Introduction
The incidence of colorectal cancer (CRC) has
increased over time, and it has become a major health problem
around the world. A previous study demonstrated that disease
recurred in 25–50% of examined patients with stage II/III CRC
within 5 years, despite receiving curative surgery (1). The most common site of recurrence and
distant metastases is the liver, and therefore, it is important to
control liver metastases to improve the prognosis of patients with
CRC (2).
5-Fluorouracil (5FU) is a significant standard
chemotherapy agent for advanced CRC (node positive cancer or
metastatic cancer) together with leucovorin and irinotecan or
oxaliplatin (3,4). 5FU improves the median overall and
disease-free survival rates and reduces recurrence rates in
patients who have undergone curative surgical resection (5). However, in the adjuvant setting, the
mechanism by which 5FU acts on liver metastases from CRC remains
unclear. It has been hypothesized that this delayed recurrence is
due to cancer dormancy. Cancer dormancy is a stage of
micrometastasis but the dormant cells are clinically undetectable
(6). To the best of our knowledge, no
previous studies have used intravital imaging to examine the
efficacy of 5FU on the microenvironment at the single-cell level in
an adjuvant setting.
Since Denk et al (7) first described 2-photon laser scanning
microscopy (TPLSM) in 1990, it has been used in the field of
biological imaging and has become a common tool in biological
laboratories. Although TPLSM has been used to observe various
structures, including blood vessels, neurons, and bone marrow
(8–10), little information is available on the
use of TPLSM to investigate liver metastasis from CRC in
vivo. Intravital TPLSM imaging has enabled the imaging of
metastatic tumors, platelets in vessels, and tumor angiogenesis in
addition to time-series imaging of liver metastases in the same
mouse at the single-cell level (11–17). The
present authors previous study established a method to visualize
the real-time in vivo response to chemotherapy using
intravital TPLSM, which enabled the study of dynamic interactions
in living mice (11). Using this
technique, the intravital imaging of the effects of 5FU on the
microenvironment after tumor formation were determined (11); however, intravital imaging of
micrometastasis has not previously been examined in the adjuvant
setting (11). The aim of the present
study was to confirm the mechanism in early stages of liver
metastasis by which 5FU affects tumor cells at the single-cell
level in living mice by using intravital TPLSM.
In the present study, the cell number in vessels in
the observation field was quantified to evaluate the response to
chemotherapy of the tumor microenvironment of liver metastatic
xenografts. In addition, the effects of 5FU on the microenvironment
in the adjuvant setting were studied and how 5FU acted on
micrometastasis was observed using TPLSM.
Materials and methods
GFP nude mice and human CRC cell
line
Green fluorescent protein (GFP)-expressing nude mice
(C57BL/6-BALB/c-nu/nu-EGFP) were purchased from AntiCancer Japan
Inc. (Osaka, Japan). GFP nude mice (8–9 weeks old) were housed in
groups of 5/sterile cage. The mice were kept in the animal house,
according to the Institutional Animal Care Guidelines. The
experimental protocols were reviewed and approved by the Animal
Care and Use Committee of the Mie University Graduate School of
Medicine (Tsu, Japan). A red fluorescent protein (RFP)-expressing
human CRC cell line (HT29) was purchased from AntiCancer Japan
Inc.
Experimental micrometastasis
model
A total of 30 mice were divided into 6 groups (n=5).
RFP-HT29 cells were injected into the spleens of GFP nude mice as a
xenogenic tumor model. The tumor cells were adjusted to
1×107 cells/ml as single-cell suspensions. GFP nude mice
were anesthetized using isoflurane inhalation (4%, Abbott
Laboratories, Chicago, IL, USA). A total of 1×106 cells
were injected into the spleens of anesthetized GFP nude mice using
a 30-gauge needle through a small incision in the left lateral
abdomen.
Surgical procedures for intravital
TPLSM
Immediately following injection, GFP nude mice were
anesthetized via 1.0% isoflurane inhalation (300 ml/min). The liver
lobe was then put into an organ-stabilizing system (Japanese Patent
Application number; P2007-129723) (11).
TPLSM setup
The procedures for TPLSM setup were performed as
previously described (11).
Experiments were performed using an upright microscope (BX61WI;
Olympus Corporation, Tokyo, Japan) and an FV1000-2P laser-scanning
microscope system (Fluoview FV1000MPE, Olympus Corporation). The
excitation source in TPLSM mode was Mai Tai® Ti:Sapphire lasers
(Spectra-Physics, Santa Clara, CA, USA), tuned and mode locked at
910 nm. The Mai Tai laser produced light pulses of ~100 femtosecond
width (repetition rate, 80 MHz). Laser light reached the sample
through the microscope objectives, connected to an upright
microscope (BX61WI). According to the depth of imaging, mean laser
power at the sample was altered from 10 to 40 mW, depending on the
depth of imaging. The microscope objective lenses used in this
study were as follows: UPLSAPO 4x(numerical aperture, 0.16),
UPLSAPO 10x (numerical aperture, 0.4), and LMPLNFI/IR 60x (water
dipping; numerical aperture, 0.9; working distance, 2 mm) (Olympus
Corporation). Data were analyzed using the FV10-ASW version 2.0
system (Olympus Corporation). TPLSM images were acquired with
512×512 pixels spatial resolution, from 210 µm field of view
dimension, using a pixel dwelling time of 4 µsec (11).
