental
invasive tubular carcinoma and lobular carcinoma in situ component:
A case report. J Breast Cancer. 14:237–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuo YJ, Ho DM, Tsai YF and Hsu CY:
Invasive ductal carcinoma arising in phyllodes tumor with isolated
tumor cells in sentinel lymph node. J Chin Med Assoc. 73:602–604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugie T, Takeuchi E, Kunishima F,
Yotsumoto F and Kono Y: A case of ductal carcinoma with squamous
differentiation in malignant phyllodes tumor. Breast Cancer.
14:327–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korula A, Varghese J, Thomas M, Vyas F and
Korula A: Malignant phyllodes tumour with intraductal and invasive
carcinoma and lymph node metastasis. Singapore Med J. 49:e318–e321.
2008.PubMed/NCBI
|
|
22
|
Choi Y, Lee KY, Jang MH, Seol H, Kim SW
and Park SY: Invasive cribriform carcinoma arising in malignant
phyllodes tumor of breast: A case report. Korean J Pathol.
46:205–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nio Y, Iguchi C, Tsuboi K and Maruyama R:
Ductal carcinoma in situ arising within a benign phyllodes tumor: A
case report with a review of the literature. Oncol Lett. 2:223–228.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimura R, Hasebe T, Imoto S and Mukai
K: Malignant phyllodes tumour with a noninvasive ductal carcinoma
component. Virchows Arch. 432:89–93. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim SM and Tan PH: Ductal carcinoma in
situ within phyllodes tumour: A rare occurrence. Pathology.
37:393–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leong AS and Meredith DJ: Tubular
carcinoma developing within a recurring cystosarcoma phyllodes of
the breast. Cancer. 46:1863–1867. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grove A and Kristensen Deibjerg L:
Intraductal carcinoma within a phyllodes tumor of the breast: A
case report. Tumori. 72:187–190. 1986.PubMed/NCBI
|
|
28
|
Ward RM and Evans HL: Cystosarcoma
phyllodes. A clinicopathologic study of 26 cases. Cancer.
58:2282–2289. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yasumura T, Matsui S, Hamajima T,
Nagashima K, Yamagishi H, Aikawa I, Oka T, Nakae T and Shimada N:
Infiltrating ductal carcinoma developing within cystosarcoma
phyllodes-a case report. Jpn J Surg. 18:326–329. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kodama T, Kameyama K, Mukai M, Sugiura H,
Ikeda T and Okada Y: Invasive lobular carcinoma arising in
phyllodes tumor of the breast. Virchows Arch. 442:614–616.
2003.PubMed/NCBI
|
|
31
|
Ramdass MJ and Dindyal S: Phyllodes breast
tumour showing invasive squamous-cell carcinoma with invasive
ductal, clear-cell, secretory and squamous components. Lancet
Oncol. 7:8802006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deodhar KK, Baraniya JB, Naresh KN, Shinde
SR and Chinoy RF: Cancerization of phyllodes tumour.
Histopathology. 30:98–99. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seemayer TA, Tremblay G and Shibata H: The
unique association of mammary stromal sarcoma with intraductal
carcinoma. Cancer. 36:599–605. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klausner JM, Lelcuk S, Ilia B, Inbar M,
Hammer B, Skornik Y and Rozin RR: Breast carcinoma originating in
cystosarcoma phyllodes. Clin Oncol. 9:71–74. 1983.PubMed/NCBI
|
|
35
|
Hunger E, Turk R and Wurster K: Malignant
cystosarcoma phylloides and squamous cell carcinoma of the breast.
A rare tumor combination. Geburtshilfe Frauenheilkd. 44:640–642.
1984.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alò PL, Andreano T, Monaco S, Sebastiani
V, Serpieri Eleuteri D and Di Tondo U: Malignant phyllode tumor of
the breast with features of intraductal carcinoma. Pathologica.
93:124–127. 2001.(In Italian). PubMed/NCBI
|
|
37
|
Nomura M, Inoue Y, Fujita S, Sakao J,
Hirota M, Souda S and Ohshima M: A case of noninvasive ductal
carcinoma arising in malignant phyllodes tumor. Breast Cancer.
13:89–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Macher-Goeppinger S, Marme F, Goeppert B,
Penzel R, Schirmacher P, Sinn HP and Aulmann S: Invasive ductal
breast cancer within a malignant phyllodes tumor: Case report and
assessment of clonality. Hum Pathol. 41:293–296. 2010. View Article : Google Scholar : PubMed/NCBI
|