Introduction
Dermatomyositis is a type of idiopathic inflammatory
myopathy (1). Bohan and Peter
(1) proposed five major criteria for
the diagnosis dermatomyositis and another idiopathic inflammatory
myopathy, polymyositis, in 1975: Progressive symmetrical weakness,
muscle-biopsy evidence, including muscle fiber swelling, absence of
striations and muscle fibers revealing vacuolar degeneration,
elevated enzymes in skeletal muscle serum, abnormal electromyogram
and dermatological features. Bohan and Peter (1) also suggested five subsets of myositis:
Polymyositis, dermatomyositis, dermatomyositis associated with
neoplasia, child dermatomyositis associated with vasculitis and
polymyositis or dermatomyositis with associated collagen-vascular
disease. Subsequent to this, Saoud et al (2) recognized an additional subset:
Amyopathic dermatomyositis. Furthermore, Callen (3) previously reported that dermatomyositis
is associated with malignant tumors. Teratomas are a type of germ
cell tumor comprising well-differentiated tissues and 3 germ cell
layers: Ectoderm, mesoderm and endoderm. They are divided into
mature (benign) and immature (malignant) teratomas, and
dermatomyositis may be associated with malignant or benign tumors.
The current study presents a case of dermatomyositis accompanied by
a benign ovarian teratoma.
Case report
A 27-year-old female patient with a 3-month history
of bilateral orbital and facial edema accompanied by skin erythema
and rash accepted treatment at the Department of Rheumatology of
the Affiliated Hospital of Qingdao University (Qingdao, China) and
was admitted on December 13, 2012. The left lesion was more
critical than the right. Limb muscles were occasionally sore,
however, the patient reported no joint pain, proximal limb
weakness, fever or hypersensitivity to light. A muscle biopsy did
not demonstrate characteristic alterations of perifascicular
atrophy and inflammation in the patient. The patient accepted
hydrocortisone (20 mg, orally once a day) treatment in the
Department of Dermatology and Ophthalmology (Affiliated Hospital of
Qingdao University). Following receipt of this treatment regime the
patient's symptoms were mildly relieved. However, once treatment
was terminated two weeks later, the symptoms became worse than upon
presentation. The patient reported no weight loss, decreased
appetite or history of infections, but had undergone an
appendectomy due to appendicitis 20 years previously and was
allergic to levofloxacin. The patient was unmarried, had no
children, had reached menarche at 13 years of age and reported a
regular menstrual cycle with no dysmenorrhea. Furthermore, a family
history revealed that the mother had hypertension, the father was
healthy and there was no family history of genetic diseases.
Examination of the patient revealed a temperature of
36.6°C (normal range, 36.9–37.3°C), a pulse of 80 beats/min (normal
range, 60–100 beats/min), a respiratory rate of 20 breaths/min
(normal range, 18–22 breaths/min) and blood pressure of 120/80 mmHg
(normal range, 90–130/60–85 mmHg). Scattered hemorrhagic rashes
were observed on the patient's face and neck, a number of which had
formed ulcerations. There was no lymphadenopathy. Edema was
observed on the face and eyelid, accompanied by characteristic
dermatological lesions (heliotrope rash). However, the ears,
eyeballs, nose, throat and mouth were normal. Stethoscopy of both
lungs revealed clear and smooth breathing. The patient's cardiac
rate was regular, and sounded strong with no murmur. The abdomen
was flat, soft and not painful. Upon examination of the limbs and
spine, the strength and tension of the limb muscles were normal.
There were no erythematous macules on elbows, knees or medial
ankles. Urinary, reproductive system and nervous system
examinations were normal. The patient did not have weak neck flexors
or Gower maneuver.