The timing of intravital TPLSM
imaging
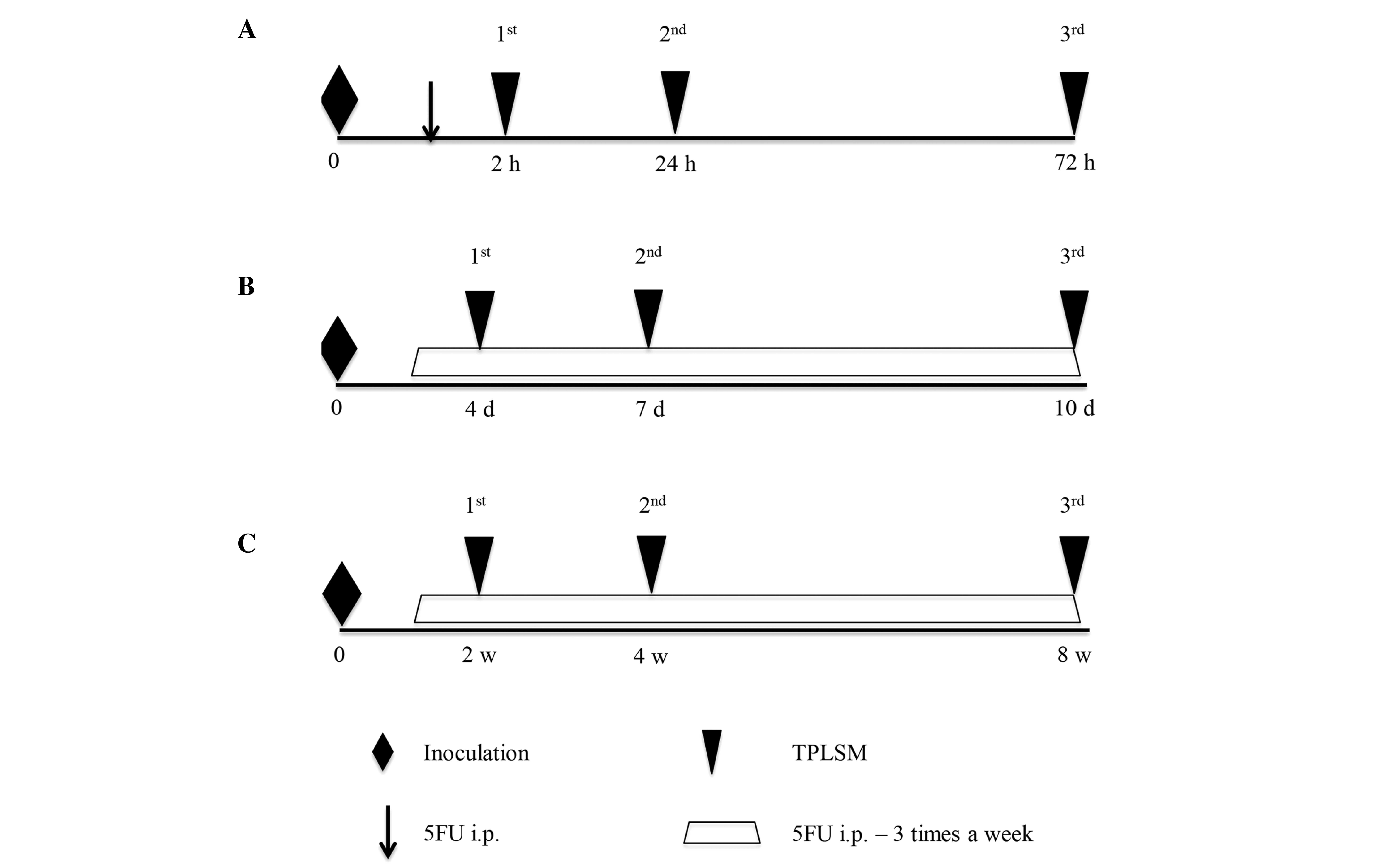
TPLSM was performed in 3 stages of metastasis. The
experimental protocol and timing of intravital TPLSM are described
in Fig. 1. TPLSM imaging was
performed using an upright microscope (BX61W1) and an FV1000 laser
scanning microscope system (Fluoview FV1000MPE). Three stages
(early, middle, and late stages) were studied in the different mice
and they kept alive during the entire time. For each stage, 10 mice
were used and divided into 2 groups (n=5). One treatment group was
injected with 5FU (Sigma-Aldrich, St. Louis, MO, USA), and the
other control group was injected with phosphate-buffered saline
(PBS). In the early stage (Fig. 1A),
TPLSM was first performed 2 h following the injection of RFP-HT29
cells into the GFP nude mice to observe early extravasation from
circulating tumor cells. TPLSM imaging was represented clearly as a
Z-stack 3-dimensional movie by scanning the images from the liver
surface. Tumors were counted in a voxel of 210×210 nm × depth (~100
µm). The livers of mice in the early stage group (5 mice), were
observed by first measuring the depth of the observation fields to
quantify the cell number in the vessel in the observation fields.
TPLSM was repeated at 24 and 72 h after the injection of RFP-HT29
cells to observe early hepatic metastasis. They were then monitored
at regular intervals and randomized to receive 5FU (25 mg/kg in a
50-µl volume) or an equivalent volume of PBS (50 µl) via
intraperitoneal injection. Injections were performed 3 times a week
from 1 h after the first injection.
