Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide and can be categorized into two major
histopathological groups: Non-small cell lung cancer (NSCLC) and
small cell lung cancer (1).
Approximately 80% of cases of human lung cancer are NSCLC, which
itself can be subcategorized into adenocarcinoma, squamous cell
carcinoma, adenosquamous carcinoma, large cell carcinoma and
sarcomatoid carcinoma (2). Current
treatments, including radiotherapy, chemotherapy and surgery, for
NSCLC exhibit limited effectiveness and the prognosis remains poor,
with an overall 5-year survival rate of only 15% (3). The poor outcome of lung cancer is
primarily explained by the difficulty of early detection and
anatomic localization of the tumors (4). Therefore, the identification of
biomarkers that are expressed in NSCLC is important to elucidate
the critical molecular events of these tumors, to accurately
predict patient prognosis and to identify the most suitable
pathways to target using novel therapeutic agents. A number of
independent prognostic factors have been suggested for predicting
survival and aiding in the management of patients with lung cancer
(5).
Wild-type p53-induced phosphatase 1 (Wip1), also
termed PPM1D, is a member of the protein phosphatase 2C family
(6). It was originally identified in
Burkitt's lymphoma cells during a screen for p53 target genes
induced by ionizing radiation (7).
Subsequently, Wip1 has been implicated as a negative regulator of
p53 via its ability to attenuate p38 mitogen-activated protein
kinase (MAPK) activity. Wip1 dephosphorylates at least six
proteins: Ataxia telangiectasia mutated, checkpoint kinase 1
(Chk1), Chk2, p53, p38 and Mdm2 (8).
These proteins are DNA damage response markers and there expression
is commonly decreased in DNA damage response pathways, which
contribute to cancer progression (8,9). Previous
studies have demonstrated that Wip1 is characterized by distinctive
oncogenic properties and is overexpressed in a wide range of tumor
tissues (10–14), including lung adenocarcinoma (15). However, previous studies have not
investigated the expression of Wip1 in different types of NSCLC or
the association between Wip1 expression and overall survival (OS)
of patients with NSCLC.
The present study aimed to detect the expression of
Wip1 in NSCLC tissues and to analyze its prognostic value in
patients with NSCLC. Wip1 protein expression was detected in 117
NSCLC and 15 normal lung tissue samples using immunohistochemistry.
Detailed clinical and demographic information was retrospectively
collected from the patients up to 5 years after surgery.
Kaplan-Meier survival and Cox's regression analyses were performed
to evaluate the prognosis of these patients.
Materials and methods
Tumor specimens
A total of 117 patients with NSCLC were admitted for
surgical treatment at the Hebei Chest Hospital (Shijiazhuang,
China) between January 2001 and December 2010. The cohort included
87 male and 30 female patients with a mean age of 56.9 years. Only
the NSCLC patients with no metastasis and suitable for surgery were
included in the present study. In addition, no patients received
any treatment, including radiation or chemotherapy, prior to the
surgery. Demographic and pathological tumor characteristics of the
patients were collected prior to the initial surgery. Normal lung
tissue samples (n=15) were obtained from patients who undergone
bronchiectasis surgery. These samples were dehydrated in an alcohol
series (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) (75%
for 1 h, 85% for 1 h, 95% for 4 h and 100% for 2 h), cleaned in
xylene (Sinopharm Chemical Reagent Co., Ltd.) and embedded in
paraffin (Wanyao Chemical Technology Co., Ltd, Shijiazhuang, China)
to prepare 5 µm serial paraffin sections, and then stained by
hematoxylin and eosin solution (Sigma-Aldrich, St. Louis, MO, USA)
and confirmed as normal using light microscopy (DM IL LED; Leica
Microsystems GmbH, Wetzlar, Germany). According to the World Health
Organization classification system (16), tumor specimens were
histopathologically diagnosed by two or more experienced
pathologists as adenocarcinoma (n=53), squamous cell carcinoma
(n=44), adenosquamous carcinoma (n=9), large cell carcinoma (n=6)
and sarcomatoid carcinoma (n=5). According to the TNM staging
system (17), the specimens
represented 25 pathological (p)-Stage I, 20 p-Stage II, 65 p-Stage
III and 7 p-Stage IV tumors. The histological type and grade were
confirmed by microscopic examination (DM IL LED; Leica Microsystems
GmbH) of hematoxylin and eosin-stained tissue slides
(Sigma-Aldrich, St. Louis, MO, USA). All patients were
consecutively enrolled in this study, and prognostic factors and
disease progression were retrospectively collected. The study was
approved by the Ethics Committee of Hebei Chest Hospital
(Shijiazhuang, China) and informed consent was obtained from all
recruited subjects.
