Introduction
Breast cancer is the most frequently diagnosed type
of cancer and the leading cause of cancer mortality among women
(1). Despite the recent trend in
decreasing in mortality rates due to the improvements in early
detection and treatment, 458,400 mortalities were attributed to
breast cancer in 2008 (1).
The development of breast cancer is generally
considered to be a result of complex genetic and epigenetic
alternations (2,3). Although cancer initiation and
progression are predominantly driven by acquired genetic
alterations, it is becoming clear that microenvironment-mediated
epigenetic perturbations have an important role in neoplastic
development. A number of well-characterized epigenetic
modifications, including the DNA methylation of Ras association
domain family member 1 (RASSF1), estrogen receptor 1 (ER),
progesterone receptor (PR), retinoic acid receptor β, cyclin D2 and
paired like homeodomain 2, and the acetylation and methylation of
histones, are associated with aberrant gene functions and altered
patterns of gene expression that are critical in breast cancer
(4). In addition, secretoglobin
family 3A member 1, O-6-methylguanine-DNA methyltransferase and
RASSF1 promoter methylation had been previously considered as
suitable biomarkers for the detection of field cancerization in
breast cancer (5).
E-cadherin protein is encoded by the E-cadherin gene
(CDH1; 16q22.1) and is the prototypical type I cadherin, a
transmembrane glycoprotein mediating hemophilic cell-cell adhesion
between neighboring cells (6). The
E-cadherin-catenin complex, comprised of intracellular domains of
E-cadherin and catenin, can activate certain signaling cascades and
has an active role in epithelial-mesenchymal transition (EMT)
(7,8).
During tumorigenesis, E-cadherin has an important role in
suppressing invasion and metastasis of breast cancer cells. As a
result, decreased expression of E-cadherin is associated with an
increased aggressive behavior in clinical breast cancer (9).
Complete loss of E-cadherin protein expression was
identified in 84% of lobular breast carcinomas (10). Mutational inactivation of CDH1
accompanied by loss of heterozygosity (LOH) of the wild-type allele
was detected in 56% of lobular breast carcinomas (11). Furthermore, gene knock-out experiments
in mice demonstrated that CDH1 mutations were causal for the
lobular breast cancer phenotype (12). Thus, invasive lobular breast
carcinomas are characterized by the complete loss of E-cadherin
expression caused by inactivating mutations and deletions.
It has also been reported that E-cadherin protein
expression is reduced or absent in breast invasive ductal
carcinoma; however, CDH1 mutations were rare or absent (13). It is of note that CDH1 promoter
methylation is another important mechanism for inhibition of
E-cadherin protein expression. This mechanism has been confirmed in
breast cancer cell lines, however, data derived from primary breast
cancer tissues is limited (14,15).
Therefore, more research is required to precisely identify the
correlation between CDH1 methylation and the prognosis of patients
with breast cancer.
In the present study, the methylation status and
mRNA expression level of CDH1 were detected in breast cancer
tissues and the matched normal breast tissues. In addition, the
correlation of CDH1 promoter region methylation was analyzed with
the characteristics and prognosis of patients with breast
cancer.
Materials and methods
Patients
A total of 245 specimens (137 primary breast
cancers, 85 matched normal breast specimens, 13 lung metastasis
specimens and 10 normal breast tissues corresponding to benign
lesions) were obtained from 160 patients at the First Affiliated
Hospital of Xi'an Jiaotong University (Xi'ian, China) between March
2007 and October 2009. The specimens were resected and frozen in
liquid nitrogen immediately after surgery. This study was approved
by the Ethics Committee of Xi'an Jiaotong University First
Affiliated Hospital and each participant signed an informed consent
document. The patients were accrued consecutively and the inclusion
criteria were no previous histological diagnosis of breast cancer.
All patients had undergone segmental resection or mastectomy and
none had received radiotherapy or chemotherapy prior to surgery.
The evidence of cancer, including the lymph node metastasis, tumor
size and status of ER, PR, human epidermal growth factor receptor-2
(Her-2), tumor protein 53 (p53), Ki-67 and E-cadherin, was based on
documented medical records. The clinical tumor node metastasis
(TNM) stage was determined according to American Joint Committee on
Cancer staging manual (16).
Histological grading was made according to the Elston-Ellis
modification of Scarff-Bloom-Richardson grading system (17). The 137 patients with primary breast
cancer were followed up for 72 months to determine overall survival
(OS) and disease-free survival (DFS).
Reverse transcription-quantitative
polymerase chain reaction (PCR)
Following mechanical tissue homogenization, total
RNA was isolated from the fresh clinical specimens using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacture's protocol. Concentration and
purity of total RNA were assessed using a NanoDrop 1000
Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). Approximately 1 µg of total RNA from each sample was
reverse-transcribed into single strand cDNA (final volume, 10 µl)
using PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's protocol.
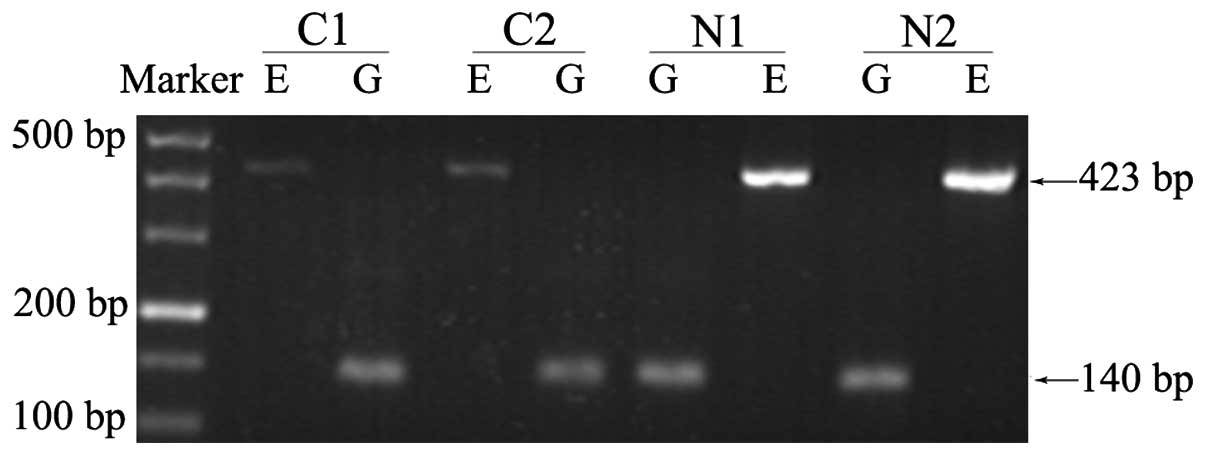
The following primers were used for amplification:
Forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′ for GAPDH (140-bp fragment); and
forward, 5′-TGCTCTTGCTGTTTCTTCGG-3′ and reverse,
5′-TGCCCCATTCGTTCAAGTAG-3′ for CDH1 (423-bp fragment). The reaction
was performed using Takara Taq DNA Polymerase Hot Start Version
(Takara Biotechnology Co., Ltd.) in a total volume of 25 µl,
containing 0.125 µl Takara Taq HS (5 U/µl), 0.25 µl of each pair of
primers (20 µM), cDNA template 2 µl, 2 µl dNTP Mixture (2.5 mM
each) and 2.5 µl 10X PCR Buffer. The amplification was conducted by
performing 35 cycles of denaturation at 94°C for 30 sec, annealing
at 56°C for 30 sec and extension at 72°C for 1 min in a PTC-200
thermal cycler (Bio-Rad Laboratories), according to the
manufacturer's protocol. The post-amplification specific PCR
products were separated on a 2.5% Biowest Regular Agarose G-10
(Gene Company Ltd., Chi Wan, Hong Kong) and analyzed using
electrophoresis.
