Introduction
Cisplatin (cis-diamminedichloroplatinum II) is one
of the clinical chemotherapeutic agents used against a broad
spectrum of human malignancies, such as ovarian, cervical, prostate
and lung cancer (1). However, relapse
following cisplatin therapy is inevitable. Thus, it is particularly
important to elucidate the cell-killing mechanism of cisplatin
(2–4).
Cisplatin is generally considered to kill cancer cells by damaging
DNA and inhibiting DNA synthesis, which induces
mitochondria-mediated apoptosis and consequently, cell death
(5–8).
Recent findings have revealed that cisplatin triggers apoptotic
events via endoplasmic reticulum (ER) stress (9,10).
The ER is a crucial organelle in eukaryotic cells
that is essential for the biological processes required for cell
survival and normal cell function, such as protein folding and
secretion, lipid biosynthesis and calcium homeostasis. Multiple
stimuli can cause the accumulation of unfolded and incompletely
folded proteins in the ER, which initiates the unfolded protein
response (UPR) and ER stress (11,12). In
the resting state, the ER, an intracellular store of calcium,
regulates basic calcium oscillations via calcium ion channels on
the ER membrane in order to transmit intracellular biological
information. Once balance is disrupted, a regulatory network of
downstream signaling pathways is activated (13–15).
Previous studies have suggested that chemotherapeutic agents target
the metabolism of reactive oxygen species and ATP production in the
mitochondria, activate mitochondria-mediated cell apoptosis, and
inhibit cell growth and proliferation via an increase in cellular
calcium mobilization (16–18). However, the function of calcium
signaling in ER-derived apoptosis induced by chemotherapy drugs is
not clear.
Thus, we presume that calcium signaling plays an
important role in the mitochondria-mediated and ER-mediated
apoptosis pathways following cisplatin exposure in human cervical
cancer HeLa cells. In the present study, it was found that
treatment with cisplatin significantly increased cellular calcium
concentrations and triggered the mitochondria-dependent and
ER-dependent apoptosis pathways in HeLa cells, which could be
inhibited by blocking calcium signaling. These results demonstrated
that calcium efflux from the ER plays a significant role in the
regulation of apoptosis triggered by cisplatin in HeLa cells.
Materials and methods
Reagents and antibodies
In the present study, the cisplatin,
bis-(o-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid
acetoxymethyl ester (BAPTA/AM), inositol triphosphate receptor
(IP3R) inhibitor 2-aminoethyl diphenylborinate (2-APB) and
3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal
bovine serum (FBS) and Iscove's modified Dulbecco's medium (IMDM)
were purchased from Life Technologies (Thermo Fisher Scientific
Inc., Waltham, MA, USA). Fluo-4/AM, Alexa Fluor 546 donkey
polyclonal anti-rabbit immunoglobulin (Ig)G (catalog no., A10040),
Alexa Fluor 546 donkey polyclonal anti-mouse IgG (catalog no.,
A10036) and Alexa Fluor 488 donkey polyclonal anti-rabbit IgG
(catalog no., A-21206) were purchased from Invitrogen (Thermo
Fisher Scientific Inc.). Rhod-2/AM was purchased from AAT Bioquest
Inc. (Sunnyvale, CA, USA). Enhanced chemiluminescence (ECL)
reagents were obtained from Thermo Fisher Scientific Inc. Mouse
monoclonal anti-C/EBP homologous protein (CHOP; catalog no.,
ab11419), rabbit polyclonal anti-caspase-3 (catalog no., ab13847)
and rabbit polyclonal anti-activated-caspase-3 (catalog no.,
ab2302) antibodies were purchased from Abcam (Cambridge, MA, USA),
while rabbit polyclonal anti-caspase-4 (catalog no., 24287) and
rabbit polyclonal anti-calpain-1 catalytic subunit (CAPN1; catalog
no., 32201) antibodies were purchased from Signalway Antibody Co.
(College Park, MD, USA), and mouse monoclonal
anti-glucose-regulated protein (GRP78; 78 kDa; catalog no.,
sc-376768) antibody was purchased from Santa Cruz Biotechnology
Inc. (Dallas, TX, USA). Rabbit polyclonal anti-β-actin (catalog
no., 20536-1-AP) and peroxidase-conjugated Affinipure goat
anti-mouse- and anti-rabbit Ig were purchased from Proteintech
Group Inc. (Chicago, IL, USA; catalog nos., SA00001-1 and
SA00001-2). All other reagents and antibodies were purchased from
Changchun Baoxin Biotechnology Co. (Changchun, China).