In the middle stage mice (Fig. 1B), TPLSM was performed on day 4, 7,
and 10, to observe middle-stage liver metastasis. In the late stage
mice (Fig. 1C), TPLSM was performed
at 2, 4, and 8 weeks after the first injection of RFP-HT29 cells
into the spleens of GFP nude mice to observe late-stage liver
metastasis, when micrometastatic changes and macroscopic metastatic
changes would have occurred.
Statistical analysis
Statistical analysis was performed using
Mann-Whitney's-U-test on StatView version 5.0 software (SAS
Institute, Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Early stage
The results of the TPLSM imaging were represented
clearly as a Z-stack 3-dimensional movie by scanning the images
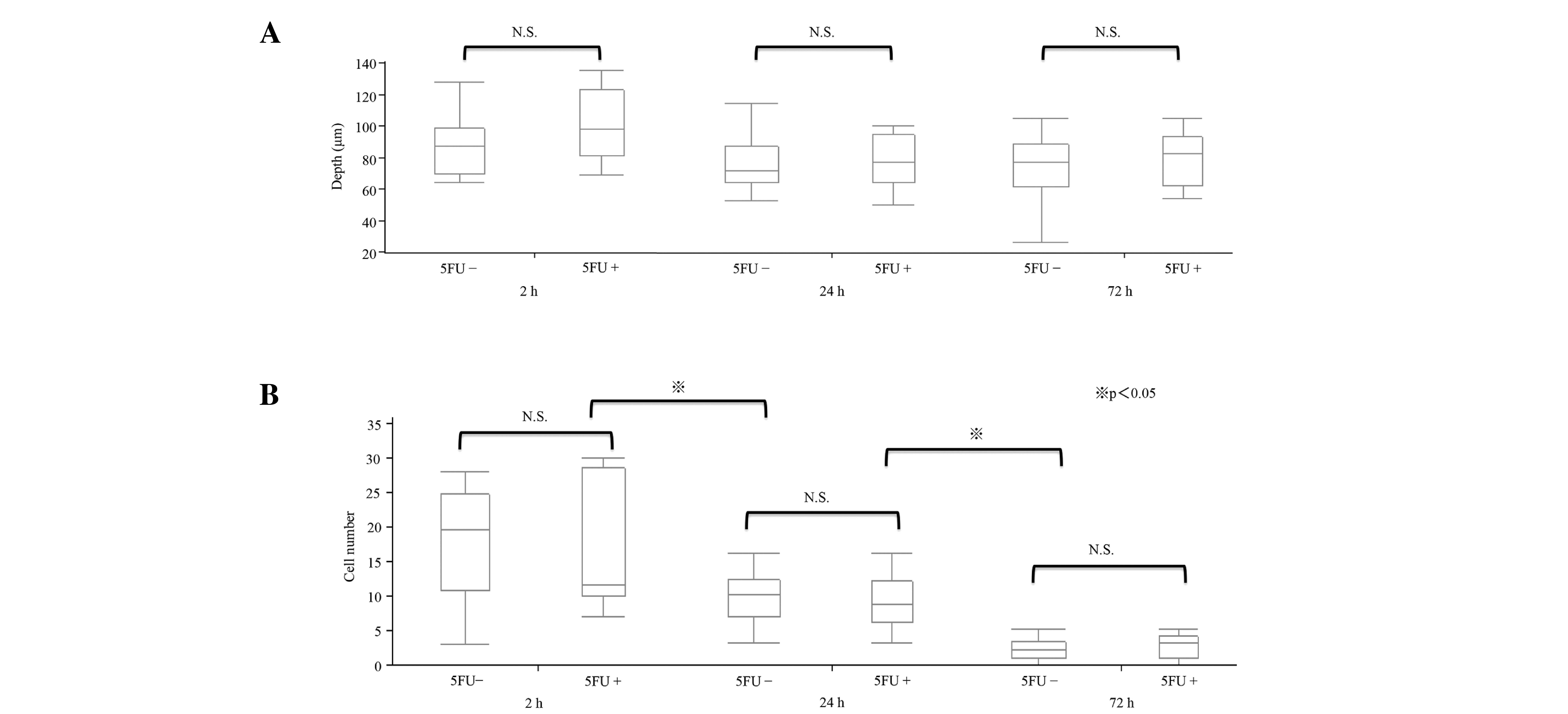
from the liver surface to a depth of ~100 µm. On observing the
livers of mice in the early stage group (5 mice), the depth of the
observation fields was observed to quantify the cell number in the
vessel in the observation fields, and the depth of ~100 µm was
observed multiple times (Fig. 2A).
The cells were counted in a voxel of 210×210 nm × depth (~100 µm).
The number of tumor cells caught in the hepatic sinusoid was
counted and the mean was calculated for 5 mice. The depth observed
was not significantly different among the different fields of the
liver. The number of cells in the hepatic sinusoid gradually
reduced over time (P<0.05; Fig.
2B). At 2 h, 17.9±7.9 cells were counted in the voxel. At 24
and 72 h, 9.5±3.7 and 2.3±0.4 cells were counted in the voxel,
respectively.
Regarding the early stage in vivo response to
5FU, similar results were observed for the 5FU-treated groups at
the 3 time points (2, 24, and 72 h). The tumor cell count at 2 h
was 16.5±9.2 in the 5FU group, compared with 17.9±7.9 in the
control group. At 24 and 72 h, 8.8±3.9 and 2.2±1.8 cells were
counted in the voxel, respectively, in the 5FU-treated groups.