Immunohistochemistry
The tissue sections were deparaffinized in xylene
(Sinopharm Chemical Reagent Co., Ltd.), hydrated with 100% and 95%
ethanol (Sinopharm Chemical Reagent Co., Ltd.), and then rinsed in
distilled water. Endogenous peroxidase was blocked with 0.1%
H2O2 (Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) for 20 min. The sections were prepared
by microwave antigen retrieval in 10 mM citrate buffer (pH 6.0;
Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 10 min. The
slides were subsequently incubated with serum blocking solution
(Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 1 h at 37°C,
rabbit anti-human polyclonal anti-Wip1 primary antibody (catalog
no., SC-20712; 1:100 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C, goat anti-rabbit biotinylated
secondary antibody (catalog no., ZB2010; dilution, 1:100; Zhongshan
Golden Bridge Biotechnology Co., Ltd.) for 1 h at 37°C and
streptavidin-horseradish peroxidase. 3,3′-Diaminobenzidine solution
(Sigma-Aldrich) was used as a chromogen. The slides were then
counterstained in a hematoxylin solution (Sigma-Aldrich) and
visualized on the DM IL LED microscope (Leica Microsystems GmbH).
Negative controls were performed by omitting the primary antibody
incubation step.
Scoring of immunohistochemistry
Immunostaining of the tissues was graded
semi-quantitatively considering the staining intensity results
determined by two pathologists that were blinded to the
clinicopathological variables. The staining intensity was scored
using the following scale of four grades: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining of cancer
cells. Wip1 expression in the cancer tissue was defined as positive
when the staining intensity score was 2 or 3.
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 statistical software (SPSS Inc., Chicago, IL, USA).
Data were presented as the mean ± standard deviation. The
associations between Wip1 expression and categorical variables were
analyzed by Pearson's χ2 test; excluding age, which was
analyzed by Student's t-test. The survival curves were estimated
using the Kaplan-Meier method and the statistical significance
between survival curves was assessed using the log-rank test. OS
was determined from the date of surgery to the date of mortality.
Significant variables from the univariate analysis were entered
into the Cox proportional hazard model. P<0.05 indicated a
statistically significant difference and all tests were
two-sided.