Laboratory analysis revealed a white blood cell
count of 10.75×109 cells/l (71.40% neutrophils, 18.90%
lymphocyte, 9.30% mononuclear cells, 0.30% eosinophils and 0.10%
basophils; normal range, 4.00–10.00×109 cells/l), a red
blood cell count of 4.28×1012 cells/l (normal range,
3,50–5.00×1012 cells/l), a hemoglobin level of 130.00
g/l (normal range, 110.00–150.00 g/l), a hematocrit level of 41.10%
(normal range, 37.00–47.00%) and a platelet count of
202.00×109/l (normal range, 100.00–300.00×109
cells/l). Creatine kinase (CK) was markedly elevated [1,543.2 U/l
(normal range, 0.0–170.0 U/l)], and CK-MB isoenzyme [27.4 U/l
(normal range, 0.0–17.0 U/l)], α-hydroxybutyric dehydrogenase
[188.4 U/l (normal range, 72.0–182.0 U.l)] and aspartate
aminotransferase [AST; 65.3 U/l (normal range, 0.0–42.0 U/l)] were
mildly elevated. However, alanine aminotransferase (48.2 U/l;
normal range, 0–60 U/l), lactate dehydrogenase (237.6 U/l; normal
range, 91–245 U/l) and γ-glutamyl transferase levels (9.5 U/l;
normal range, 0–64 U/l) were normal. Rheumatological evaluation
revealed that the expression of the following anti-nuclear
antibodies were all negative: Anti-SSA, anti-SSB, anti-Scl-70,
anti-polymyositis/Scl, anti-Jo-1, anti-Sm and anti-double stranded