The primers for target gene (CDH1) and internal
control gene [β-actin (ACTB)] were as follows: Forward,
5′-CAGCACGTACACAGCCCTAA-3′ and reverse, 5′-ACCTGAGGCTTTGGATTCCT-3′
for CDH1; and forward, 5′-TTCTACAATGAGCTGCGTGTG-3′ and reverse,
5′-GGGGTGTTGAAGGTCTCAAA-3′ for ACTB. qPCR was conducted in a total
reaction volume of 25 µl, containing 2 µl cDNA, 12.5 µl SYBR Premix
Ex Taq II (Tli RNaseH Plus, 2X; Takara Biotechnology Co., Ltd.),
8.5 µl dH2O and 1.0 µl of each pair of primers (10 µM).
The amplification was performed as follows: Pre-denaturation at
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 30 sec, according to the manufacturer's protocol. The
quantification cycle (Cq) was automatically calculated using iQ5
Optical System Software version 2.1 (Bio-Rad Laboratories,
Hercules, CA, USA). Relative expression levels of the target gene
were determined using internal control (ACTB). Data were analyzed
using the quantitative threshold cycle (2−ΔΔCq) method
(18). All amplification reactions
were performed in triplicate. The CDH1 mRNA expression level was
detected by qPCR, and the change fold relative expression level was
calculated as a ratio of the average expression level of normal
control tissues, from patients with benign lesions.
DNA extraction and treatment with
sodium bisulfite
Genomic DNA was isolated from tissues using a
TIANamp Genomic DNA kit (Tiangen, Beijing, China), according to the
manufacturer's protocol. DNA concentrations and purity were
measured by a NanoDrop 1000 Spectrophotometer (Thermo Fisher
Scientific, Inc.).
The conversion of DNA by sodium bisulfite was
performed according to the following established protocol.
Initially, 2 µg of genomic DNA were denatured with 3 M NaOH at 42°C
for 30 min (final concentration, 0.3 M NaOH), followed by
incubation with freshly prepared 2.5 M sodium bisulfite (cat no.
S9000) and 1 M hydroquinone (pH 5.0; cat no. H9003; Sigma-Aldrich,
St. Louis, MO, USA) to a total volume of 520 µl at 55°C for 16 h.
The DNA was purified with the Wizard DNA Clean-Up System (Promega,
Madison, WI, USA), according to the manufacturer's protocol.
Modification of the DNA was terminated by the addition of 5.5 µl
NaOH (3 M) at room temperature for 15 min. The precipitation was
conducted through the addition of 33 µl ammonium acetate (10 M, pH
7.0), 270 µl ethanol and 4.0 µl glycogen (10 µg/µl; Invitrogen;
Thermo Fisher Scientific, Inc.), at −20°C for 12 h. The modified
DNA was resuspended in 30 µl elution buffer and stored at
−20°C.
Methylation-specific-PCR (MS-PCR)
The sodium bisulfite-converted DNA was amplified
from 50 ng with Takara Taq Hot Start Version (Takara Biotechnology
Co., Ltd.), using the following protocols. The polymerase chain
reaction amplification was performed using Takara Taq Hot Start
Version (Takara Biotechnology Co., Ltd.), with a total reaction
volume of 25 µl (composition as above). The cycling conditions were
as follows: 38 cycles of denaturation at 94°C for 30 sec, annealing
at 60°C (unmethylated) or 62°C (methylated) for 30 sec and
extension at 72°C for 1 min, according to the manufacturer's
protocol. The post-MS-PCR products were separated on 3.0% Regular
Agarose (Biowest) and analyzed using electrophoresis. DL500 DNA
Marker (Takara Biotechnology Co., Ltd.) was the molecular weight
marker. The following primers were used for amplification: Sense,
5′-TTAGGTTAGAGGGTTATCGCGT-3′ and antisense,
5′-TAACTAAAAATTCACCTACCGAC-3′ for a 116-bp fragment corresponding
to the CDH1 methylated sequence; and sense,
5′-TAATTTTAGGTTAGAGGGTTATTGT-3′ and antisense,
5′-CACAACCAATCAACAACACA-3′ for a 97-bp fragment corresponding to
the CDH1 unmethylated sequence. Regardless of whether the
unmethylated allele was amplified, positivity was determined as a
sample with a methylated allele. All other samples were classified
as negative.
Statistical analysis
Descriptive analyses, such as mean, standard
deviation, median, frequency, and percentage, were performed to
explore the clinicopathological characteristics and methylation
status. Pearson's χ2 test for categorical variables and Student's
t-test for continuous variables were used for comparing clinical
factors between tumors demonstrating hypermethylation versus no
hypermethylation. Bonferroni correction was applied to adjust
P-values for the multiple comparisons. Kaplan-Meier survival curves
and log-rank statistics were employed to evaluate DFS and OS.
Multivariate regression analysis was performed using the Cox
proportional hazards model. All statistical analyses were performed
using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA).
Reported P-values were two-sided, and P<0.05 indicated a
statistically significant difference.
Results
Patient characteristics
The present study analyzed 160 patients. At the time
of diagnosis, patients with breast cancer ranged in age from 34 to
71 years old (mean age, 50.2±11.0 years; median age, 51 years). The
age of patients with benign lesions ranged from 25 to 58 years
(mean age, 39.8±11.9 years; median age, 38 years). Other
histopathological features of the primary breast cancer patients
are shown in Table I.
 | Table I.Association between CDH1 methylation
and clinical variables in patients with breast cancer. |
Table I.
