Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous
group of clonal disorders that originate from early hematopoietic
progenitor cells. These syndromes are characterized by maturation
defects that lead to ineffective hematopoiesis, resulting in
anemia, leucopenia and thrombocytopenia (1). In the USA, the annual incidence rate for
MDS is 2–4 cases per 100,000 individuals. Notably, MDS are most
common in elderly individuals; the annual MDS incidence rate ranges
between 15 and 50 cases per 100,000 individuals in patients aged
>70 years (1). The majority of
patients ultimately succumb to infection and/or hemorrhage.
Thrombocytopenia (platelet count, <100×109/l) is
common in MDS, occurring in 40–65% of patients (2). In severe cases of thrombocytopenia
and/or hemorrhage, thrombocytopenia in MDS is treated via platelet
transfusions. However, platelet transfusions often lead to a brief
increase in platelet count and antibodies may be produced
subsequent to multiple transfusions, causing human leukocyte
antigen-alloimmunization. Furthermore, ≥30% of platelet
transfusions result in complications, including bacteremia,
graft-versus-host disease, acute pulmonary injury and exacerbated
thrombocytopenia (3,4).
5-Aza-2′-deoxycytidine (decitabine) is a known
inhibitor of DNA methylation and has been shown to exhibit a marked
antileukaemic effect (5). Decitabine
has been shown to induce response rates of 45–50% in elderly
high-risk MDS patients, even inducing trilineage response and
cytogenetic complete remission (CR) in ~30% of patients with
cytogenetic abnormalities, by increasing platelet count (6). However, the mechanism by which
decitabine increases the platelet count in patients with MDS
remains unclear (6–9).
The association between decitabine and cellular
differentiation was initially identified in the human
erythroleukemia cell line, K562. Decitabine was found to induce
irreversible hemoglobinization and morphological differentiation in
a dose-dependent manner (7,8). A number of previous studies have
suggested that the effects of decitabine treatment for
thrombocytopenia in MDS patients may be due to enhanced
megakaryocyte differentiation (6–12).
Therefore, to elucidate the mechanisms by which decitabine
increases platelet count in patients with MDS, and to investigate
the effects of decitabine on megakaryocyte differentiation and
platelet release, the present study used cluster of differentiation
(CD)41 (integrin, α IIb) as a cell surface marker for megakaryocyte
maturation (13). Furthermore, the
associated clinical data was collected prior to and after
decitabine treatment, allowing analysis of the association between
the clinical data and laboratory results.
Materials and methods
Patient characteristics
Patients with a hematological diagnosis of de
novo primary MDS were considered eligible for the present
study. A total of 20 MDS patients with thrombocytopenia (11 males,
9 females) with a median age of 55 years (range, 35–75 years) were
enrolled at the General Hospital of Tianjin Medical University
(Tianjin, China) between March 2013 and February 2014. According to
the World Health Organization criteria (14), 16 patients exhibited refractory anemia
with excess blasts (RAEB)-1 and 4 patients exhibited RAEB-2. Two
control groups were also enrolled between March 2013 and February
2014. The first control group included 20 acute myeloid leukemia
patients (12 males, 8 females) with a median age of 46 years
(range, 20–69 years) who had achieved CR after induction
chemotherapy [1 M2, 8 M4, 6 M3 and 5 M5 patients (15)]. The second control group included 20
healthy donors (10 males, 10 females) with a median age of 40 years
(range, 22–57 years). Written informed consent was obtained from
all patients and healthy donors, and the study was approved by the
Ethics Committee of the General Hospital of Tianjin Medical
University (Tianjin, China).
Treatment
All patients were administered with intravenous
decitabine (20 mg/m2/day; Xian Janssen Pharmaceutical
Ltd., Beijing, China) for 5 consecutive days, according to
Kantarjian's protocol proposed by the National Comprehensive Cancer
Network (NCCN) (16). After one
course of treatment, bone marrow aspiration was performed to assess
the effects of decitabine.