DNA antibody. Cytoplasmic, perinuclear, protease 3 and
myeloperoxidase anti-nuclear antibody, as well as anti-glomerular
basement membrane antibody, were normal. The patient's
immunoglobulin (Ig) levels (IgG, 950 mg/dl; normal range, 751–1560
mg/dl; IgA, 136 mg/dl; normal range, 82–453 mg/dl; IgM, 232 mg/dl;
normal range, 46–304 mg/dl; IgE, 93.24 IU/ml; normal range, 0–100
IU/ml), complement levels (C3, 91.2 mg/dl; normal range, 79–152
mg/dl; C4, 16.40 mg/dl; normal range, 16–38 mg/dl) and erythrocyte
sedimentation rate [10.5 mm/1 h (normal range, 0.0–20.0 mm/1 h)]
were also normal. Cancer marker analysis revealed positivity for
carbohydrate antigen 125 [CA125; 68.15 U/ml (normal range, 0–35
U/ml)], and negativity for carcinoembryonic antigen, α-fetoprotein,
carbohydrate antigen 19–9, neuron-specific enolase, squamous cell
carcinoma antigen and β-human chorionic gonadotropin.
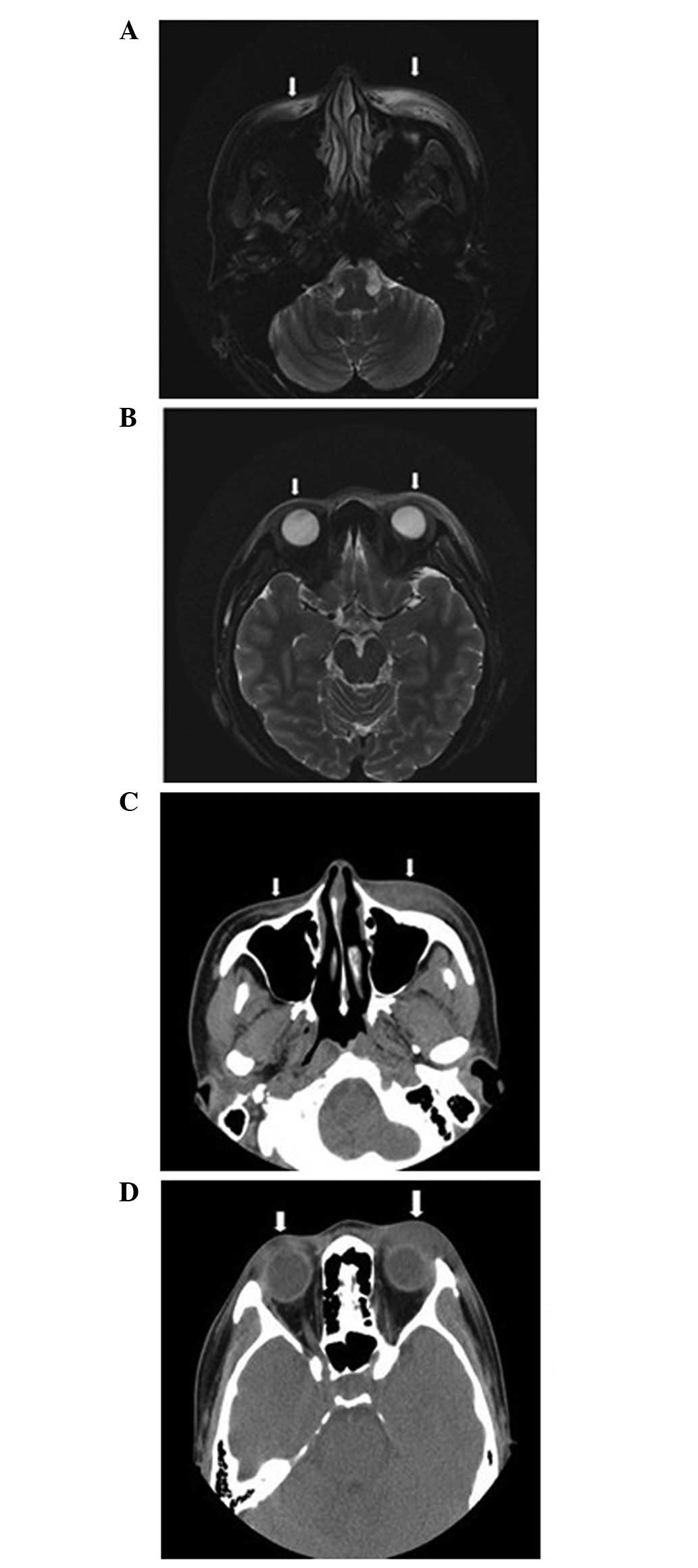
Magnetic resonance imaging (MRI; Signa HDxt 3.0T; GE
Healthcare Life Sciences, Chalfont, UK) revealed thickening and
swelling of the soft tissue of the bilateral eyelids and bilateral
buccal regions; furthermore, subcutaneous long T1- and T2-weighted
signal intensity (fat saturation) was compatible with inflammatory
infiltration (Fig. 1A and B). There