Association between CDH1 methylation
and clinical variables in patients with breast cancer.
|
|
| Methylation status
of CDH1 |
|
|---|
|
|
|
|
|
|---|
| Variable | Sample size, n | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| Age, years |
|
|
| 0.341 |
|
≤50 | 58 | 21 (36.2) | 37 (63.8) |
|
|
>50 | 79 | 35 (44.3) | 44 (55.7) |
|
| Histological
grade |
|
|
| 0.410a |
| I | 26 | 8
(30.7) | 18 (69.2) |
|
|
II | 64 | 26 (40.6) | 38 (59.4) |
|
|
III | 47 | 22 (46.8) | 25 (53.2) |
|
| TNM stagec |
|
|
| 0.258b |
| I | 17 | 5
(29.4) | 12 (70.6) |
|
|
II | 81 | 32 (39.5) | 49 (60.5) |
|
|
III | 39 | 19 (48.7) | 20 (51.3) |
|
|
IV | 13 | 8
(61.5) | 5 (38.5) |
|
| Lymph node
metastasis |
|
|
| 0.022 |
|
Positive | 77 | 38 (49.4) | 39 (50.6) |
|
|
Negative | 60 | 18 (30.0) | 42 (70.0) |
|
| Histological
type |
|
|
| 0.052 |
|
Ductal | 106 | 48 (45.3) | 58 (54.7) |
|
|
Lobular | 31 | 8 (25.8) | 23 (74.2) |
|
| ER |
|
|
| 0.018 |
|
Positive | 96 | 33 (34.4) | 63 (65.6) |
|
|
Negative | 41 | 23 (56.1) | 18 (43.9) |
|
| PR |
|
|
| 0.407 |
|
Positive | 67 | 25 (37.3) | 42 (62.7) |
|
|
Negative | 70 | 31 (44.3) | 39 (55.7) |
|
| Her-2 |
|
|
| 0.284 |
|
Positive | 36 | 12 (33.3) | 24 (66.7) |
|
|
Negative | 101 | 44 (43.6) | 57 (56.4) |
|
| p53 |
|
|
| 0.634 |
|
Positive | 53 | 23 (43.4) | 30 (56.6) |
|
|
Negative | 84 | 33 (39.3) | 51 (60.7) |
|
| Ki-67 |
|
|
| 0.379 |
|
Positive | 82 | 36 (43.9) | 46 (56.1) |
|
|
Negative | 55 | 20 (36.4) | 35 (63.6) |
|
| E-cadherin |
|
|
| <0.001 |
|
Positive | 79 | 22 (27.8) | 57 (72.2) |
|
|
Negative | 58 | 34 (58.6) | 24 (41.4) |
|
Methylation analyses
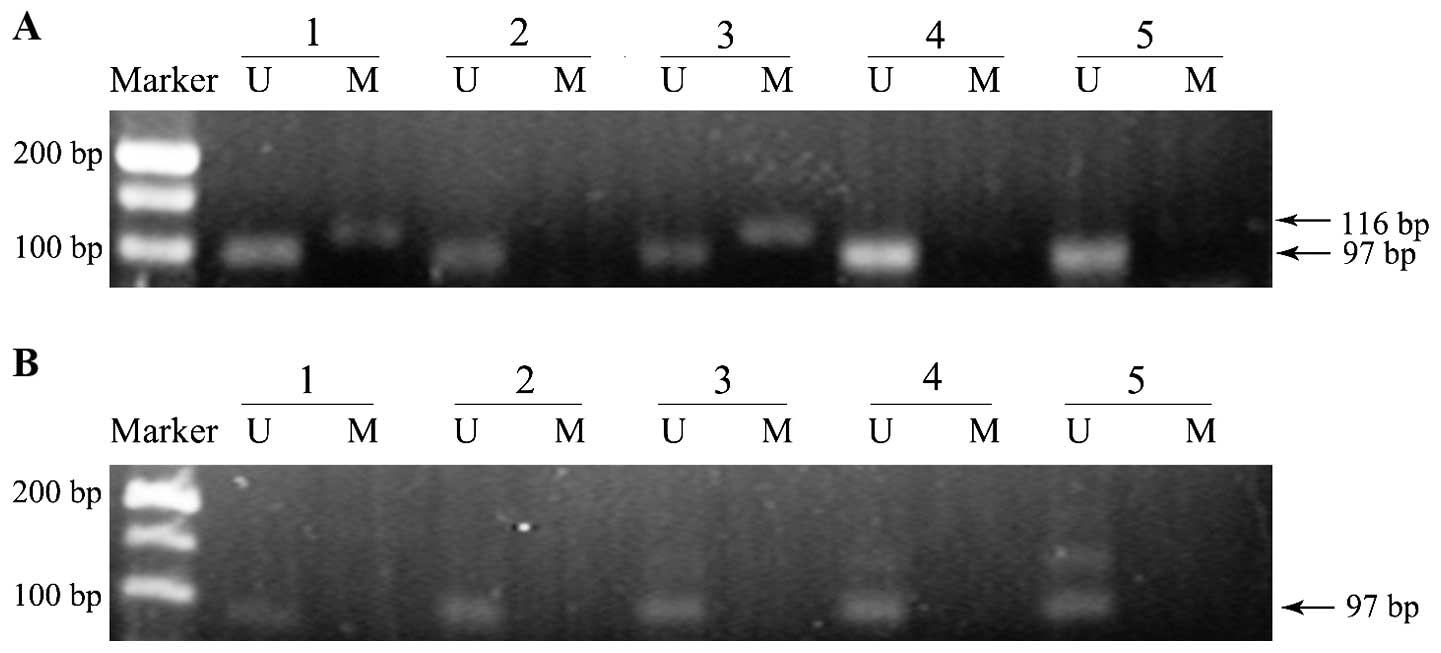
A group of representative examples of MS-PCR
analysis from breast cancer and matched normal breast tissues are
shown in Fig. 1. As shown, two of the
five breast cancer samples exhibited promoter methylation
positivity, while no methylation band was observed in matched
normal tissues. The presence of a methylated band was the standard
for methylation positivity.