Determination of treatment
efficacy
MDS patient subtypes, blast cell count and treatment
response was determined according to NCCN guidelines (16). After one cycle of decitabine
treatment, all the patients underwent bone marrow aspiration to
determine treatment efficacy. All bone marrow smears were evaluated
by one hematologist. A bone marrow sorting counter (WZR-BM2; Ai
Lin, Suzhou, China) was used by the hematologist to count nucleic
cells in the bone marrow smears, and a total of 500 nucleic cells
were counted in each smear. If the blast cell percentage increased,
this was considered as disease progression, and if the blast cell
percentage decreased by <50%, this was considered as a poor
response. In all leukemia CR patients and donors, the blast cell
count was <5% in the bone marrow.
Megakaryocyte count
The number of megakaryocytes in a 1.5×3.5-cm area of
a standard bone marrow smear were determined under microscope
(BX53; Olympus Corporation, Tokyo, Japan) using Wright's staining
(Sigma-Aldrich, St. Louis, MO, USA) in all patients and donors, as
described previously (17,18). Megakaryocyte morphology was also
analyzed in all patients and donors (17,18).
Platelet count
Prior to and following one course of decitabine
treatment, patients' peripheral blood platelet count was performed
using a CytoFLEX blood cell counter (Beckman Coulter, Inc., Brea,
CA, USA).
In vitro induction of primary bone
marrow mononuclear cell (BMMNC) differentiation
A total of 5 ml bone marrow aspirate was obtained
from each subject at the Department of Hematology (General Hospital
of Tianjin Medical University). Overall, 80 bone marrow samples
were collected; 40 samples were obtained from MDS patients (prior
to and following one cycle of decitabine treatment), 20 samples
from the leukemia CR patients and 20 samples from the healthy
donors. EDTA (BD Biosciences, San Diego, CA, USA) was added to the
aspirate at the time of collection. The samples were washed
immediately with sterile phosphate-buffered saline (PBS; BD
Biosciences). BMMNCs were separated with Facoil buffer
(Sigma-Aldrich) and seeded in a 6-well culture plate. Cells
(1.10–1.20×106 cells/well) were cultured at 37°C in a
1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12
(Gibco; Thermo Fisher Scientific, Waltham, MA, USA) with 10 ng/ml
recombinant human thrombopoietin (Thermo Fisher Scientific) and 5
ng/ml stem cell factor (Thermo Fisher Scientific) for 7 days. Cells
were counted using a cell count plate (Eppendorf, Hamberg, Germany)
prior to and following culture. To assess cell viability Trypan
Blue (Sigma-Aldrich) was used to stain cells and only viable cells
were counted. Decitabine was added to the wells in a concentration
ladder (0.0, 2.0, 2.5 and 3.0 µM). Megakaryocyte differentiation
was evaluated following 7 days of culture at 37°C in at atmosphere
containing 5% CO2. At harvesting, 1 ml PBS was added to
each well. Following gentle mixing with a plastic pipette (1 ml
pipette; Thermo Fisher Scientific), cells were counted and
harvested with the pipette. Megakaryocytes remained suspended in
PBS and were incubated with 20 µl mouse anti-human
CD41α-fluorescein isothiocyanate immunoglobulin G antibody (ready
to use; cat. no. 340929; BD Biosciences) at 4°C for 30 min in the
dark. Following incubation, the cells were washed twice with PBS.
Approximately 30,000 cells were acquired and analyzed for CD41
expression using fluorescence-activated cell sorting analysis
(FACS; FACScalibur™; BD Biosciences) and CellQuest™ software
version 6.0 (BD Biosciences).
Statistical analysis
All results are expressed as the mean or median ±
standard deviation (SD). Data were analyzed using the independent
samples t-test for comparisons between groups. All statistical
analyses were performed using SPSS 19.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
In vivo effects of decitabine
In the present study, all MDS patients enrolled were
thrombocytopenic at the commencement of therapy. After one cycle of
decitabine treatment, an increase in platelet count was identified
in 16 patients (80%), however, platelet count remained stable
(increased or decreased by <10×109/l) in 4 patients.