Cell culture
Human cervical cancer HeLa cells were obtained from
China Academy of Chinese Medical Sciences (Beijing, China), and
were cultured at 37°C in a 5% (v/v) CO2 and 95% (v/v)
air atmosphere, using IMDM containing 10% (v/v) FBS, and 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were divided into
six groups: Non-treated cells, cells treated with 5 µg/ml
cisplatin, cells treated with 2.5 µM BAPTA/AM, cells treated with
100 µM 2-APB, cells treated with 5 µg/ml cisplatin combined with
2.5 µM BAPTA/AM, and cells treated with 5 µg/ml cisplatin combined
with 100 µM 2-APB.
Determination of intracellular and
mitochondrial free Ca2+ levels
The fluorescent calcium indicator Fluo-4/AM (5 µM)
and Rhod-2 (5 µM) were used to measure intracellular and
mitochondrial free Ca2+ levels, as previously described (19). The cells were monitored using an
Olympus FV1000 confocal laser microscope (Olympus Corporation,
Tokyo, Japan). Fluorescence images labeled with Fluo-4/Rhod-2 were
collected using an excitation wavelength of 488/546 nm. The same
parameters of illumination and detection were maintained digitally
for consistency throughout the experiments.
MTT assay
Cell viability was determined using the MTT assay.
In the experiments, exponentially growing HeLa cells were seeded in
96-well culture plates at a density of 1×104 cells/well. After 24 h
of incubation, cisplatin with or without BAPTA/AM and 2-APB was
added for 24 h in four parallel wells. MTT solution (5 mg/ml) was
added for 4 h, followed by the addition of 150 µl dimethyl
sulfoxide. After 10 min of shaking, the absorbance was measured at
570 nm using a microplate reader (iMark; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The survival rate was calculated as
follows: Survival (%) = absorbance of experimental group /
absorbance of control group × 100. In each experiment, the mean
value of four wells per treatment group was calculated.
Flow cytometry analysis
The MUSE™ Annexin-V Dead Cell kit (EMD Millipore,
Billerica, MA, USA) was used to monitor cell death. In the
experiments, exponentially growing HeLa cells were seeded in 6-well
culture plates at a density of 2×105 cells/well. Following exposure
to the different experimental conditions, the cells were
trypsinized and resuspended in IMDM medium with 10% FBS at a
concentration of 1×106 cells/ml. The cells were incubated with
Annexin-V in the dark at room temperature for 20 min. Finally,
samples were detected using the MUSE Cell Analyzer (EMD Millipore).
All experiments were performed in triplicate.
Immunofluorescence staining and
confocal laser microscopy
The cells were cultured on coverslips overnight, and
after treatment with the indicated dose of cisplatin with or
without BAPTA/AM and 2-APB for 24 h, were fixed with 4% (w/v)
paraformaldehyde, stained with nuclear Hoechst 33342 (1 µg/ml;
Sigma-Aldrich) for 5 min, washed with phosphate-buffered solution
(PBS), and examined using an Olympus FV1000 confocal laser
microscope to reveal cell chromatin condensation. The expression of
GRP78, active caspase-3 and CHOP was examined by indirect
immunofluorescence. The cells were cultured on coverslips
overnight, then treated with the indicated dose of cisplatin with
or without BAPTA/AM and 2APB for 24 h, and rinsed with PBS three
times. After incubation, the cells were fixed with 4% (w/v)
paraformaldehyde for 20 min, permeabilized with 0.1% (v/v) Triton
X-100 for 5 min, blocked with bovine serum albumin, and incubated
with the primary antibodies for GRP78 (1:50 dilution), active
caspase-3 (1:250 dilution), and CHOP (1:100 dilution) overnight at
4°C. The cells were then incubated in Alexa Fluor 488 Donkey
Anti-Rabbit IgG, Alexa Fluor 546 Donkey Anti-Rabbit IgG and Alexa
Fluor 546 Donkey Anti-Mouse IgG secondary antibodies (1:400
dilution) for 30 min, stained with Hoechst 33342 (1 µg/ml) for 5
min and washed with PBS three times. After mounting, the cells were
examined under an Olympus FV1000 confocal laser microscope. The
same parameters of illumination and detection were maintained
digitally throughout the experiments.