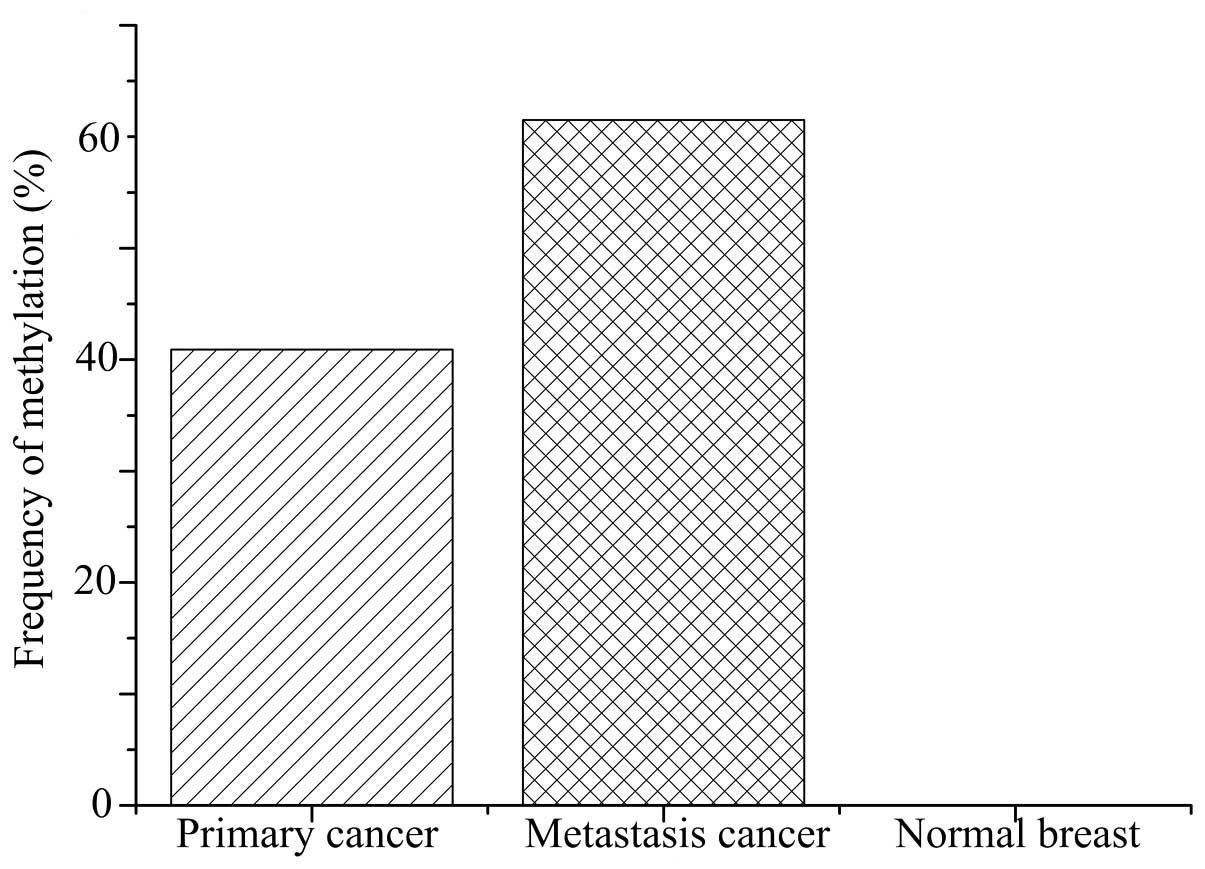
The methylation frequency of the CDH1 promoter in
breast cancer tissues was 42.7% (56/137 primary cancer and 8/13
lung metastasis), significantly higher than that 0% (0/85) in the
normal breast tissues (P<0.001; Fig.
2). Furthermore, the methylation status was significantly
associated with lymph node metastasis (P=0.022) and ER expression
(P=0.018) in patients with breast cancer. However, no statistically
significant differences between CDH1 promoter methylation frequency
and age (≤50 vs. >50 years), TNM stage, histological grade,
histological type, PR, Her-2, p53 or Ki-67 status were identified
in patients with breast cancer (Table
I).
Notably, 60.7% (34/56) of primary carcinoma samples
with CDH1 methylation showed negative expression of E-cadherin.
However, only 29.6% (24/81) of primary carcinoma samples without
CDH1 methylation showed negative expression of E-cadherin.
Therefore, positive CDH1 methylation of appears to be significantly
correlated with decreased E-cadherin expression (P<0.001).
However, there were a number of discrepancies in the data; for
example, 22 samples with positive CDH1 promoter methylation showed
positive expression of E-cadherin, while 24 samples showed negative
expression of E-cadherin without CDH1 methylation (Table I).
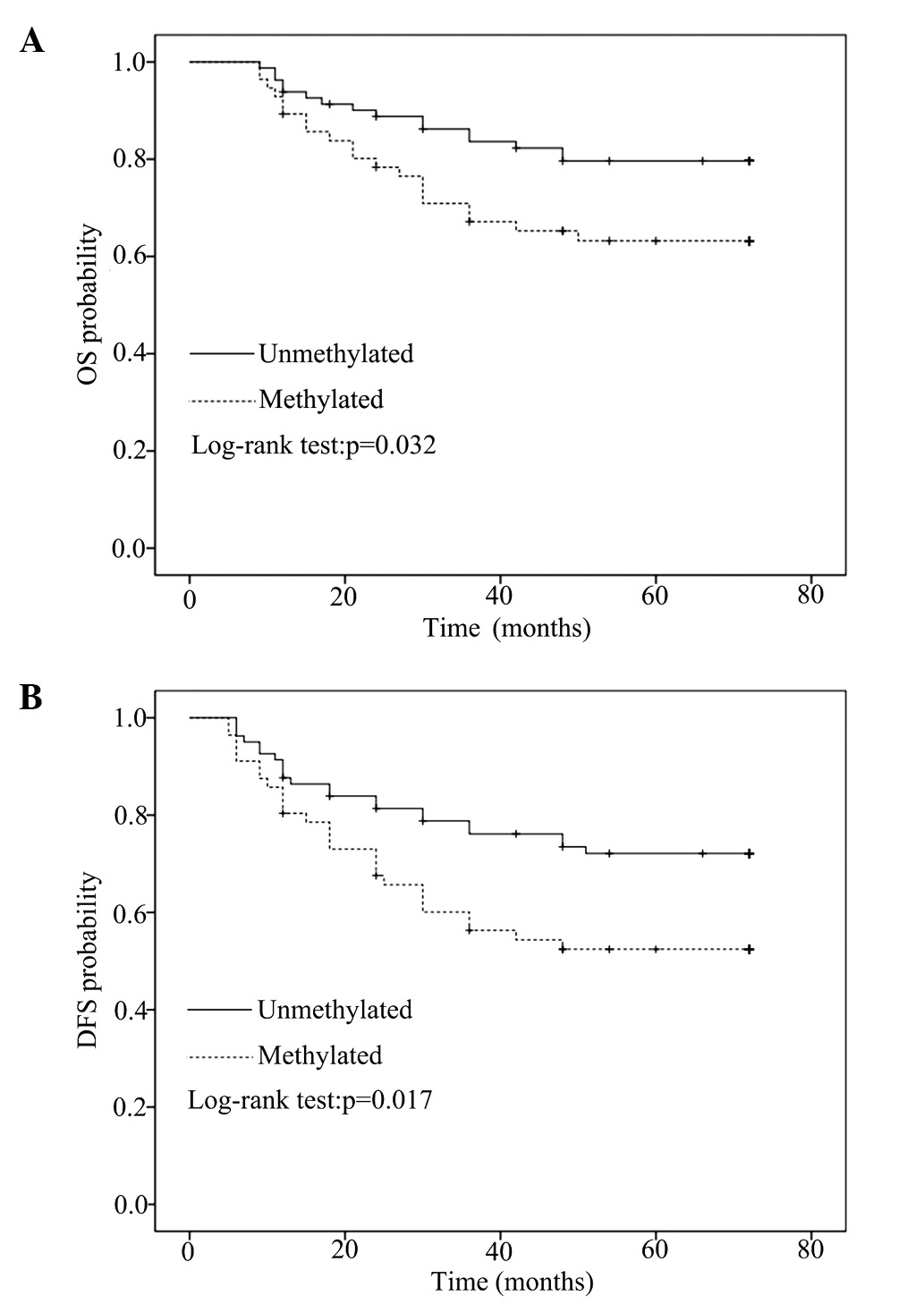
After a median follow-up of 72 months, OS and DFS
data of the patients with primary breast cancer (n=137) were
compared between methylated and unmethylated CDH1 promoter
sequences by univariate Kaplan-Meier analysis using log-rank
statistics. CDH1 methylation in primary breast cancer was
significantly associated with poor OS (5-year survival: 64.3% in
the methylated group vs. 80.0% in the unmethylated group, P=0.032)
and DFS (5-year survival: 53.6% in the methylated group vs. 72.8%
in the unmethylated group, P=0.017), as indicated by the
Kaplan-Meier survival curves (Fig.