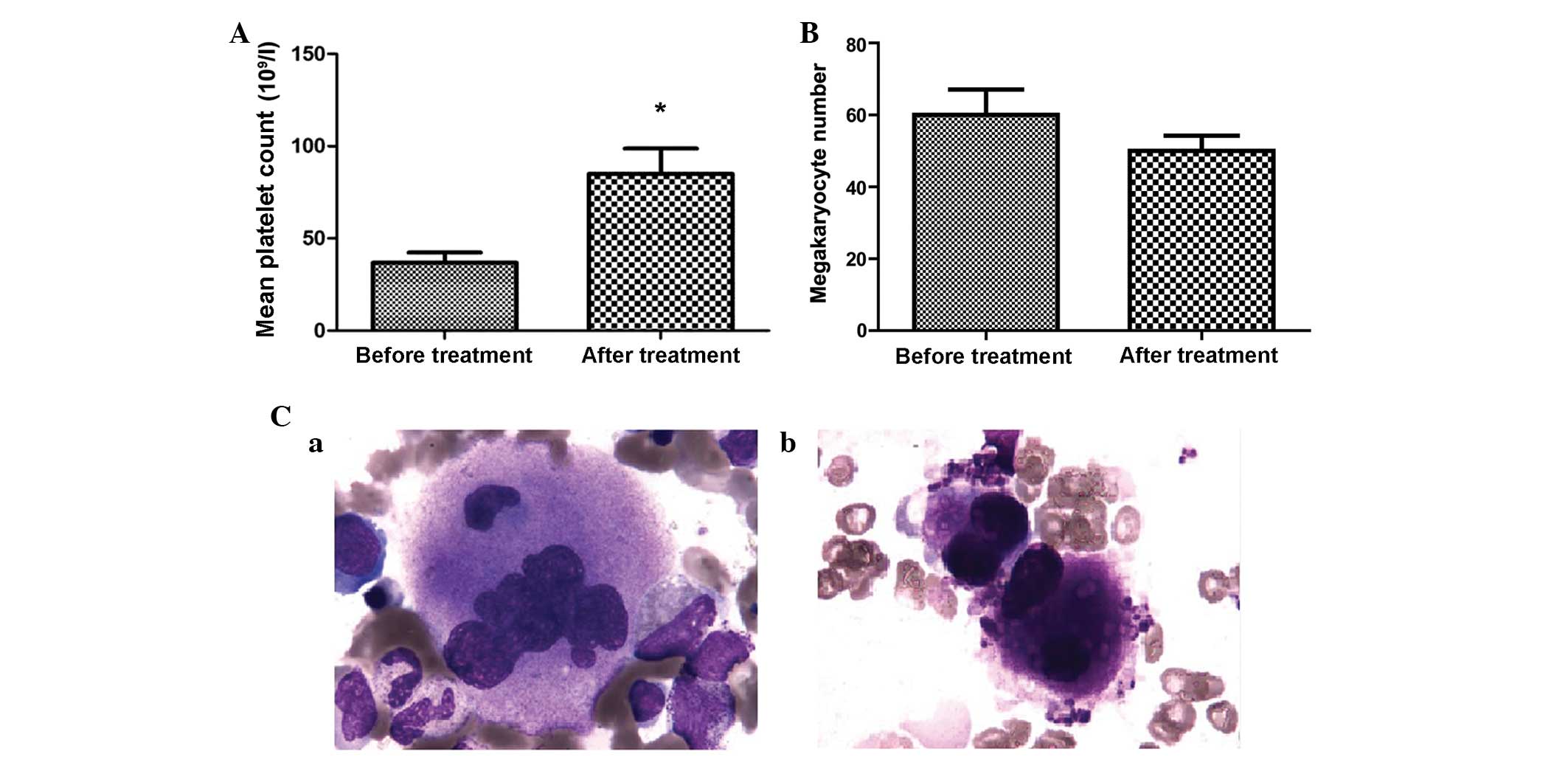
The mean platelet counts were significantly increased in the MDS
patient group, from 36.85±24.54×109/l before treatment
to 84.90±61.85×109/l after treatment (P=0.001; Fig. 1A). However, no significant differences
in megakaryocyte count were identified in the MDS patient group
following one cycle of decitabine therapy (P>0.05). For the
healthy donor group and leukemia CR patient group, the mean
platelet counts were 198.55±54.25×109/l and
224.13±72.33×109/l, respectively, and these patients did
not receive any treatment in the present study. Morphological
analysis revealed megakaryocyte maturation and the production of
platelets following treatment of MDS patients with 20
mg/m2/day decitabine (Fig. 1B
and C). Furthermore, in 13 patients, bone marrow blast cell
count decreased by >50%. For 3/7 poorly responded patients,
blast cell count increased after one cycle of decitabine
chemotherapy. Notably, the platelet count increased to
>30×109/l in all of the 3 progressed patients (data
not shown). In the control group, the size and maturation of
maturation of megakaryocytes was normal, and the blast cell
percentage was <0.5%.