Results
Wip1 expression in NSCLC by
immunohistochemistry
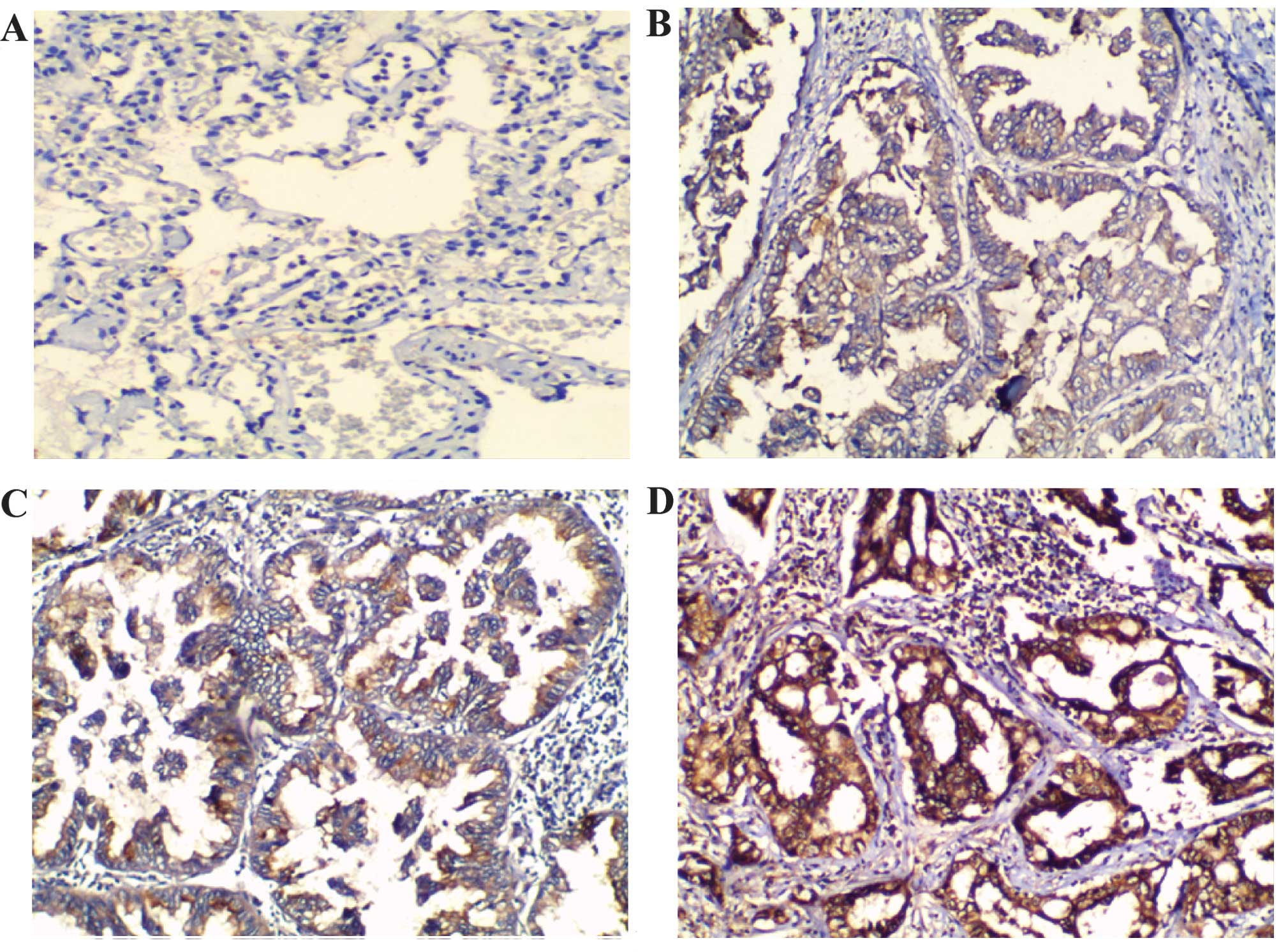
Immunohistochemistry was used to analyze the
expression of Wip1 protein in the NSCLC tissues. In the normal lung
tissues, the expression of Wip1 was not detected or was weakly
expressed (Fig. 1A). However,
cytoplasmic staining was observed in the human NSCLC tissue samples
(Fig. 1B–D). Scoring of the
immunohistochemical slides revealed positive Wip1 expression
(immunostaining score, 2/3) in 69.3% (81/117) of NSCLC samples.
Weak Wip1 expression (immunostaining score, 0/1) was observed in
30.7% (36/117) of NSCLC samples (Table
I).
 | Table I.Association between Wip1
immunostaining scores and NSCLC histological subtype. |
Table I.