3). To verify whether CDH1 methylation was an independent
prognostic factor, multivariate Cox regression analysis was
conducted with various factors, including tumor size, lymph node
metastasis, histological grade, and ER, PR and Her-2 status. CDH1
methylation in breast carcinoma represented an independent and
strong risk factor for OS (HR, 1.737; 95% CI, 0.957–3.766; P=0.041)
and DFS (HR, 2.018; 95% CI, 2.057–3.845; P=0.033) (Table II).
 | Table II.Multivariate Cox regression analysis
of CDH1 promoter methylation with regard to OS and DFS of
patients. |
Table II.
Multivariate Cox regression analysis
of CDH1 promoter methylation with regard to OS and DFS of
patients.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor size |
|
| 0.336 |
|
| 0.074 |
| T1–2
vs. T3–4 | 1.842 | 0.531–6.393 |
| 1.730 | 0.950–3.050 |
|
| Lymph node
metastasis |
|
| 0.015 |
|
| 0.045 |
| N0 vs.
N1–3 | 0.294 | 0.110–0.789 |
| 1.937 | 0.997–3.776 |
|
| Histological
grade |
|
| 0.579 |
|
| 0.305 |
| I/II
vs. III | 1.266 | 0.550–2.917 |
| 0.635 | 0.267–1.512 |
|
| ER status |
|
| 0.274 |
|
| 0.224 |
|
Negative vs. positive | 0.602 | 0.243–1.494 |
| 0.623 | 0.267–1.364 |
|
| PR status |
|
| 0.026 |
|
| 0.380 |
|
Negative vs. positive | 3.938 | 1.177–13.176 |
| 1.450 | 0.633–3.321 |
|
| Her-2 status |
|
| 0.437 |
|
| 0.763 |
|
Negative vs. positive | 1.580 | 1.499–5.005 |
| 1.134 | 0.501–2.565 |
|
| CDH1
methylation |
|
| 0.033 |
|
| 0.041 |
|
Negative vs. positive | 2.018 | 1.057–3.854 |
| 1.737 | 0.957–3.766 |
|
Gene expression analyses
qPCR was used to quantitatively detect the relative
expression level of CDH1 mRNA. The mean expression level of normal
breast specimens derived from benign lesion patients was used as a
baseline to calculate relative expression level change fold. By
conducting RT-PCR in advance, a series of electrophoretograms were
obtained to intuitively present differences in expression between
cancer and normal groups (Fig.
4).
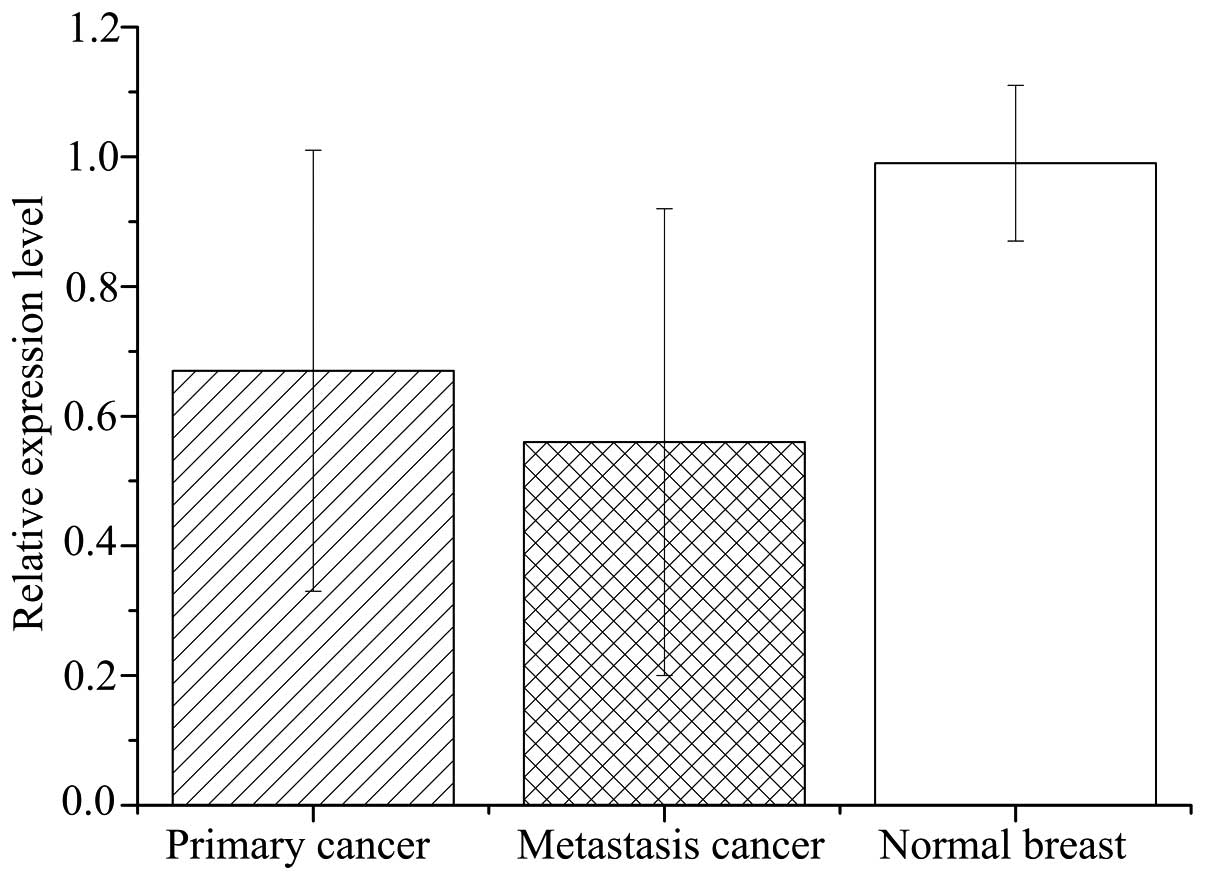
Relative expression levels of CDH1 mRNA in primary
breast cancer tissues (0.67±0.34) were significantly lower than the
matched normal tissues (0.99±0.12; P<0.001; Fig. 5). However, the mRNA levels varied
considerably among breast cancer samples, with certain samples even
exhibiting a higher expression level than the matched normal
samples (Fig. 6). Relative expression
of CDH1 mRNA was significantly lower in metastasis specimens
(0.56±0.36) compared with primary cancer (P=0.831) and normal
breast (P<0.001) tissues (Fig.