In vitro effects of decitabine
To investigate the effect of decitabine on
megakaryocyte differentiation, megakaryocytes were cultured in
vitro and exposed to various concentrations of decitabine (0.0,
2.0, 2.5 and 3.0 µM) to identify the optimal concentration required
for megakaryocyte maturation. These results may provide a partial
guide for the clinical use of decitabine in MDS patients with
refractory thrombocytopenia. Briefly, BMMNC cells were incubated
with various concentrations of decitabine (0.0, 2.0, 2.5 and 3.0
µM) for 7 days. The BMMNC cells were harvested, stained for the
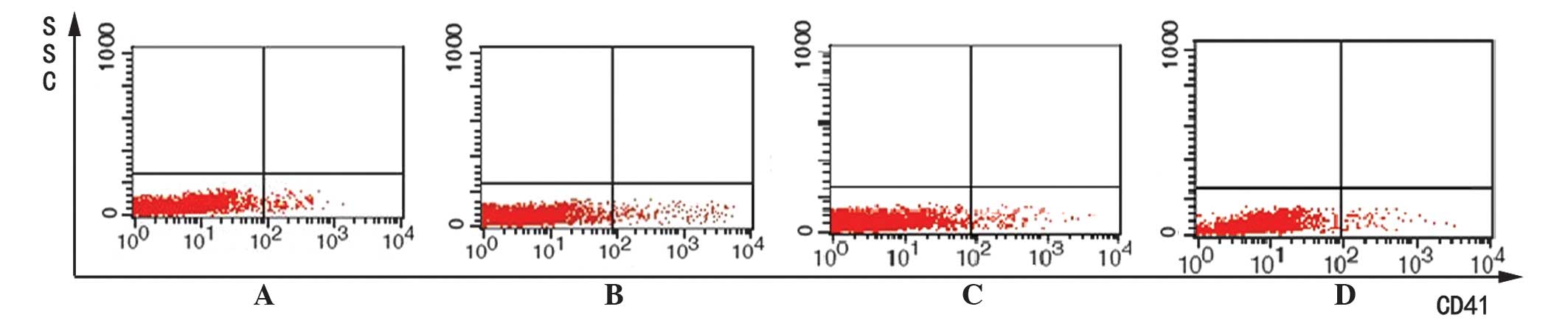
megakaryocyte marker CD41, and evaluated by flow cytometry. The
mean fluorescence intensity (MFI) of CD41 was compared between the
four different treatment groups. In the MDS patients group, the
results indicated that 2.0 µM decitabine induced the highest
expression of CD41 following treatment (MFI, 258.95±28.05;
P<0.05; Table I; Fig. 2), however, CD41 expression remained
consistently and significantly lower than the healthy control group