were no abnormal signals near the bilateral eyeballs or muscles
around the eyes and posterior orbital. Computed tomography (CT;
Brilliance iCT; Philips Healthcare, DA Best, The Netherlands) scans
of the bilateral eyelids and bilateral buccal regions were
consistent with the MRI findings (Fig. 1C
and D). There were no obvious abnormalities upon pelvic X-ray
and thoracic CT. B-mode ultrasound of the neck indicated no
abnormalities in the thyroid or in lymph nodes located in neck
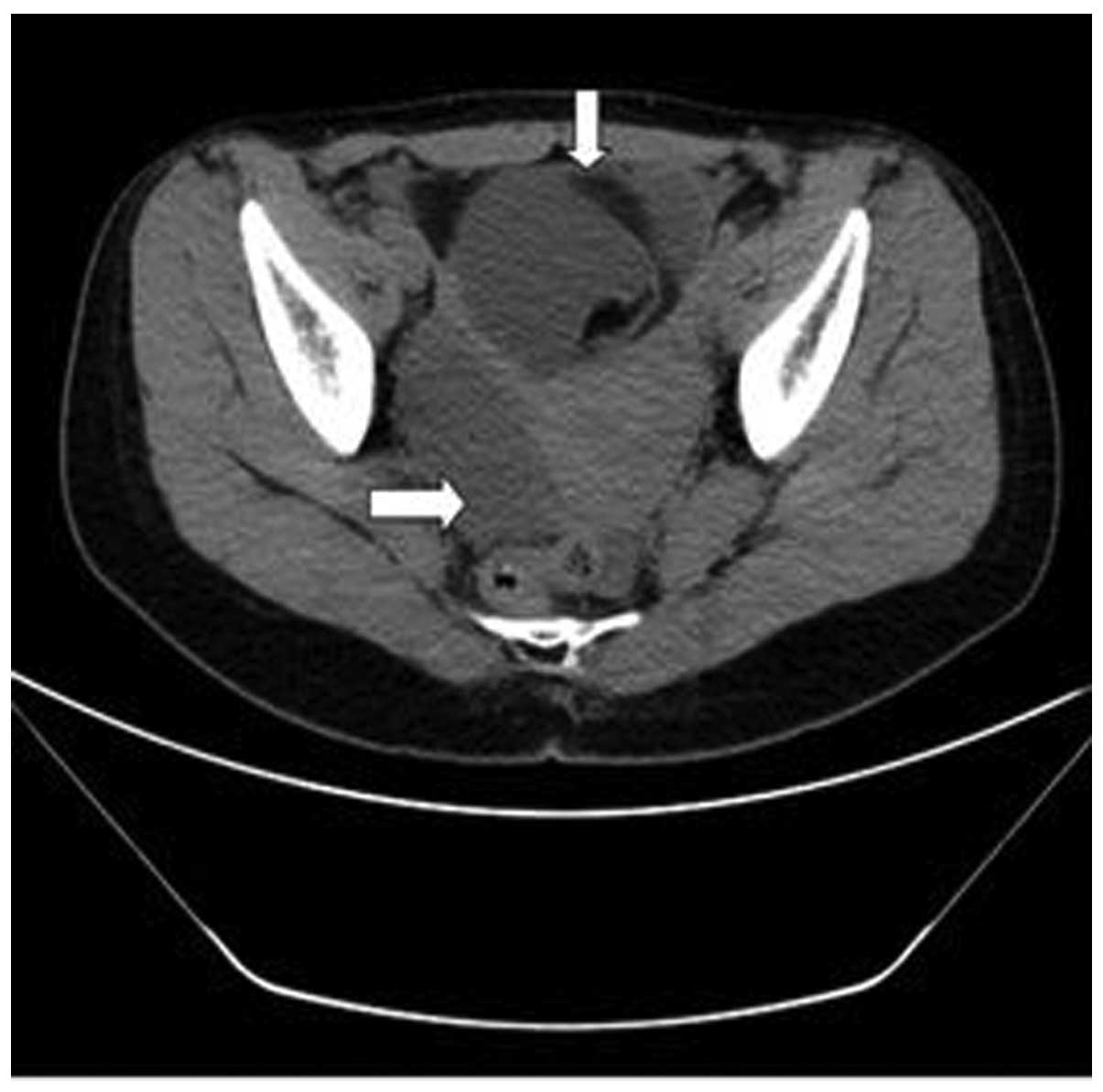
subcutaneous tissue. Non-enhanced CT of the upper abdomen, lower
abdomen and pelvic cavity revealed no significant abnormalities of
the liver, kidney or bladder, respectively. However, a multilocular
cystic mass in the pelvic cavity with a significant fat component
was observed at the right accessory region, and the anterior wall
of the uterus was thickened (Fig. 2).
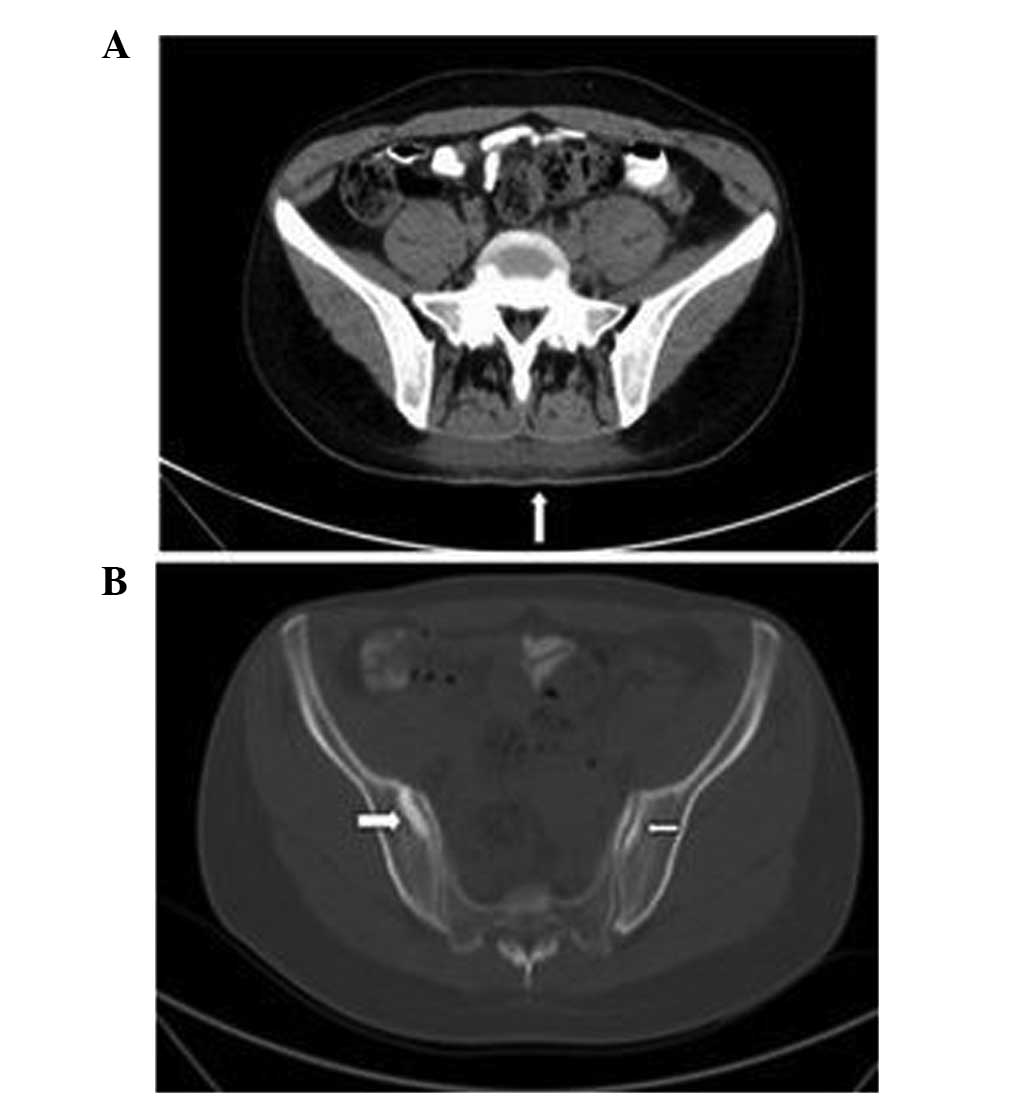
Additionally, CT indicated blurred subcutaneous fat clearance of
the back and high-density signals (Fig
3A). Bone destruction and high-density signals were also
observed on the bilateral sacroiliac joint surface (Fig. 3B). Gynecological ultrasound findings
indicated an ovarian teratoma (8.7×7.8×9.3 cm) and uterine myoma
(3.6×3.1 cm).