Association between Wip1
immunostaining scores and NSCLC histological subtype.
|
| Wip1 immunostaining
score, n (%) |
|---|
|
|
|
|---|
| NSCLC subtype | 0 | 1 | 2 | 3 | Total |
|---|
| Adenocarcinoma | 3 (5.7) | 3 (5.6) | 44 (83.0) | 3 (5.7) | 53 (88.7) |
| Squamous cell
carcinoma | 5 (11.4) | 14 (31.8) | 23 (52.3) | 2 (4.5) | 44 (37.6) |
| Othersa | 4 (2.0) | 9 (45.0) | 6 (30.0) | 1 (5.0) | 20 (17.2) |
| Total | 12 (10.2) | 24 (20.5) | 75 (64.1) | 6 (5.2) | 117 (100) |
Association between Wip1 expression,
and demographic and pathological factors of NSCLC
Statistical analysis using the χ2 test
identified that of the status of Wip1 expression was correlated
with demographic and pathological factors of the tumors (Table II). Notably, Wip1 overexpression was
predominantly observed in lung adenocarcinoma compared with other
histological subtypes of NSCLC (P<0.01; Table II); in p-Stage III–IV compared with
p-Stage I–II (P=0.045); and in pT2–4 tumors compared with pT1
tumors (P=0.004). However, no statistically significant correlation
was identified between Wip1 expression and the other demographic
and pathological factors of NSCLC analyzed, such as age, gender,
histological differentiation or pN classification (Table II).
 | Table II.Association between Wip1 expression,
and demographic and pathological tumor characteristics of patients
with NSCLC. |
Table II.
Association between Wip1 expression,
and demographic and pathological tumor characteristics of patients
with NSCLC.
|
| Wip1 protein status,
na |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | + | − | P-valueb |
|---|
| Age, years |
57.8±11.5c | 56.1±9.1c | 0.268 |
| Gender |
|
| 0.138 |
| Male | 57 | 30 |
|
|
Female | 24 | 6 |
|
| Histology |
|
| <0.001 |
|
Adenocarcinoma | 49 | 4 |
|
| Squamous
cell carcinoma | 25 | 19 |
|
|
Others | 7 | 13 |
|
| Differentiation |
|
| 0.249 |
| Well | 24 | 7 |
|
|
Moderate/poor | 57 | 29 |
|
| pT
classification |
|
| 0.004 |
| T1 | 12 | 14 |
|
| T2–4 | 69 | 22 |
|
| pN
classification |
|
|
0.157 |
| N0 | 45 | 25 |
|
| N1–3 | 36 | 11 |
|
| p-Stage |
|
| 0.045 |
| I–II | 55 | 11 |
|
|
III–IV | 26 | 15 |
|
Overexpression of Wip1 correlates with
the poor prognosis of NSCLC
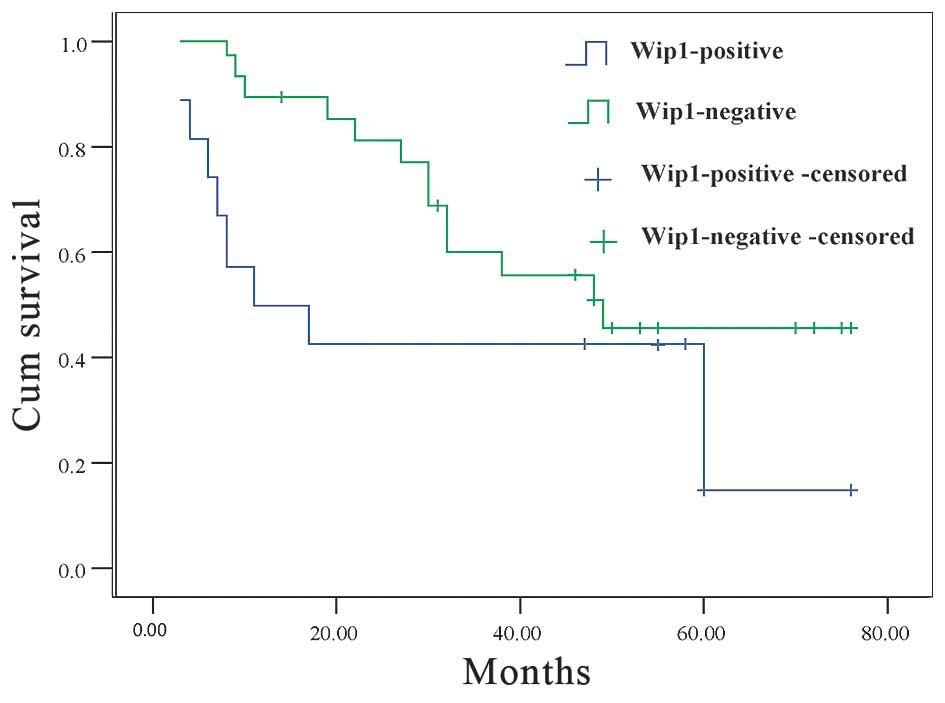
The OS of Wip1-negative and Wip1-positive groups
were examined. A statistically significant difference between the
two groups was observed using the log-rank test. The survival of
Wip1-negative patients was significantly longer than that observed
for the Wip1-positive patients (45.6 and 18.5% 5-year survival
rate, respectively; P=0.014; Fig.