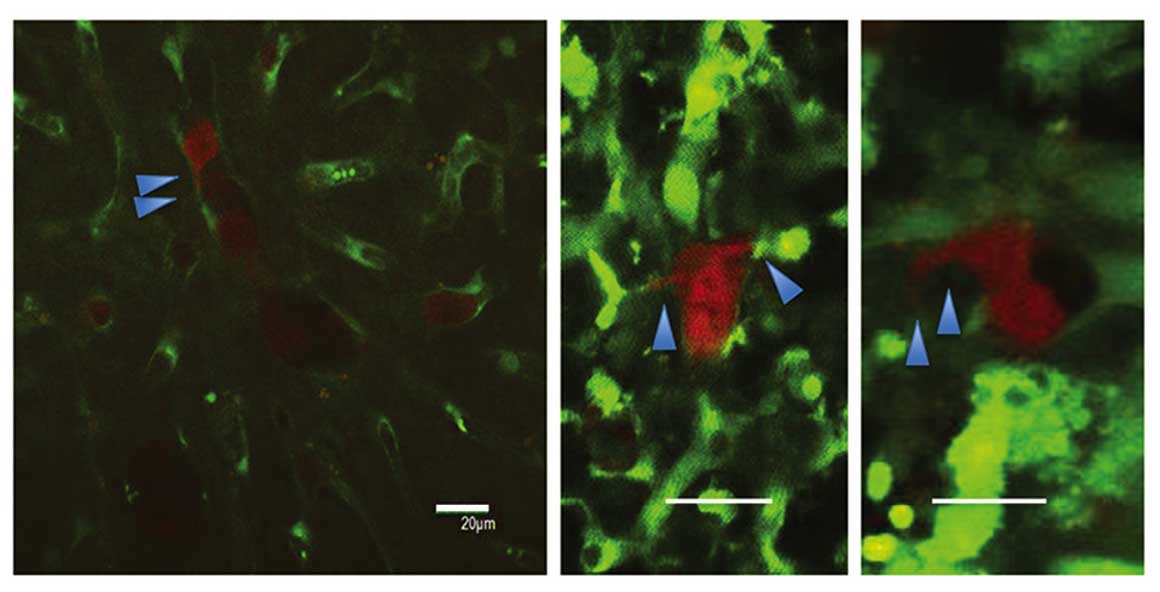
Regarding the 2-h intravital TPLSM, several RFP-HT29
cells were present with platelets in the hepatic sinusoids, and the
majority of these cells were circle-shaped. In the 24-h intravital
TPLSM, a number of RFP-HT29 cells had been removed from the
sinusoids and the number of adhesive platelets had increased. In
addition, invadopodia were observed in both groups (Fig. 3). A greater number of invadopodia were
observed in the control groups compared with the 5FU groups,
however the difference in number was not significant. For the 24-h
TPLSM, extravascular metastatic foci of RFP-HT29 cells were
observed and a small number of cells were present in the hepatic
sinusoids. Due to the presence of inflammation, it was not clear
whether the tumor cells within the surrounding hepatic tissues had
changed. These changes occurred under the influence of
inflammation. Similar results were observed for the 5FU groups
(Fig. 4A).
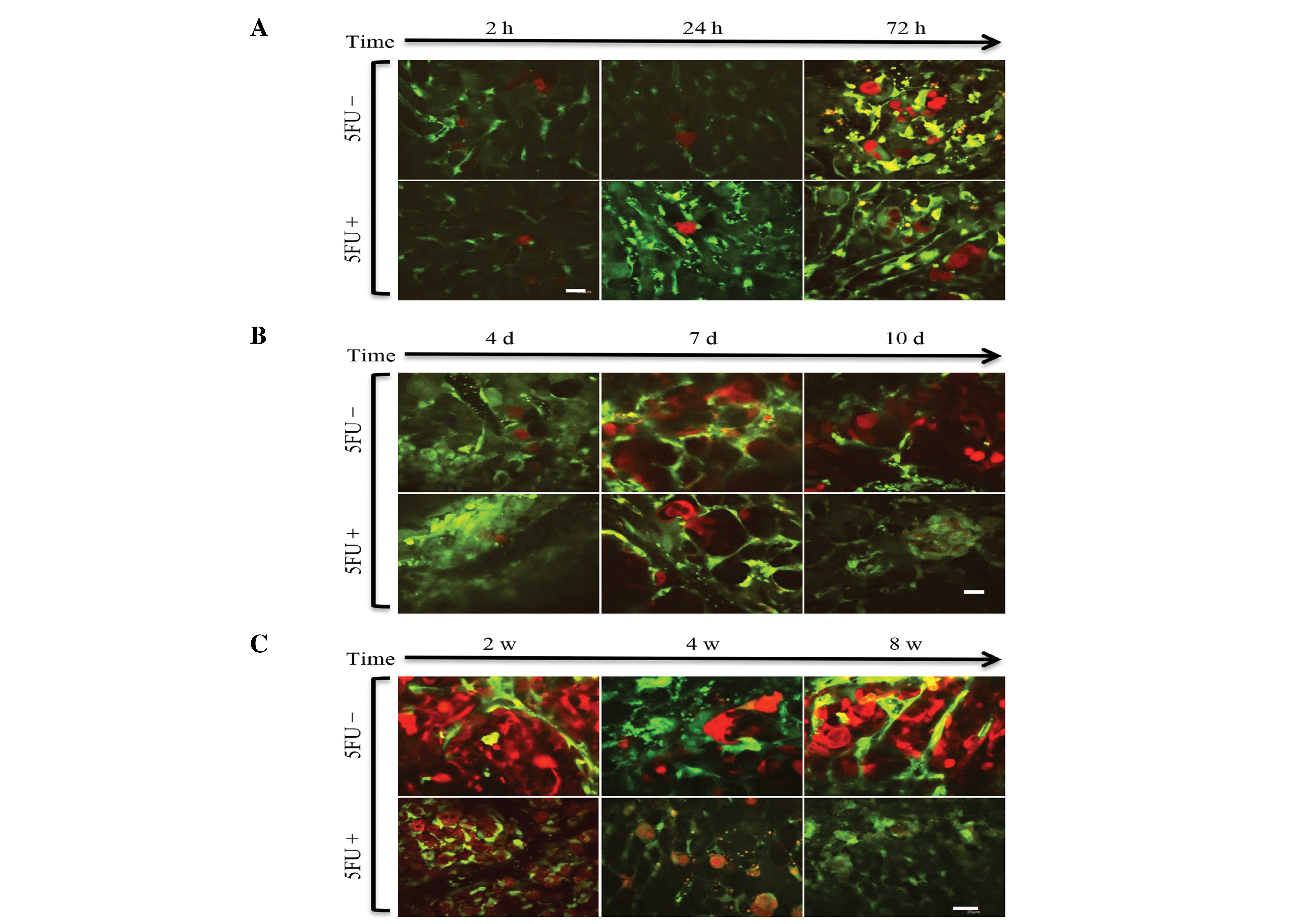 | Figure 4.(A) Early-stage TPLSM images of the
liver in each group. Representative images were taken in the same
mouse. Arrested tumor cells in the hepatic sinusoid gradually
reduced over time. Images were captured 2, 24, and 72 h after the
injection of tumor cells in 5FU-treated and untreated mice (scale
bar, 20 µm). (B) Middle stage of time course TPLSM images of the
liver in each group. Representative images were captured in the
same mouse, 4, 7 and 10 days after injection with or without 5FU
treatment. Cell fragments due to apoptosis were observed (scale
bar, 20 µm). (C) Late-stage TPLSM images of the liver in each
group. Representative images were captured in the same mouse, 2, 4,
and 8 w after the injection of tumor cells in 5FU-treated and
untreated mice. Extravasated tumor cells in the hepatic tissues
(scale bar, 20 µm). |
Middle and late stages
Certain differences in microscopic appearance were
observed 10 days after the injection of RFP-HT29 cells. In the
control group, metastatic foci were observed, but in the 5FU
treated group, cell fragments due to apoptosis were observed
(Fig. 4B).
Two weeks after the injection of RFP-HT29 cells,
additional differences were noted in the macroscopic appearance
compared with the appearance at 10 days. At the 2-week intravital
TPLSM, cell apoptosis was observed. At the 4-week TPLSM, apparent