Initially, the patient accepted treatment of
hydrocortisone (20 mg, orally once a day) in the outpatient clinic
of the Department of Dermatology and Ophthalmology (Affiliated
Hospital of Qingdao University) for two weeks between November 2012
and December 2012. Following receipt of this treatment regime the
patient's dermatological symptoms were mildly relieved. However,
when treatment was terminated the dermatological symptoms worsened,
and became more severe than at presentation. Subsequently, between
December 2012 and January 2013 methylprednisolone (40 mg,
intravenous drip; administered 4 times in the 1 month period) and
methotrexate (5 mg, intravenous injection; administered 4 times in
the 1 month period) therapy were administered at the Department of
Rheumatology (Affiliated Hospital of Qingdao University). However,
prior to surgery, there was no obvious relief in the patient's
dermatological symptoms and the level of CK remained elevated
(1131.8–1543.2 U/l). The patient was diagnosed with dermatomyositis
due to the dermatological features, elevated CK and sacroiliac
arthritis on December 25, 2012. The ovarian teratoma and uterine
myoma were laprascopically removed in the Department of Obstetrics
and Gynecology of the Affiliated Hospital of Qingdao University
(Qingdao, China) on January 10, 2013, and diagnosed as benign right
ovary cystic mature teratoma and uterine myoma by analysis of the
biopsy. In order to perform this diagnosis, tissue was embedded in
a paraffin block and sliced into 10-mm sections using a microtome
(SYD-S3020; Shenyang LongShou Electronic Instrument Co., Ltd.,
Shenyang, China), followed by hematoxylin and eosin staining
(Shanghai Biyuntian Bio-Technology Co., Ltd., Shanghai, China). One
week after surgical removal of the teratoma, the dermatological
symptoms were significantly relieved. The level of CK was 248.7
U/l, and the results of additional laboratory tests (CA125, CK-MB
isoenzyme, AST) had returned to normal. The patient was discharged
from hospital on January 21, 2013. Following discharge, the patient
continued to receive oral methotrexate (10 mg) and
methylprednisolone (28 mg). When the patient's CK was followed-up 2
weeks later, the level was normal (130 U/l) and dermatological
symptoms were completely relieved. Follow-up consisted of
observation of the facial skin lesions and the levels of CK, CKMB
and lactate dehydrogenase of the patient, which after two weeks of
follow up were as follows: CK, 160 U/l (normal range, 0–170 U/l);
CKMB, 15 U/l (normal range,0-17 U/l); lactate dehydrogenase, 230
U/l (normal range, 91–245 U/l). The patient is currently alive and
well, with no signs of recurrence.