2).
Multivariate analysis (Cox proportional hazard
model) for age, gender, histological differentiation, TNM stage and
overexpression of Wip1 was performed to examine the association
between possible prognostic factors and survival (Table III). It was identified that the pN
classification (P=0.022), p-Stage (P=0.013) and Wip1 overexpression
(P=0.009) were statistically significant predictors for OS
(Table III).
 | Table III.Cox proportional hazard model
analysis of survival time. |
Table III.
Cox proportional hazard model
analysis of survival time.
|
|
|
| 95% CI for Exp
(B) |
|
|---|
|
|
|
|
|
|
|---|
|
| Wald | Exp (B) | Lower | Upper | P-value |
|---|
| Wip1 (+ vs.
-)a | 6.815 | 5.096 | 1.501 | 17.303 | 0.009 |
| p-Stage (I–II vs.
III–IV) | 6.150 | 0.159 | 0.037 | 0.680 | 0.013 |
| pT classification
(T1 vs. T2–4) | 1.502 | 4.712 | 0.395 | 26.210 | 0.220 |
| pN classification
(N0 vs. N1–3) | 5.218 | 5.488 | 1.273 | 23.647 | 0.022 |
| Histology
(adenocarcinoma vs. non-adenocarcinomab) | 0.006 | 1.564 | 0.561 | 9.377 | 0.937 |
| Differentiation
(well vs. moderate/poor) | 1.688 | 0.306 | 0.051 | 1.825 | 0.194 |
| Gender (male vs.
female) | 1.185 | 4.439 | 0.303 | 5.005 | 0.276 |
| Age | 0.143 | 0.991 | 0.948 | 1.037 | 0.705 |
Discussion
The present study determined that: i) Wip1 is highly
expressed in NSCLCs; ii) the OS rate for patients in the
Wip1-positive expression group was significantly lower than that of
the Wip1-negative group, as determined by survival analysis; and
iii) positive Wip1 expression, pN classification and p-Stage are
significant prognostic predictors of NSCLC, as determined by Cox
multivariate analysis.
Human Wip1 protein has a molecular weight of ~61 kD
and is composed of 605 amino acids (6). Wip1 has been identified in colon cells
expressing a mutant form of p53, and mediates a negative feedback
regulation on p53 through the p38MAPK/p53 signaling pathway,
leading to decreased expression of p53 and p53 mutants, suggesting
a close association between Wip1 and p53 (18). A number of previous reports also
identified Wip1 overexpression in mouse embryonic fibroblasts and in
accelerated cancer progression (19–21). To
the best of our knowledge, the present study is the first to
quantify Wip1 expression in different types of lung cancer and
normal lung tissue using immunohistochemistry. It was observed that
Wip1 protein levels were significantly higher in lung cancer tissue
compared with normal tissue, and Wip1 overexpression was observed
predominantly in lung adenocarcinoma. The current results are
consistent with a previous finding that Wip1 mRNA expression is
significantly higher in the early stages of NSCLC (22) and with the observation of increased
Wip1 expression in lung adenocarcinoma (15). Using univariate analysis, Zhao et
al identified that Wip1 mRNA levels were correlated with the
p-Stage of NSCLC (22). The present
study also revealed that Wip1 protein levels were correlated with
p-Stage and pT classification: Overexpression was predominantly
observed in p-Stage III–IV (versus p-Stage I–II) and pT2–4 tumors
(versus pT1 tumors). However, no statistically significant
correlations were found in NSCLCs between Wip1 expression, and
other demographic and pathological factors, such as age, gender,
histological differentiation and pN classification.