(P<0.05). In the leukemia CR patients group, the expression of
CD41 was significantly higher than that of the healthy controls
prior to decitabine treatment (MFI, 318.91±24.70 vs. 284.53±38.12;
P<0.05; Table I); however, CD41
expression significantly decreased in the leukemia CR group with
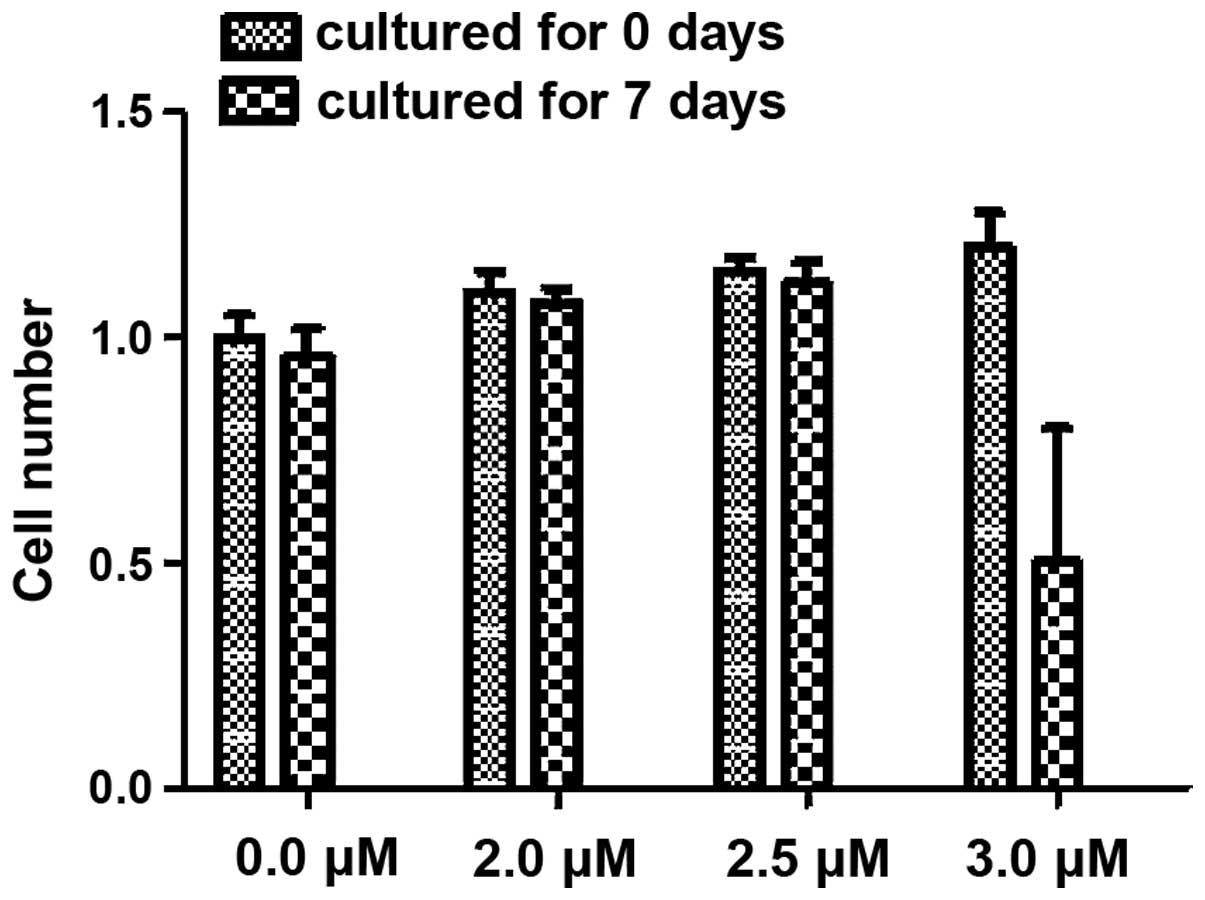
increasing decitabine concentration (P<0.05; Table I). In addition, the cell number of
every well was calculated prior to and after cell culture. A total
of 1.10–1.20×106 cells were seeded in every well.
Following culture, no significant differences in cell number were
identified in the 0.0, 2.0 or 2.5 µM treatment subgroups. However,
following treatment with 3.0 µM decitabine, the cell numbers of all
three groups decreased to 0.5–0.8×106/well and these
differences were determined to be significant (P<0.05; Fig. 3).
 | Table I.Mean fluorescence intensity of
membrane cluster of differentiation 41 in the bone marrow
mononuclear cells of MDS patients, following treatment with
decitabine at various concentrations. |
Table I.