differences were noted between the 5FU and control groups. The
number of extravasated HT29 cells was reduced in the 5FU treatment
group, and an increase in cell fragments was observed. An increase
in the number of metastatic foci of HT29 cells was observed at the
4-week TPLSM compared with at the 2-week TPLSM. At the 8-week
TPLSM, increased liver metastatic colonization and tumor vessels
were visualized in the control groups; in the 5FU-treated groups, a
markedly reduced number of HT29 cells were observed in the voxel
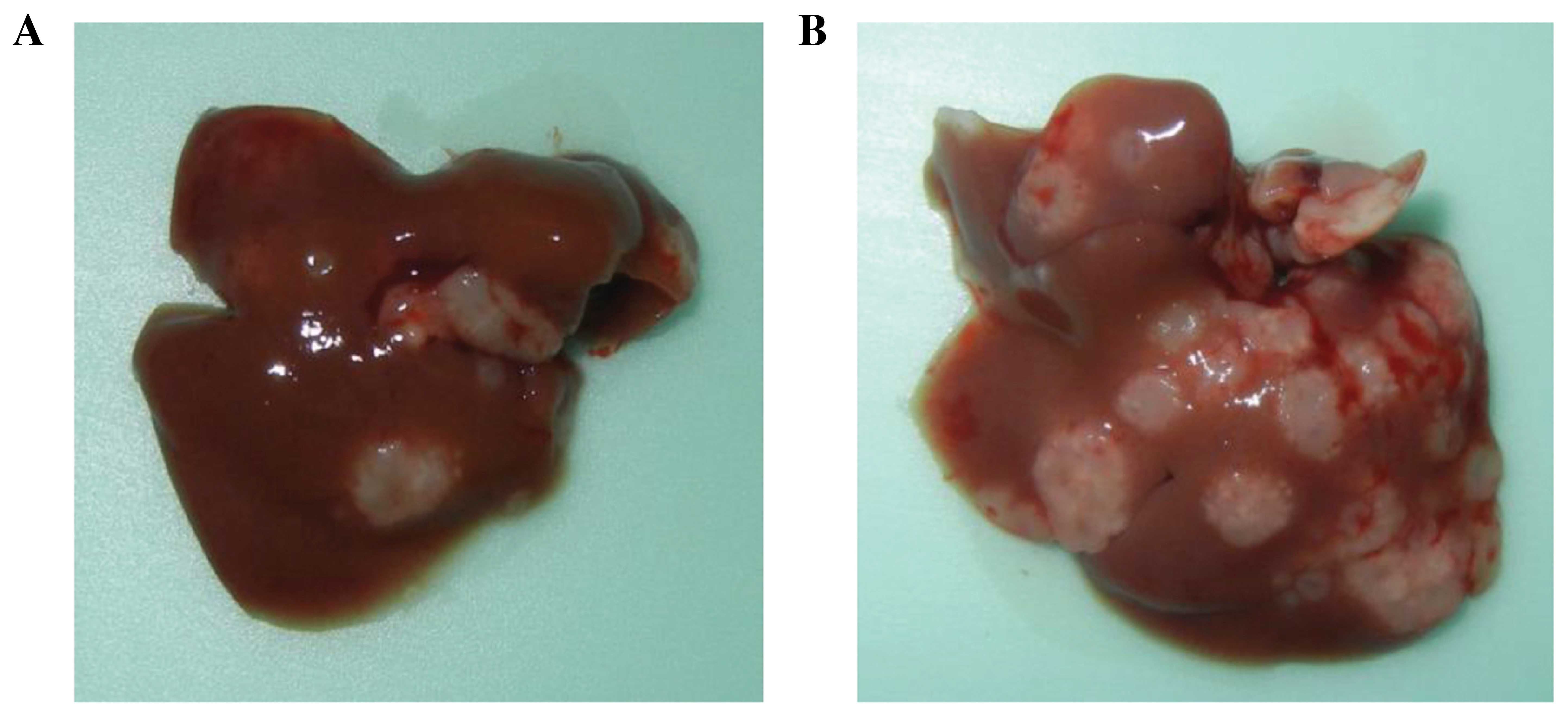
(Fig. 4C). In addition, liver
metastases were identified as macroscopic findings (Fig. 5). The results indicate that 5FU
inhibited the growth of CRC tumors in vivo.
Discussion
The findings of the present study revealed a
correlation between the inhibition of recurrence by 5FU and the
mechanism by which 5FU acts on liver metastases from CRC, at the
single-cell level. For stage II/III CRC, surgical removal of the
primary tumor is the only available curative intervention; however,
25–50% of patients relapse within 5 years (1). Relapse may be due to undetectable tumor
cells remaining following curative surgery. After curative surgery,
the need for effective chemotherapy to inhibit recurrence is clear.
In the 1990s, 2 clinical trials revealed that 5FU-based regimens
improved overall or recurrence-free survival in the adjuvant
setting (5,18). The aim of adjuvant chemotherapy is to
reduce the risk of local and distal recurrence of CRC.
5FU, a widely used cytotoxic agent, is a pyrimidine
analog that is converted intracellularly to a number of products
that bind to the enzyme thymidylate synthase and interfere with DNA
replication. At present, previous studies have investigated the
efficacy of 5FU in vitro, and a number of studies have
examined the effects of 5FU in subcutaneous xenografts
preclinically (19,20). However, to the best of our knowledge,
no previous study has evaluated the effect of 5FU on
micrometastatic foci in the process of suppressing liver metastasis
following surgery in vivo. Previous studies have reported
differences in the effects of treatment on subcutaneous and liver
metastatic xenografts (21,22). Therefore, the present study used a
liver metastatic xenograft as a preclinical model to evaluate an
anticancer drug. In the present authors previous study, a method
for intravital TPLSM imaging of liver metastasis was established at