An underlying hypothesis of the modern era of cancer
research has been that prediction of a patient's prognosis or
response to therapy may be improved by combining standard clinical
variables (i.e., tumor size, differentiation or stage) with
intrinsic genetic or biochemical characteristics of the tumors
(23). Overexpression of Wip1 in lung
adenocarcinoma, pancreatic neuroendocrine tumors, endometrial
cancer and gliomas is has previously been identified as a
prognostic indicator for certain patients at a high risk of
tumor-related mortality (13,15,24,25). Over
the past several decades, hundreds of papers have proposed a
variety of molecular markers or proteins that may have prognostic
significance in NSCLC; for example, p53 (26). Satoh et al reported that there
was a statistically significant association between increased Wip1
expression and lower OS rate (15).
In the current study, survival analysis identified that the OS rate
for patients with NSCLC in the Wip1-positive expression group was
significantly lower than that of the Wip1-negative group.
Furthermore, Cox multivariate analysis revealed that positive Wip1
expression, as well as the two clinical variables pN classification
and p-Stage, were significant prognostic predictors of NSCLC.
In conclusion, the present study demonstrated that
Wip1 is overexpressed in NSCLC and that Wip1 overexpression is
significantly associated with poorer OS in NSCLC. Therefore, Wip1
status appears to be an independent molecular marker associated
with worse clinical outcomes and prognosis of NSCLC. However, the
mechanism responsible for the role of Wip1 in tumorigenesis and its
biological function merits further evaluation.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Hebei Province (no. H2013315011).
References
|
1
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun S, Schiller JH, Spinola M and Minna
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciancio N, Galasso MG, Campisi R, Bivona
L, Migliore M and Di Maria GU: Prognostic value of p53 and Ki67
expression in fiberoptic bronchial biopsies of patients with non
small cell lung cancer. Multidiscip Respir Med. 7:292012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moorhead GB, Trinkle-Mulcahy L and
Ulke-Lemée A: Emerging roles of nuclear protein phosphatases. Nat
Rev Mol Cell Biol. 8:234–244. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiscella K, Franks P and Shields CG:
Perceived family criticism and primary care utilization:
Psychosocial and biomedical pathways. Fam Process. 36:25–41. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Nguyen TA, Moon SH, Darlington Y,
Sommer M and Donehower LA: The type 2C phosphatase Wip1: An
oncogenic regulator of tumor suppressor and DNA damage response
pathways. Cancer Metastasis Rev. 27:123–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gorgoulis VG, Vassiliou LV, Karakaidos P,
Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr,
Kastrinakis NG, Levy B, et al: Activation of the DNA damage
checkpoint and genomic instability in human precancerous lesions.
Nature. 434:907–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castellino RC, De Bortoli M, Lu X, Moon
SH, Nguyen TA, Shepard MA, Rao PH, Donehower LA and Kim JY:
Medulloblastomas overexpress the p53-inactivating oncogene
WIP1/PPM1D. J Neurooncol. 86:245–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito-Ohara F, Imoto I, Inoue J, Hosoi H,
Nakagawara A, Sugimoto T and Inazawa J: PPM1D is a potential target
for 17q gain in neuroblastoma. Cancer Res. 63:1876–1883.