5).
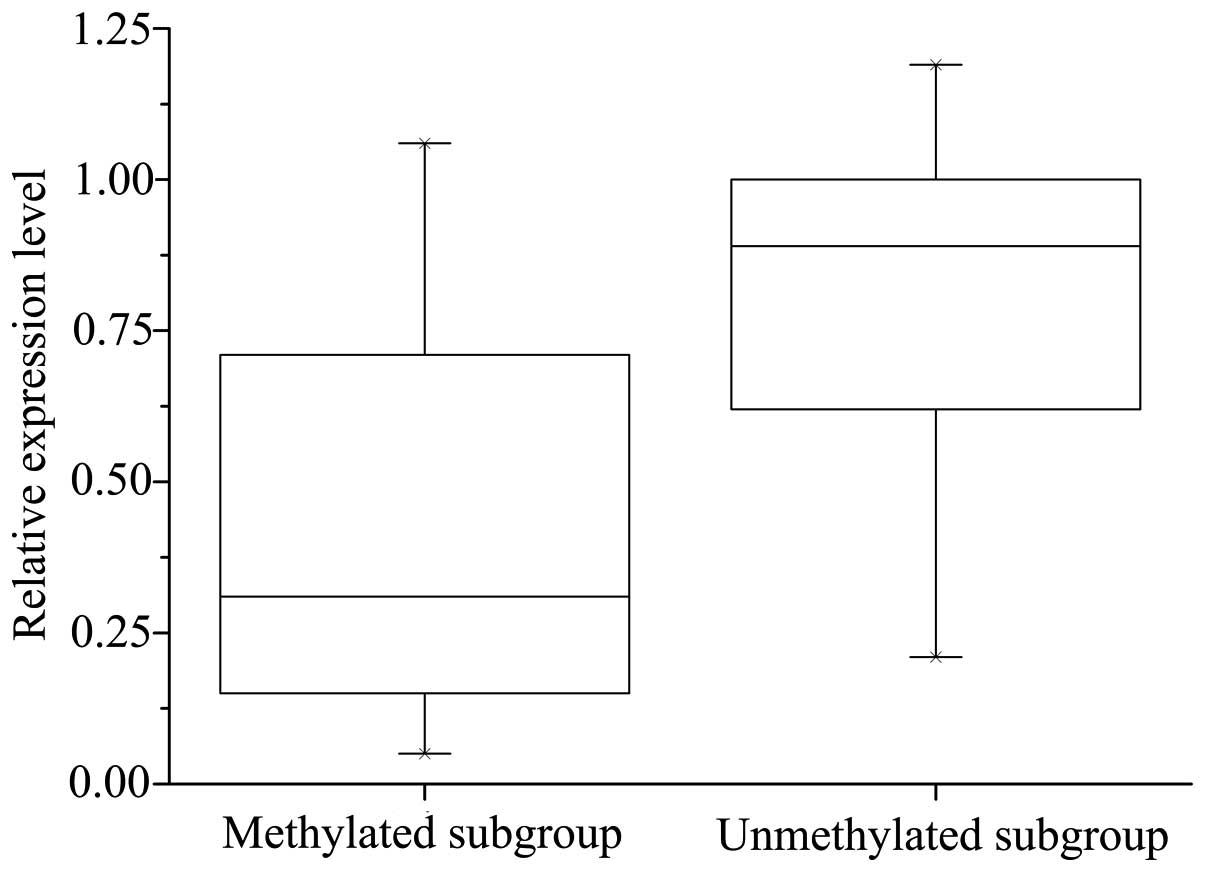
Significant correlation was observed between CDH1
methylation and decreased expression of CDH1 mRNA: The mRNA levels
of CDH1 were significantly lower in the CDH1 methylated group
(0.55±0.31) compared with the unmethylated group (0.74±0.29;
P=0.020). However, the methylation of CDH1 did not necessarily
result in a corresponding downregulation of CDH1 mRNA expression
(Fig. 6).
Discussion
The present study investigated the promoter
methylation status and expression levels of CDH1 in breast cancer
tissues and matched normal breast tissues. The methylation status
of CDH1 was detected by MS-PCR at a sensitivity level of 1/1,000
requiring only small quantities of DNA sample (19).
In breast cancer, the incidence of CDH1 promoter
methylation ranges between 21 and 72% (20–25). In
agreement with this, the current study identified CDH1 promoter
hypermethylation in 42.7% of patients with breast cancer (40.9% in
primary cancer and 61.5% in lung metastasis). The differences in
the methylation incidence observed in previous studies may result
from different tissue specimen preservation (for example, fresh
tissues and formalin-fixed paraffin embedded tissues). Developments
in DNA methylation detection methods, such as quantitative MS-PCR,
may also contribute to the variable detectable rate. Additionally,
the clinical samples included in the previous studies vary in
clinical stage, histological grade, histological type, metastatic
status and familial breast cancer status, possibly leading to
variable results.
Several previous studies have demonstrated an
association between CDH1 methylation or abnormal expression of
E-cadherin and breast cancer progression (23,26,27). A
previous experiment in breast cancer cell lines also confirmed that
CDH1 promoter methylation regulates gene expression level (14). In the present study, CDH1 methylation
was correlated with the expression level of CDH1 mRNA in matched
breast cancer and normal tissues. Consistent with previous studies,
the incidence of CHD1 promoter methylation in the primary cancer
samples (40.9%) was significantly different from that in the
matched normal tissues (P<0.001), and the CDH1 mRNA levels were
significantly lower than in the matched normal specimens
(P<0.001). Furthermore, the current study analyzed the
association between CDH1 methylation status and the expression
levels in the breast cancer group. The analysis demonstrated that
CDH1 methylation was significantly correlated with the
downregulation of CDH1 mRNA (P=0.020) and E-cadherin expression
levels (P<0.001). The aforementioned results suggest that
abnormal promoter methylation is one of the mechanisms of
downregulating CDH1 expression and may correlate with breast
carcinogenesis.