the single-cell level using GFP-expressing mice (15,16). In
addition, the authors reported that TPLSM enabled observation at 3
time points and that the response of metastatic tumor cells to 5FU
in the microenvironment could be visualized (11). Martin et al (23) reported the ex vivo examination
of individual tumor cells in the liver and micrometastasis in
situ by using 2-photon microscopy, and they also reported the
influence of different microenvironments on tumor cell
extravasation. However, no prior study has investigated the
interaction of tumor cells with their microenvironment at the
earliest steps of metastasis in vivo. In the present study,
the response of circulating tumor cells to 5FU at the single-cell
level in the 3-dimensional microenvironment was visualized using
Z-stack imaging in the early stages of liver metastasis. To the
best of our knowledge, this is the first demonstration of the
effect of 5FU on liver micrometastasis at the single-cell level
prior to the development of liver metastasis in the murine model at
the earliest steps of metastasis. The point at which 5FU affects
the metastatic process was determined. In the early stage, there
was little change in the number of metastatic foci in response to
5FU. Morphological changes such as invadopodia and apoptosis were
also visualized.
The results of the present study revealed that 5FU
did not prevent tumor adhesion to the liver sinusoids and supported
the arguments of Warusavitarne et al (24) that 5FU treatment does not influence
invasion and metastasis in microsatellite-unstable colorectal
cancer in vitro. The results of the present study also
demonstrated that 5FU acted on HT29 cells as a cytotoxic agent
after the cells extravasated into the tissue parenchyma. These
results may lead to a better understanding of the earliest steps of
tumor-host interactions in the liver microenvironment.
In the middle and late stage groups, the mechanism
by which 5FU acted on the extravasated cells and the tumor cells
that began to proliferate were observed. As expected, 5FU inhibited
the growth of tumors. The present authors previously reported that
cancer cells in metastatic foci were involved in peripheral tumor
cell fragmentation and central tumor necrosis in the body and that
platelet aggregation occurred on endothelial cells within the
metastatic-focus tumor vessel (11).