2003.PubMed/NCBI
|
|
12
|
Hirasawa A, Saito-Ohara F, Inoue J, Aoki
D, Susumu N, Yokoyama T, Nozawa S, Inazawa J and Imoto I:
Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas
with poor prognosis and identification of PPM1D and APPBP2 as
likely amplification targets. Clin Cancer Res. 9:1995–2004.
2003.PubMed/NCBI
|
|
13
|
Hu W, Feng Z, Modica I, Klimstra DS, Song
L, Allen PJ, Brennan MF, Levine AJ and Tang LH: Gene amplifications
in well-differentiated pancreatic neuroendocrine tumors inactivate
the p53 pathway. Genes Cancer. 1:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Yang Y, Peng Y, Austin RJ, van
Eyndhoven WG, Nguyen KC, Gabriele T, McCurrach ME, Marks JR, Hoey
T, et al: Oncogenic properties of PPM1D located within a breast
cancer amplification epicenter at 17q23. Nat Genet. 31:133–134.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satoh N, Maniwa Y, Bermudez VP, Nishimura
K, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Hurwitz J and
Hayashi Y: Oncogenic phosphatase Wip1 is a novel prognostic marker
for lung adenocarcinoma patient survival. Cancer Sci.
102:1101–1106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Tumours of the lung. World Health Organization
Classification of Tumours. Pathology and Genetics of Tumours of the
Lung, Pleura, Thymus and Heart. IARC Press. (Lyon). 102004.
|
|
17
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JY, Song JY, Kim HM, Han HS, Seol HS,
Jang SJ and Choi J: p53-Independent expression of wild-type
p53-induced phosphatase 1 (Wip1) in methylmethane sulfonate-treated
cancer cell lines and human tumors. Int J Biochem Cell Biol.
44:896–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulavin DV, Demidov ON, Saito S,
Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G,
Nebreda AR, Anderson CW, et al: Amplification of PPM1D in human
tumors abrogates p53 tumor-suppressor activity. Nat Genet.
31:210–215. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nannenga B, Lu X, Dumble M, Van Maanen M,
Nguyen TA, Sutton R, Kumar TR and Donehower LA: Augmented cancer
resistance and DNA damage response phenotypes in PPM1D null mice.
Mol Carcinog. 45:594–604. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demidov ON, Kek C, Shreeram S, Timofeev O,
Fornace AJ, Appella E and Bulavin DV: The role of the MKK6/p38 MAPK
pathway in Wip1-dependent regulation of ErbB2-driven mammary gland
tumorigenesis. Oncogene. 26:2502–2506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao JX, ZW, Sun L, Luo HH and Gu Y:
Expression of Wip1 in non small cell lung cancer tissue and cells.
Chin J Pathophysiol. 25:1731–1735. 2009.(In Chinese).
|
|
23
|
Singhal S, Vachani A, Antin-Ozerkis D,
Kaiser LR and Albelda SM: Prognostic implications of cell cycle,
apoptosis, and angiogenesis biomarkers in non-small cell lung
cancer: A review. Clin Cancer Res. 11:3974–3986. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirasawa A, Aoki D, Inoue J, Imoto I,
Susumu N, Sugano K, Nozawa S and Inazawa J: Unfavorable prognostic
factors associated with high frequency of microsatellite
instability and comparative genomic hybridization analysis in
endometrial cancer. Clin Cancer Res. 9:5675–5682. 2003.PubMed/NCBI
|
|
25
|
Liang C, Guo E, Lu S, Wang S, Kang C,
Chang L, Liu L, Zhang G, Wu Z, Zhao Z, et al: Over-expression of
wild-type p53-induced phosphatase 1 confers poor prognosis of
patients with gliomas. Brain Res. 1444:65–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campling BG and El-Deiry WS: Clinical
implication of p53 mutation in lung cancer. Mol Biotechnol.
24:141–156. 2003. View Article : Google Scholar : PubMed/NCBI
|