Western blotting
Whole-cell protein extracts from the HeLa cells were
prepared with cell lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1 mM sodium-EDTA, 1 mM EDTA, 1% (v/v) Triton X-100, 2.5 mM
sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 mM NaF, 1 µg/ml leupeptin and 1 mM
PMSF] for western blotting. Protein concentration was quantified
using the BCA Protein Assay kit (Pierce™; Thermo Fisher Scientific
Inc.). For western blot analysis, lysates (30 µg) were resolved on
10% (w/v) sodium dodecyl sulfate-polyacrylamide gels and
transferred onto immobilon-P transfer membranes (EMD Millipore).
The membranes were blocked with 5% (w/v) skimmed dry milk in buffer
[10 mM Tris-HCl (pH 7.6), 100 mM NaCl and 0.1% Tween 20] for 1 h at
room temperature and then incubated with the desired primary
antibody overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (1:2,000; Proteintech
Group Inc.) for 1.5 h at room temperature. Immunodetection was
performed using the ECL reagents and images were captured using
Syngene Bio Imaging (Synoptics, Cambridge, UK). The level of
protein was normalized to that of actin and the ratios of
normalized protein to actin are presented as the mean ± standard
deviation from three independent experiments. Protein levels were
quantified by densitometry using Quantity One version 4.6.2
software (Bio-Rad Laboratories Inc.).
Statistical analysis
Data are representative of three independent
experiments each performed in triplicate. Statistical analysis of
the data was performed using a one-way analysis of variance on IBM
SPSS version 22.0 (IBM SPSS, Armonk, NY, USA). Tukey's post-hoc
test was used to determine the significance for all pairwise
comparisons of interest. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cisplatin increases free Ca2+ levels
in the cytosol and mitochondria
For the determination of cytosolic and mitochondrial
free Ca2+ levels, the Ca2+-sensitive fluorescent dyes, Fluo-4/AM
and Rhod-2/AM, were used. Based on previous studies (10), the HeLa cells were treated with