This observation indicated that the tumor vessel endothelial cell
is an obstacle to successfully treating metastasis. A number of
phenomena, including peripheral tumor cell fragmentation and
central tumor necrosis, are observed when 5-FU is prescribed to
patients by metronomic scheduling. The findings of the present
study supported this hypothesis at the single-cell level by using a
liver metastatic mouse model under real-time TPLSM. As Kerbel et
al (25) and Colleoni et
al (26) proposed, the
endothelial damage or coagulation abnormality or tumor vascular
disorder may be associated with the mechanisms of metronomic
chemotherapy.
In metastatic cancer, the extravasation and
proliferation of cancer cells in the target organ and the
interactions between cancer cells and target organ stromal cells
serve important roles. To determine the effect of novel anticancer
agents for the purpose of metastatic control, it is important to
evaluate their therapeutic effects on cancer cells and host cells
in the microenvironment of metastatic foci in a liver metastasis
model. Therefore, the tumor-host interaction needs to be observed
at the single-cell level.
TPLSM has 2 major functions: i) The observation of
living tissues and cells (low toxicity) and ii) deep observation
(high permeability). TPLSM is attracting attention as a tool to
visualize the target and anticancer mechanism of novel cancer
drugs. TPLSM has been used to investigate bone marrow, blood
vessels, and the thymus (13,27–29).
Intravital TPLSM may reveal information missed by conventional
histopathological and molecular biological analyses. TPLSM may be
used to monitor the spatiotemporal relationships between cancer
cells and endothelial or blood cells.
Adjuvant chemotherapy often consists of cytotoxic
drugs, and is commonly used to target CRC. However, adjuvant
chemotherapy does not only target CRC cells. For example, the
target of bevacizumab is the host tissue (blood vessels).
A previous study demonstrated whole-body imaging in
metastatic models (30). Compared
with whole-body imaging, the strengths of TPLSM include the ability
to visualize tumors clearly at the single-cell level to evaluate
drug efficacy.
The present authors previously reported that TPSLM
enables the observation of tumor vessels in the surrounding stroma
in the same mouse at different time points (11). In the present study, the effects of a
cytotoxic drug at different time points were observed at the
cellular level. This strategy may be applied to observe the
efficacy of host-targeted drugs such as Bevacizumab by using the
methodology of quantification.
A limitation of the present study is that intravital
TPLSM only captures images to a depth of 200 µm in liver tissues,
and thus, it is not easily applicable to human tissues. In
addition, the organ-stabilizing system minimizes the artifacts
caused by heart and respiratory movements, but it is a technically
challenging procedure. There is therefore a need to improve the
procedure. However, technological advancements in fluorescence
microscopy will aid in revealing the mechanisms of cancer
progression and provide visual targets for novel therapeutics.
In conclusion, in the field of biological imaging,
intravital TPLSM may be a powerful tool to reveal the mechanisms of
the effects of drugs on cancer progression at the single-cell level
and to enable the visualization of targets of novel anticancer
therapeutics.
Acknowledgements
The present study was supported by grants from the
Ministry of Education, Culture, Sports, Science, and Technology of
Japan (KAKENHI; grant nos., 22591484, 21591723 and 21390377).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
5FU
|
5-Fluorouracil
|
|
RFP
|
red fluorescent protein
|
|
GFP mouse
|
green fluorescent protein-transgenic
mouse
|
|
TPLSM
|
2-photon laser-scanning microscopy
|
References
|
1
|
Desch CE, Benson AB III, Somerfield MR,
Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS
and Petrelli NJ: American Society of Clinical Oncology: Colorectal
cancer surveillance: 2005 update of an American society of clinical
oncology practice guideline. J Clin Oncol. 23:8512–8519. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed: Chemotherapy of
metastatic colorectal cancer: Fluorouracil plus folinic acid and
irinotecan or oxaliplatin. Prescrire Int. 14:230–233.
2005.PubMed/NCBI
|
|
4
|
Kong L, Wang X, Zhang K, Yuan W, Yang Q,
Fan J, Wang P and Liu Q: Gypenosides synergistically enhances the
anti-tumor effect of 5-fluorouracil on colorectal cancer in
vitro and in vivo: A role for oxidative stress-mediated
DNA damage and p53 activation. PLoS One. 10:e01378882015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA,
Tormey DC and Glick JH: Levamisole and fluorouracil for adjuvant
therapy of resected colon carcinoma. N Engl J Med. 322:352–358.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wikman H, Vessella R and Pantel K: Cancer
micrometastasis and tumour dormancy. APMIS. 116:754–770. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Denk W, Strickler JH and Webb WW:
Two-photon laser scanning fluorescence microscopy. Science.
248:73–76. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hickey MJ and Kubes P: Intravascular
immunity: The host-pathogen encounter in blood vessels. Nat Rev
Immunol. 9:364–375. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grutzendler J, Yang G, Pan F, Parkhurst CN
and Gan WB: Transcranial two-photon imaging of the living mouse
brain. Cold Spring Harb Protoc. 2011:pdb.prot0654742011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sumen C, Mempel TR, Mazo IB and von
Andrian UH: Intravital microscopy: Visualizing immunity in context.
Immunity. 21:315–329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka K, Okigami M, Toiyama Y, Morimoto
Y, Matsushita K, Kawamura M, Hashimoto K, Saigusa S, Okugawa Y,
Inoue Y, et al: In vivo real-time imaging of chemotherapy response
on the liver metastatic tumor microenvironment using multiphoton
microscopy. Oncol Rep. 28:1822–1830. 2012.PubMed/NCBI
|
|
12
|
Toiyama Y, Mizoguchi A, Okugawa Y, Koike
Y, Morimoto Y, Araki T, Uchida K, Tanaka K, Nakashima H, Hibi M, et
al: Intravital imaging of DSS-induced cecal mucosal damage in
GFP-transgenic mice using two-photon microscopy. J Gastroenterol.