However, CDH1 promoter methylation was not uniformly
associated with the downregulation of CDH1 expression levels in the
current study. In the unmethylated subgroup of the breast cancer
samples, 24 samples exhibited absent expression of E-cadherin and
downregulation of CDH1 mRNA. Various studies have demonstrated that
CDH1 expression can be repressed by mechanisms other than promoter
methylation, such as changes in chromatin structure, LOH at
16q22.1, inactivating gene mutations, specific transcriptional
factors, and translational and post-translational regulation
(28,29). By contrast, 5 samples of the
methylated subgroup presented CDH1 mRNA expression levels that were
comparable with the normal breast group, as well as positive
E-cadherin expression. The presence of an unmethylated band in
these specimens indicated that the high CDH1 mRNA expression level
resulted from a large proportion of unmethylated cells in the
specimen, and the presence of a methylated band was caused by a
small number of methylated cells due to intratumoral
heterogeneity.
In all samples exhibiting CDH1 methylation,
unmethylated alleles invariably coexisted with methylated alleles.
These unmethylated alleles may reflect the contribution of normal
breast cells mixed in the samples. In addition, the unmethylated
alleles may be derived from cancer cells only possessing
unmethylated bases in the promoter region, as stated above.
Considering that DNA methylation is a dynamic and reversible
regulation mechanism, it is not unforeseen that not all cytosines
in the gene promoter region were methylated or not all bases in one
chain of DNA molecule were methylated (hemimethylation) by DNA
methyltransmethylase 3 (DNMT3) (30).
Hemimethylation also occurs during DNA replication if the
maintenance mechanism of methylation based on DNMT1 is disrupted
(9,31,32). Above
all, the dynamic features of epigenetic regulation and complicacy
of gene expression regulation are responsible for the inconformity
of CDH1 promoter hypermethylation and the decreased expression of
CDH1 observed in the current study.
Consistent with previous studies, the present study
revealed CDH1 methylation to be significantly associated with
ER-negative samples (23,33). Promoter hypermethylation is common in
numerous cancer-related genes, such as RASSF1, ESR1, PGR, APC,
GSTP1, BRCA, CDH13 and RARB, which also exhibit promoter
hypermethylation and decreased gene expression in breast cancer
(3,34,35). The
correlation between ER expression and CDH1 methylation may result
from the coexistence of ER and CDH1 promoter methylation, as stated
in a previous study (25).
Furthermore, lymph node metastasis was identified to
be significantly correlated with CDH1 methylation (P<0.05) in
the current study. As patients with metastasis lesions rarely
undergo surgery, only a small number of metastasis specimens were
included in the present study. The incidence of methylation in
metastasis was higher than that in primary cancer samples
(P>0.05) and normal samples (P<0.001). The current findings
indicate that CDH1 hypermethylation predominates in breast cancer
cases with a more aggressive phenotype. The traditional view is
that distant metastatic lesions are accompanied by a higher
frequency of gene methylation, however, the majority studies do not
deny the existence of unmethylated cases (36). Studies have shown that promoter
hypermethylation of CDH1 independent of any gene mutation is
associated with EMT and downstream events, such as aggressiveness,
invasion and metastasis (37,38). Cancer cells may undergo EMT then
migrate to a secondary site in the body where they occasionally
undergo mesenchymal-epithelial transition (MET), reverting back to
a more epithelial phenotype (39).
Therefore, it should not be surprising that CDH1 hypermethylation
and downregulation of CDH1 mRNA were not found in 5 distant
metastasis lesions in the current study.
Notably, an association between CDH1 methylation
status and clinical outcome was observed in the present study. CDH1
methylation in primary cancer indicated poor prognosis, and may be
an independent prognostic factor for the OS and DFS of patients
with breast cancer. However, the results may be considered weak as
different postoperative treatments and a limited number of patients
were included. Therefore, further investigations are required to
determine the impact of CDH1 methylation on the prognosis of
patients with breast cancer.
Loss of E-cadherin protein expression is most
frequent in infiltrating lobular tumor types and is commonly a
bi-allelic event resulting from any combination of gene promoter
hypermethylation, mutation or allelic loss (40,41). While
ductal cancer frequently presents with varying levels of
expression, the mechanism involved in ductal breast cancer may be
different from the lobular tumor types (13). However, the frequency of CDH1
methylation demonstrated no significant difference between lobular
tumors and ductal tumors in the present study, although the
methylation percentage in ductal cancer was larger than the lobular
(P=0.052). This may be a reflection of sample selection, as the
majority of cases analyzed in the present study were infiltrating
ductal tumors.
No significant association was identified between
CDH1 methylation, and age, clinical stage, histological grade or
Her-2, PR, P53 or Ki67 status, although the majority of these
parameters have been reported to be correlated with gene
methylation status (21,22,25). The
observation difference may result from disparate sample types. For
example, patients with invasive breast cancer (stage II, grade II)
accounted for majority of the total cases. Also, different
standards for grouping cases based on these parameters may lead to
different outcomes (21,33). Additionally, more samples and a more
effective methylation detection method may aid in uncovering the
association between CDH1 methylation and breast cancer.
In conclusion, the present study demonstrated that
CDH1 promoter methylation may be correlated with breast
carcinogenesis and indicate poor prognosis in patients with breast
cancer.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (approval ID: 81272418). The authors
thank the assistants at the Department of Thoracic Surgery and the
staff of the Key Laboratory of Environment and Genes Related to
Disease (Ministry of Education, Faculty of Public Health, College
of Medicine, Xi'an Jiaotong University, Xi'an, China) for their
technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorincz AT: Cancer diagnostic classifiers
based on quantitative DNA methylation. Expert Rev Mol Diagn.
14:293–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanwal R and Gupta S: Epigenetics and
cancer. J Appl Physiol (1985). 109:598–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Nayak S, Jankowitz R, Davidson NE
and Oesterreich S: Epigenetics in breast cancer: What's new? Breast
Cancer Res. 13:2252011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spitzwieser M, Holzweber E, Pfeiler G,
Hacker S and Cichna-Markl M: Applicability of HIN-1, MGMT and
RASSF1A promoter methylation as biomarkers for detecting field
cancerization in breast cancer. Breast Cancer Res. 17:1252015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drees F, Pokutta S, Yamada S, Nelson WJ
and Weis WI: Alpha-catenin is a molecular switch that binds
E-cadherin-beta-catenin and regulates actin-filament assembly.
Cell. 123:903–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada S, Pokutta S, Drees F, Weis WI and
Nelson WJ: Deconstructing the cadherin-catenin-actin complex. Cell.
123:889–901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrews JL, Kim AC and Hens JR: The role
and function of cadherins in the mammary gland. Breast Cancer Res.
14:2032012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Leeuw WJ, Berx G, Vos CB, Peterse JL,
Van de Vijver MJ, Litvinov S, Van Roy F, Cornelisse CJ and
Cleton-Jansen AM: Simultaneous loss of E-cadherin and catenins in
invasive lobular breast cancer and lobular carcinoma in situ. J
Pathol. 183:404–411. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berx G, Cleton-Jansen AM, Strumane K, de
Leeuw WJ, Nollet F, van Roy F and Cornelisse C: E-cadherin is
inactivated in a majority of invasive human lobular breast cancers
by truncation mutations throughout its extracellular domain.