Mean fluorescence intensity of
membrane cluster of differentiation 41 in the bone marrow
mononuclear cells of MDS patients, following treatment with
decitabine at various concentrations.
|
| Mean fluorescence
intensity (±SD) |
|---|
|
|
|
|---|
| Decitabine (µM) | Control group | Leukemia CR | de novo
MDS |
|---|
| 0.0 | 284.53±38.12 | 318.91±24.70 | 226.19±17.61 |
| 2.0 | 294.07±47.34 | 307.42±55.40 | 258.95±28.05 |
| 2.5 | 273.25±34.26 | 273.05±47.54 | 242.89±24.11 |
| 3.0 | 272.93±38.36 | 232.43±33.90 | 224.23±16.05 |
Discussion
Previously, the treatment of elderly MDS patients
with decitabine was found to result in a response rate of 45–50%
(10,11). Increased platelet count is a major
response observed in these patients. This is of particular
importance, as supportive care involves platelet transfusions,
which may be a burden for the patient, often leading to a
refractory response to expensive platelet transfusions (1–3).
In the current study, after one cycle of decitabine
treatment, a platelet response was observed in 16/20 (80%) MDS
patients, with an mean increase of >30×109/l. In
2004, Van den Bosch et al (12) investigated 162 high-risk MDS patients.
The authors reported that 58% of thrombocytopenic patients
exhibited a platelet response following one cycle of decitabine
therapy, and 69% of patients with a low platelet count exhibited a
response during therapy. In addition, an increase in platelet count
was found to be preceded by a positive trilineage response. The
platelet responses observed in MDS patients following decitabine
treatment in the present study and the study by Van den Bosch et
al (12) (80 and 65%,
respectively) were higher than the previously reported platelet
response rates of 45–50% (6,10,11).
Therefore, it is hypothesized that the megakaryocytic lineage
pathways affected by decitabine may be independent from its effects
on other lineages. Notably, in the current study, after one cycle
of decitabine, the three progressed patients exhibited an evident
platelet response, which enforces our hypothesis. Alternatively,
dysplastic megakaryocytes may exhibit more sensitivity and thus
respond to treatment earlier than other cell lineages (12). The maturation of megakaryocytes
involves endomitosis (19), whereby
the latter stages of mitosis are bypassed to allow an increase in
DNA content and size of cells; therefore, megakaryocytes may be
more sensitive to hypomethylation agents, such as decitabine, due
to high levels of DNA replication (9).
In the present study, a concentration ladder of
decitabine was used for in vitro cell culture. The results
indicated that the MFI of CD41 was highest in the 2.0 µM decitabine
subgroup (Fig. 2; Table I). Wang et al (9) performed a similar experiment in
vitro using the mouse cell line, L8057, and demonstrated that
decitabine induced the highest expression of CD41 at a
concentration of 2.5 µM. Notably, these concentrations of
decitabine are lower than the concentration used clinically,
according to NCCN guidelines (16).
CD41 is the surface marker of the megakaryocytic lineage which
represents megakaryocyte maturation (20). In the current study, the higher
concentration subgroup (3.0 µM) expressed a lower MFI and a
decreased cell number after 7 days culture, which may be a result
of severe cytotoxic side effects of decitabine. However, the 3.0 µM
subgroup, which approximates to the clinical dose used, resulted in
higher CD41 expression than the 0.0 µM decitabine treatment group.
These results indicate that a therapeutic regimen using a lower
concentration of decitabine may be of clinical use for the
induction chemotherapy of MDS patients. Furthermore, in the present
study, although CD41 expression was significantly increased in the
MDS group following treatment with 2.0 µM decitabine, the
expression levels remained lower than those observed in the two
control groups, which indicates that additional decitabine
consolidation chemotherapy may be required for MDS patients
following treatment with induction chemotherapy.