45:544–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koike Y, Tanaka K, Okugawa Y, Morimoto Y,
Toiyama Y, Uchida K, Miki C, Mizoguchi A and Kusunoki M: In vivo
real-time two-photon microscopic imaging of platelet aggregation
induced by selective laser irradiation to the endothelium created
in the beta-actin-green fluorescent protein transgenic mice. J
Thromb Thrombolysis. 32:138–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morimoto Y, Tanaka K, Toiyama Y, Inoue Y,
Araki T, Uchida K, Kimura K, Mizoguchi A and Kusunoki M: Intravital
three-dimensional dynamic pathology of experi- mental colitis in
living mice using two-photon laser scanning microscopy. J
Gastrointest Surg. 15:1842–1850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka K, Morimoto Y, Toiyama Y, Okugawa
Y, Inoue Y, Uchida K, Kimura K, Mizoguchi A and Kusunoki M:
Intravital dual-colored visualization of colorectal liver
metastasis in living mice using two photon laser scanning
microscopy. Microsc Res Tech. 75:307–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka K, Morimoto Y, Toiyama Y,
Matsushita K, Kawamura M, Koike Y, Okugawa Y, Inoue Y, Uchida K,
Araki T, et al: In vivo time-course imaging of tumor angiogenesis
in colorectal liver metastases in the same living mice using
two-photon laser scanning microscopy. J Oncol. 2012:2654872012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka K, Toiyama Y, Inoue Y, Uchida K,
Araki T, Mohri Y, Mizoguchi A and Kusunoki M: Intravital imaging of
gastrointestinal diseases in preclinical models using two-photon
laser scanning microscopy. Surg Today. 43:123–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Gramont A, Krulik M, Cady J, Lagadec B,
Maisani JE, Loiseau JP, Grange JD, Gonzalez-Canali G, Demuynck B
and Louvet C: High-dose folinic acid and 5-fluorouracil bolus and
continuous infusion in advanced colorectal cancer. Eur J Cancer
Clin Oncol. 24:1499–1503. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torimura T, Ueno T, Taniguchi E, Masuda H,
Iwamoto H, Nakamura T, Inoue K, Hashimoto O, Abe M, Koga H, et al:
Interaction of endothelial progenitor cells expressing cytosine
deaminase in tumor tissues and 5-fluorocytosine administration
suppresses growth of 5-fluorouracil-sensitive liver cancer in mice.
Cancer Sci. 103:542–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffmann D, Bayer W and Wildner O: In situ
tumor vaccination with adenovirus vectors encoding measles virus
fusogenic membrane proteins and cytokines. World J Gastroenterol.
13:3063–3070. 2007.PubMed/NCBI
|
|
21
|
Wilmanns C, Fan D, Obrian C, Radinsky R,
Bucana C, Tsan R and Fidler I: Modulation of doxorubicin
sensitivity and level of P-glycoprotein expression in human
colon-carcinoma cells by ectopic and orthotopic environments in
nude-mice. Int J Oncol. 3:413–422. 1933.
|
|
22
|
Wilmanns C, Fan D, O'Brian CA, Bucana CD
and Fidler IJ: Orthotopic and ectopic organ environments
differentially influence the sensitivity of murine colon carcinoma
cells to doxorubicin and 5-fluorouracil. Int J Cancer. 52:98–104.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin MD, Kremers GJ, Short KW, Rocheleau
JV, Xu L, Piston DW, Matrisian LM and Gorden DL: Rapid
extravasation and establishment of breast cancer micrometastases in
the liver microenvironment. Mol Cancer Res. 8:1319–1327. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warusavitarne J, Ramanathan P, Kaufman A,
Robinson BG and Schnitzler M: 5-fluorouracil (5FU) treatment does
not influence invasion and metastasis in microsatellite unstable (M
SI-H) colorectal cancer. Int J Colorectal Dis. 21:625–631. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colleoni M, Orlando L, Sanna G, Rocca A,
Maisonneuve P, Peruzzotti G, Ghisini R, Sandri MT, Zorzino L, Nolè
F, et al: Metronomic low-dose oral cyclophosphamide and
methotrexate plus or minus thalidomide in metastatic breast cancer:
Antitumour activity and biological effects. Ann Oncol. 17:232–238.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishii M, Egen JG, Klauschen F,
Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL and Germain RN:
Sphingosine-1-phosphate mobilizes osteoclast precursors and
regulates bone homeostasis. Nature. 458:524–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hickey MJ and Kubes P: Intravascular
immunity: The host-pathogen encounter in blood vessels. Nat Rev
Immunol. 9:364–375. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bousso P, Bhakta NR, Lewis RS and Robey E:
Dynamics of thymocyte-stromal cell interactions visualized by
two-photon microscopy. Science. 296:1876–1880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang M, Baranov E, Jiang P, Sun FX, Li XM,
Li L, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T, et al:
Whole-body optical imaging of green fluorescent protein-expressing
tumors and metastases. Proc Natl Acad Sci USA. 97:1206–1211. 2000.
View Article : Google Scholar : PubMed/NCBI
|