Oncogene. 13:1919–1925. 1996.PubMed/NCBI
|
|
12
|
Derksen PW, Liu X, Saridin F, van der
Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink
J, Krimpenfort P, et al: Somatic inactivation of E-cadherin and p53
in mice leads to metastatic lobular mammary carcinoma through
induction of anoikis resistance and angiogenesis. Cancer Cell.
10:437–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA
and Zhang PJ: Differential expression of E-cadherin in lobular and
ductal neoplasms of the breast and its biologic and diagnostic
implications. Am J Clin Pathol. 115:85–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benton G, Crooke E and George J: Laminin-1
induces E-cadherin expression in 3-dimensional cultured breast
cancer cells by inhibiting DNA methyltransferase 1 and reversing
promoter methylation status. FASEB J. 23:3884–3895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT,
Wu CW and Shen CY: Mechanisms of inactivation of E-cadherin in
breast carcinoma: Modification of the two-hit hypothesis of tumor
suppressor gene. Oncogene. 20:3814–3823. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 41:154–161. 1991.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herman JG, Graff JR, Myöhänen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertolo C, Guerrero D, Vicente F, Cordoba
A, Esteller M, Ropero S, Guillen-Grima F, Martinez-Peñuela JM and
Lera JM: Differences and molecular immunohistochemical parameters
in the subtypes of infiltrating ductal breast cancer. Am J Clin
Pathol. 130:414–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sebova K, Zmetakova I, Bella V, Kajo K,
Stankovicova I, Kajabova V, Krivulcik T, Lasabova Z, Tomka M,
Galbavy S and Fridrichova I: RASSF1A and CDH1 hypermethylation as
potential epimarkers in breast cancer. Cancer Biomark. 10:13–26.
2011.PubMed/NCBI
|
|
22
|
Caldeira JR, Prando EC, Quevedo FC, Neto
FA, Rainho CA and Rogatto SR: CDH1 promoter hypermethylation and
E-cadherin protein expression in infiltrating breast cancer. BMC
Cancer. 6:482006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shinozaki M, Hoon DS, Giuliano AE, Hansen
NM, Wang HJ, Turner R and Taback B: Distinct hypermethylation
profile of primary breast cancer is associated with sentinel lymph
node metastasis. Clin Cancer Res. 11:2156–2162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tao MH, Mason JB, Marian C, McCann SE,
Platek ME, Millen A, Ambrosone C, Edge SB, Krishnan SS, Trevisan M,
et al: Promoter methylation of E-cadherin, p16 and RAR-β (2) genes
in breast tumors and dietary intake of nutrients important in
one-carbon metabolism. Nutr Cancer. 63:1143–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nass SJ, Herman JG, Gabrielson E, Iversen
PW, Parl FF, Davidson NE and Graff JR: Aberrant methylation of the
estrogen receptor and E-cadherin 5′ CpG islands increases with
malignant progression in human breast cancer. Cancer Res.
60:4346–4348. 2000.PubMed/NCBI
|
|
26
|
Parrella P, Poeta ML, Gallo AP, Prencipe
M, Scintu M, Apicella A, Rossiello R, Liguoro G, Seripa D, Gravina
C, et al: Nonrandom distribution of aberrant promoter methylation
of cancer-related genes in sporadic breast tumors. Clin Cancer Res.
10:5349–5354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horne HN, Sherman ME, Garcia-Closas M,
Pharoah PD, Blows FM, Yang XR, Hewitt SM, Conway CM, Lissowska J,
Brinton LA, et al: Breast cancer susceptibility risk associations
and heterogeneity by E-cadherin tumor tissue expression. Breast
Cancer Res Treat. 143:181–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tryndyak VP, Beland FA and Pogribny IP:
E-cadherin transcriptional down-regulation by epigenetic and
microRNA-200 family alterations is related to mesenchymal and
drug-resistant phenotypes in human breast cancer cells. Int J
Cancer. 126:2575–2583. 2010.PubMed/NCBI
|
|
30
|
Berletch JB, Phipps SM, Walthall SL,
Andrews LG and Tollefsbol TO: A method to study the expression of
DNA methyltransferases in aging systems in vitro. Methods Mol Biol.
371:81–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Plass C and Soloway PD: DNA methylation,
imprinting and cancer. Eur J Hum Genet. 10:6–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Denis H, Ndlovu MN and Fuks F: Regulation
of mammalian DNA methyltransferases: A route to new mechanisms.
EMBO Rep. 12:647–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cavusoglu Celebiler A, Sevinc AI, Saydam
S, Canda T, Baskan Z, Kilic Y and Sakizli M: Promoter methylation
and expression changes of CDH1 and P16 genes in invasive breast
cancer and adjacent normal breast tissue. Neoplasma. 57:465–472.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holm K, Hegardt C, Staaf J,
Vallon-Christersson J, Jönsson G, Olsson H, Borg A and Ringnér M:
Molecular subtypes of breast cancer are associated with
characteristic DNA methylation patterns. Breast Cancer Res.
12:R362010. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pathiraja TN, Stearns V and Oesterreich S:
Epigenetic regulation in estrogen receptor positive breast
cancer-role in treatment response. J Mammary Gland Biol Neoplasia.
15:35–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kominsky SL, Fackler MJ, Lahti-Domenici J,
Polyak K, Sacchi N, Garrett-Mayer E, Argani P and Sukumar S: Very
high frequency of hypermethylated genes in breast cancer metastasis
to the bone, brain and lung. Clin Cancer Res. 10:3104–3109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ and Cleton-Jansen AM: E-cadherin
transcriptional downregulation by promoter methylation but not
mutation is related to epithelial -to- mesenchymal transition in
breast cancer cell lines. Br J Cancer. 94:661–671. 2006.PubMed/NCBI
|
|
38
|
van Horssen R, Hollestelle A, Rens JA,
Eggermont AM, Schutte M and Ten Hagen TL: E-cadherin promotor
methylation and mutation are inversely related to motility capacity
of breast cancer cells. Breast Cancer Res Treat. 136:365–377. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chao YL, Shepard CR and Wells A: Breast
carcinoma cells re-express E-cadherin during mesenchymal to
epithelial reverting transition. Mol Cancer. 9:1792010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Droufakou S, Deshmane V, Roylance R, Hanby
A, Tomlinson I and Hart IR: Multiple ways of silencing E-cadherin
gene expression in lobular carcinoma of the breast. Int J Cancer.
92:404–408. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asgeirsson KS, Jónasson JG, Tryggvadóttir
L, Olafsdóttir K, Sigurgeirsdóttir JR, Ingvarsson S and
Ogmundsdóttir HM: Altered expression of E-cadherin in breast
cancer. patterns, mechanisms and clinical significance. Eur J
Cancer. 36:1098–1106. 2000. View Article : Google Scholar : PubMed/NCBI
|