In the present study, the megakaryocyte number was
calculated for the MDS patient group prior to and after one cycle
of decitabine chemotherapy, however, no significant differences
were identified. These results indicate that the increases in
platelet counts and CD41 expression observed were due to the
improvement of the quality of megakaryocytes, rather than
megakaryocyte number. Thus, we hypothesize that decitabine affects
the megakaryocytic lineage via the induction of differentiation and
maturation of the lineage. Furthermore, maturation of
megakaryocytes was observed directly via morphological comparison
of patients' bone marrow smears prior to and after decitabine
chemotherapy (Fig. 1B).
MDS is a malignant cloning disease. Typically, a
small number of normal and dysplastic clones usually coexist in the
bone marrow of MDS patients, (21,22). In
the current study, megakaryocyte differentiation in healthy donors
was also induced by decitabine, as indicated by increased
expression of CD41, which peaked in the 2.0 µM subgroup (Table I). Similarly, Momparler et al
(23) observed a 2–3-fold increase in
the platelet count of patients treated for metastatic lung cancer
using the same chemotherapeutic agent. In addition, in sickle cell
anemia patients treated with even lower decitabine doses (0.15–0.30
mg/kg), an increase in hemoglobin F levels and concomitant
increases in platelet counts were observed (24). These results indicate that the normal
clones that exist in the bone marrow of MDS patients may also be
induced by decitabine, which may explain the platelet response
observed following decitabine treatment.
In conclusion, decitabine, as a DNA-hypomethylating
agent, appears to induce the differentiation and maturation of
myelodysplastic megakaryocytes in MDS patients, even at low
concentrations. Therefore, the repeated administration of
decitabine at lower doses in MDS patients may be useful in clinical
practice, and may lead to the development of alternative treatments
for other diseases of abnormal megakaryocyte differentiation, such
as idiopathic thrombocytopenic purpura, however, future studies are
required to investigate this.
Acknowledgements
This study was partially supported by The National
Natural Science Foundation of China (grant nos. 81170472, 81370607
and 30971285), the Natural Science Foundation of Tianjin (grant
nos. 14JCYBJC25400, 14JCYBJC27200), the Tianjin Cancer Major
Projects Research Plan (grant nos. 12ZCDZSY17900 and
12ZCDZSY18000), the National Public Health Grand Research
Foundation (grant no. 201202017) and the Tianjin Medical University
Fund (grant no. 2011KYQ15).
References
|
1
|
Steensma DP and Tefferi A: The
myelodysplastic syndrome(s): A perspective and review highlighting
current controversies. Leuk Res. 27:95–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kantarjian H, Giles F, List A, Lyons R,
Sekeres MA, Pierce S, Deuson R and Leveque J: The incidence and
impact of thrombocytopenia in myelodysplastic syndromes. Cancer.
109:1705–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Webb JJ and Anderson KC: Risks, costs and
alternatives to platelet transfusion. Leuk Lymphoma. 34:71–84.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
List A, Kurtin S, Roe DJ, Buresh A,
Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R and Zeldis JB:
Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J
Med. 352:549–557. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richel DJ, Colly LP, Kluin-Nelemans JC and
Willemze R: The antileukemic activity of 5-aza-2-deoxycytidine
(Aza-dC) in patients with relapsed and resistant leukaemia. Br J
Cancer. 64:144–148. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lübbert M, Wijermans P, Kunzmann R,
Verhoef G, Bosly A, Ravoet C, Andre M and Ferrant A: Cytogenetic
responses in high-risk myelodysplastic syndromes following low-dose
treatment with the DNA methylation inhibitor 5-aza-2-deoxycytidine.
Br J Haematol. 114:349–357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Vos D and van Overveld W: Decitabine: A
historical review of the development of an epigenetic drug. Ann
Hematol. 84(Suppl 1): S3–S8. 2005. View Article : Google Scholar
|
|
8
|
Hennessy BT, Garcia-Manero G, Kantarjian
HM and Giles FJ: DNA methylation in haematological malignancies:
The role of decitabine. Expert Opin Investig Drugs. 12:1985–1993.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Yi Z, Wang S and Li Z: The effect
of decitabine on megakaryocyte maturation and platelet release.
Thromb Haemost. 106:337–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wijermans P, Krulder JW, Huygens PC and
Neve P: Continous infusion of low dose 5-aza-2′-deoxycytidine in
elderly patients with high-risk myelodysplastic syndromes.