Discussion
Callen and Wortmann (4) presented images of characteristic
cutaneous lesions and clinical manifestation of dermatomyositis. In
a study by Callen (2), it was
reported that dermatomyositis is associated with malignant tumors,
including those of the ovary, lung, pancreas, stomach, colon or
rectum, as well as non-Hodgkin's lymphoma. Ibarra et al
(5) described a case report of
juvenile dermatomyositis accompanied by a benign teratoma. An
8-year-old female patient presented with of right arm pain,
weakness in both legs and difficulty in arising from a seated or
squatting position that was ongoing for 4 months, as well as 1
month of pain in the hips, ankles and knees (5). Following rheumatological evaluation,
including elevated levels of IgG and IgE, the patient was diagnosed
with juvenile dermatomyositis. After surgical removal of the
teratoma, the myositis, synovitis and cutaneous findings resolved
over 4 months without additional therapy (5). Benign teratomas have also been
associated with paraneoplastic syndromes, including paraneoplastic
limbic encephalitis (6–10), opsoclonus-myoclonus syndrome (11), paraneoplastic polyarthritis (12) and autoimmune hemolytic anemia
(13). Titulaer et al
(14) performed a screen for tumors
in paraneoplastic syndromes to aid the early detection of
tumors.
In conclusion, to the best of our knowledge, the
current case report provides a novel observation of dermatomyositis
associated with a benign ovarian teratoma in an adult. The case
also provides evidence that elevated levels of CK and the presence
of heliotrope rash are associated with benign ovarian teratoma,
despite the lack of progressive symmetrical weakness, muscle-biopsy
evidence and abnormal electromyogram. We propose that benign
ovarian teratoma may cause dermatomyositis (as in the present case
it was proposed that the teratoma occurred before the
dermatomyositis, as it was thought to be unlikely that the teratoma
could have grown to a size of 8.7×7.8×9.3 cm in 3 months) in
addition to paraneoplastic syndromes, as previously reported. The
present study appears to expand the range of disease entities known
to be caused by benign ovarian teratoma beyond paraneoplastic
syndromes. The present study appears to expand the range of disease
entities known to be caused by benign ovarian teratoma beyond the
previously reported paraneoplastic syndromes. The current case also
serves as a reminder of the process of dermatomyositis clinical
diagnosis and treatment.
References
|
1
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (first of two parts). N Engl J Med. 292:344–347.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saoud B, Allali F and Hassouni NH:
Amyopathic dermatomyositis. Joint Bone Spine. 73:318–320. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Callen JP: Relation between
dermatomyositis and polymyositis and cancer. Lancet. 357:85–86.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Callen JP and Wortmann RL:
Dermatomyositis. Clin Dermatol. 24:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ibarra M, Chou P and Pachman LM: Ovarian
teratoma mimicking features of juvenile dermatomyositis in a child.
Pediatrics. 128:e1293–e1296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadalage G, Karim A and Jacob S:
Autoimmune encephalitis screen-a review of rapid diagnostic
screening in 600 patients over 5 years. J Neurol Neurosurg
Psychiatry. 84:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KG: Paraneoplastic limbic encephalitis
associated with ovarian teratoma. Clin Med. 12:95–96. 2012.
View Article : Google Scholar
|
|
8
|
Yang YW, Tsai CH, Chang FC, Lu MK and Chiu
PY: Reversible paraneoplastic limbic encephalitis caused by a
benign ovarian teratoma: Report of a case and review of
literatures. J Neurooncol. 80:309–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanyi JL, Marsh EB, Dalmau J and Chu CS:
Reversible paraneoplastic encephalitis in three patients with
ovarian neoplasms. Acta Obstet Gynecol Scand. 91:630–634. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu MH, Huang CC, Hung PL, Huang HM, Huang
LT, Huang CC, Sheen JM, Huang SC and Chang YC: Paraneoplastic
neurological disorders in children with benign ovarian tumors.
Brain Dev. 36:248–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fitzpatrick AS, Gray OM, McConville J and
McDonnell GV: Opsoclonus-myoclonus syndrome associated with benign
ovarian teratoma. Neurology. 70:1292–1293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiese W, Alansari H, Tranchilda P and
Madrid FF: Paraneoplastic polyarthritis in an ovarian teratoma. J
Rheumatol. 31:1854–1857. 2004.PubMed/NCBI
|
|
13
|
Kim I, Lee JY, Kwon JH, Jung JY, Song HH,
Park YI, Ro E and Choi KC: A case of autoimmune hemolytic anemia
associated with an ovarian teratoma. J Korean Med Sc. 21:365–367.
2006. View Article : Google Scholar
|
|
14
|
Titulaer MJ, Soffietti R, Dalmau J, Gilhus
NE, Giometto B, Graus F, Grisold W, Honnorat J, Sillevis Smitt PA,
Tanasescu R, et al: Screening for tumours in paraneoplastic
syndromes: Report of an EFNS task force. Eur J Neurol. 18:19–e3.
2011. View Article : Google Scholar : PubMed/NCBI
|