Leukemia. 11:1–5. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wijermans P, Lübbert M, Verhoef G, Bosly
A, Ravoet C, Andre M and Ferrant A: Low dose
5-aza-2′-deoxycytidine, a DNA-hypomethylating agent for the
treatment of high-risk myelodysplastic syndromes: A multicenter
phase II study in elderly patients. J Clin Oncol. 18:956–962.
2000.PubMed/NCBI
|
|
12
|
Van den Bosch J, Lübbert M, Verhoef G and
Wijermans PW: The effects of 5-aza-2′-deoxycytidine (Decitabine) on
the platelet count in patients with intermediate and high-risk
myelodysplastic syndromes. Leuk Res. 28:785–790. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng HY and Liao HF: Staurosporine induces
megakaryocytic differentiation through the upregulation of
JAK/Stat3 signaling pathway. Ann Hematol. 90:1017–1029. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brunning R, Orazi A, Germing U, et al:
Myelodysplastic syndromes. World Health Organization Classification
of Tumours of Haematopoietic and Lymphoid Tissue. Swerdlow S, Campo
E, Harris NL, et al: (4th). IARC Press. (Lyon, France). 88–103.
2008.
|
|
15
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Wiliman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: International Working Group for Diagnosis,
Standardization of Response Criteria, Treatment Outcomes, and
Reporting Standards for Therapeutic Trials in Acute Myeloid
Leukemia: Revised recommendations of the International working
group for diagnosis, standardization of response criteria,
treatment outcomes, and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greenberg PL, Attar E, Bennett JM, et al:
National Comprehensive Cancer Network: NCCN Clinical Practice
Guidelines in Oncology: Myelodysplastic syndromes. J Natl Compr
Canc Netw. 9:30–56. 2011.PubMed/NCBI
|
|
17
|
Gajendra S, Jha B, Goel S, Sahni T, Sharma
R, Shariq M, Jaiswal S and Sachdev R: Leishman and Giemsa stain: A
new reliable staining technique for blood/bone marrow smears. Int J
Lab Hematol. Jul 30–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori H, Niikura H, Terada H and Fujita K:
Morphological analysis of the megakaryocytes in myelodysplastic
syndrome. Rinsho Byori. 38:1347–1352. 1990.(In Japanese).
PubMed/NCBI
|
|
19
|
Bluteau D, Lordier L, Di Stefano A, Chang
Y, Raslova H, Debili N and Vainchenker W: Regulation of
megakaryocyte maturation and platelet formation. J Thromb Haemost.
7(Suppl 1): 227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao X, Wu J, Zou W and Dai Y: Two ellagic
acids isolated from roots of sanguisorba officinalis L. Promote
hematopoietic progenitor cell proliferation and megakaryocyte
differentiation. Molecules. 19:5448–5458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giagounidis A, Mufti GJ, Fenaux P, Germing
U, List A and MacBeth KJ: Lenalidomide as a disease-modifying agent
in patients with del (5q) myelodysplastic syndromes: Linking
mechanism of action to clinical outcomes. Ann Hematol. 93:1–11.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valent P, Bain BJ, Bennett JM, Wimazal F,
Sperr WR, Mufti G and Horny HP: Idiopathic cytopenia of
undetermined significance (ICUS) and idiopathic dysplasia of
uncertain significance (IDUS) and their distinction from low risk
MDS. Leuk Res. 36:1–5. 2012.PubMed/NCBI
|
|
23
|
Momparler RL, Bouffard DY, Momparler LF,
Dionne J, Belangerc K and Ayoub J: Pilot phase I–II study on
5-aza-2-deoxycytidine (Decitabine) in patients with metastatic lung
cancer. Anticancer Drugs. 8:358–368. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koshy M, Dorn L, Bressler L, Molokie R,
Lavelle D, Talischy N, Hoffman R, van Overveld W and DeSimone J:
5-Aza-2-deoxycytidine and foetal haemoglobin induction in sickle
cell anaemia. Blood. 96:2379–2384. 2000.PubMed/NCBI
|