increasing doses of cisplatin (2.5, 5 and 10 µg/ml) for 0, 6, 12
and 24 h, and further incubated with the calcium indicators
Fluo-4/AM and Rhod-2/AM. Confocal microscopy was used to observe
alterations in free Ca2+ levels. It was found that the free Ca2+
levels in the cytosol (Fig. 1A and B)
and mitochondria (Fig. 1C and D)
increased in a dose- and time-dependent manner in the HeLa cells
(P<0.001).
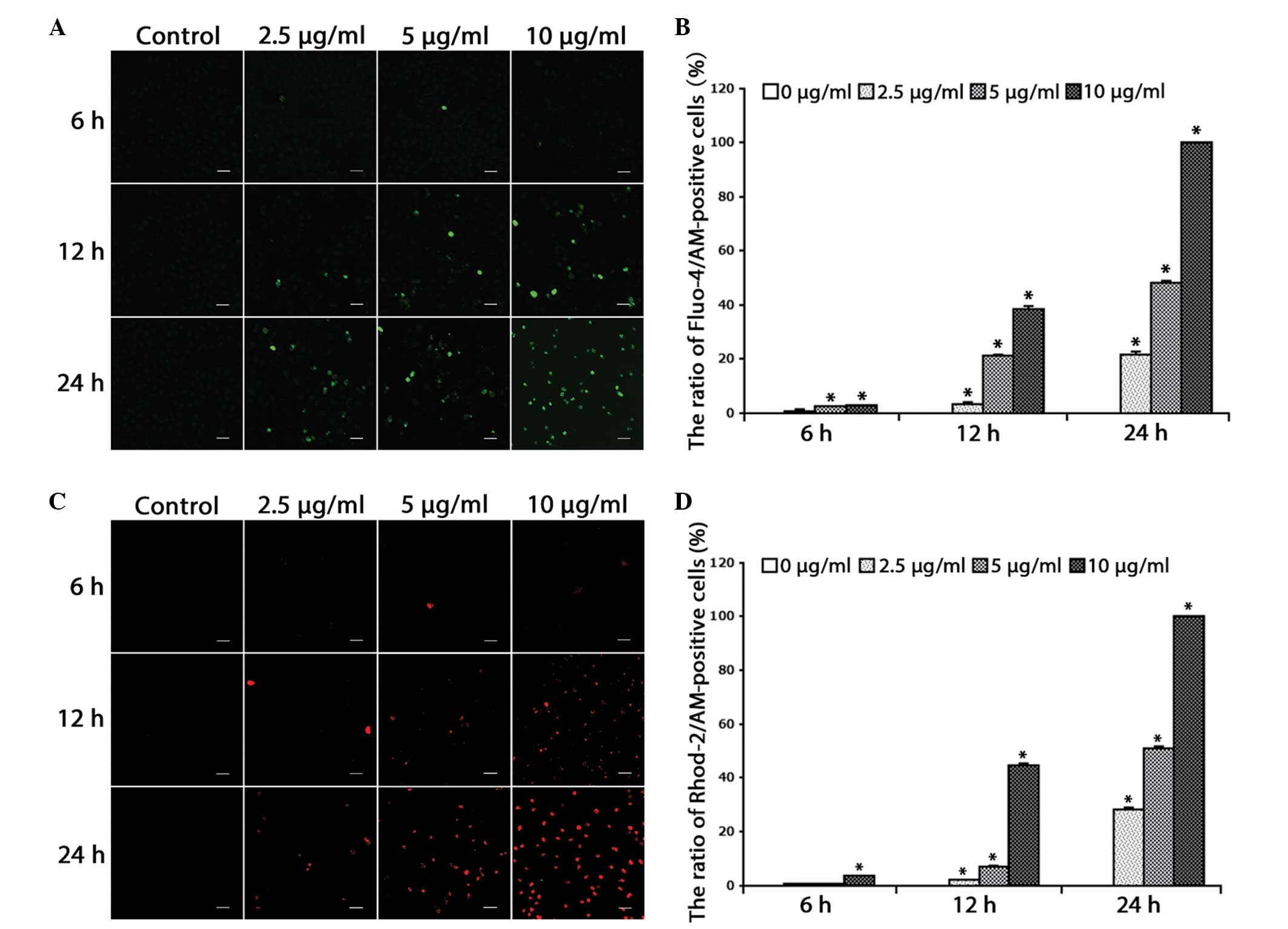 | Figure 1.Cisplatin increases free
Ca2+ levels in the cytosol and mitochondria. (A) The
cells were treated with cisplatin (2.5, 5 or 10 µg/ml) for 0, 6, 12
and 24 h, and incubated with the fluorescent calcium indicator,
Fluo-4/AM. Calcium concentrations in the cytosol were observed by
confocal microscopy (scale bar, 40 µm). (B) Quantitation of free
Ca2+ levels in the cytosol. Data are presented as the
mean ± SD (n=3). *P<0.05 vs. control. (C) Cells were treated
with cisplatin (2.5, 5 or 10 µg/ml) for 0, 6, 12 and 24 h, and
incubated with the fluorescent calcium indicator, Rhod-2. Calcium
concentrations in the mitochondria were observed by confocal
microscopy (scale bar, 40 µm). (D) Quantitation of free
Ca2+ levels in the mitochondria. Data are presented as
the mean ± SD (n=3). *P<0.05 vs. control. SD, standard
deviation. |
These results suggested that cisplatin increases
free Ca2+ levels in the cytosol and mitochondria of HeLa
cells, indicating the relevance of calcium in the cell death
induced by cisplatin.
Calcium signaling is involved in
cisplatin-induced mitochondria-dependent apoptosis
To evaluate the action of calcium in
cisplatin-induced apoptotic cell death, the calcium chelating
agent, BAPTA/AM, and the IP3R inhibitor, 2-APB, were used to alter
the free Ca2+ levels induced by cisplatin. It was observed that the
cisplatin-induced free Ca2+ levels in the cytosol and mitochondria
substantially decreased in the groups treated with cisplatin
combined with BAPTA/AM or 2-APB (Fig. 2A
and B).
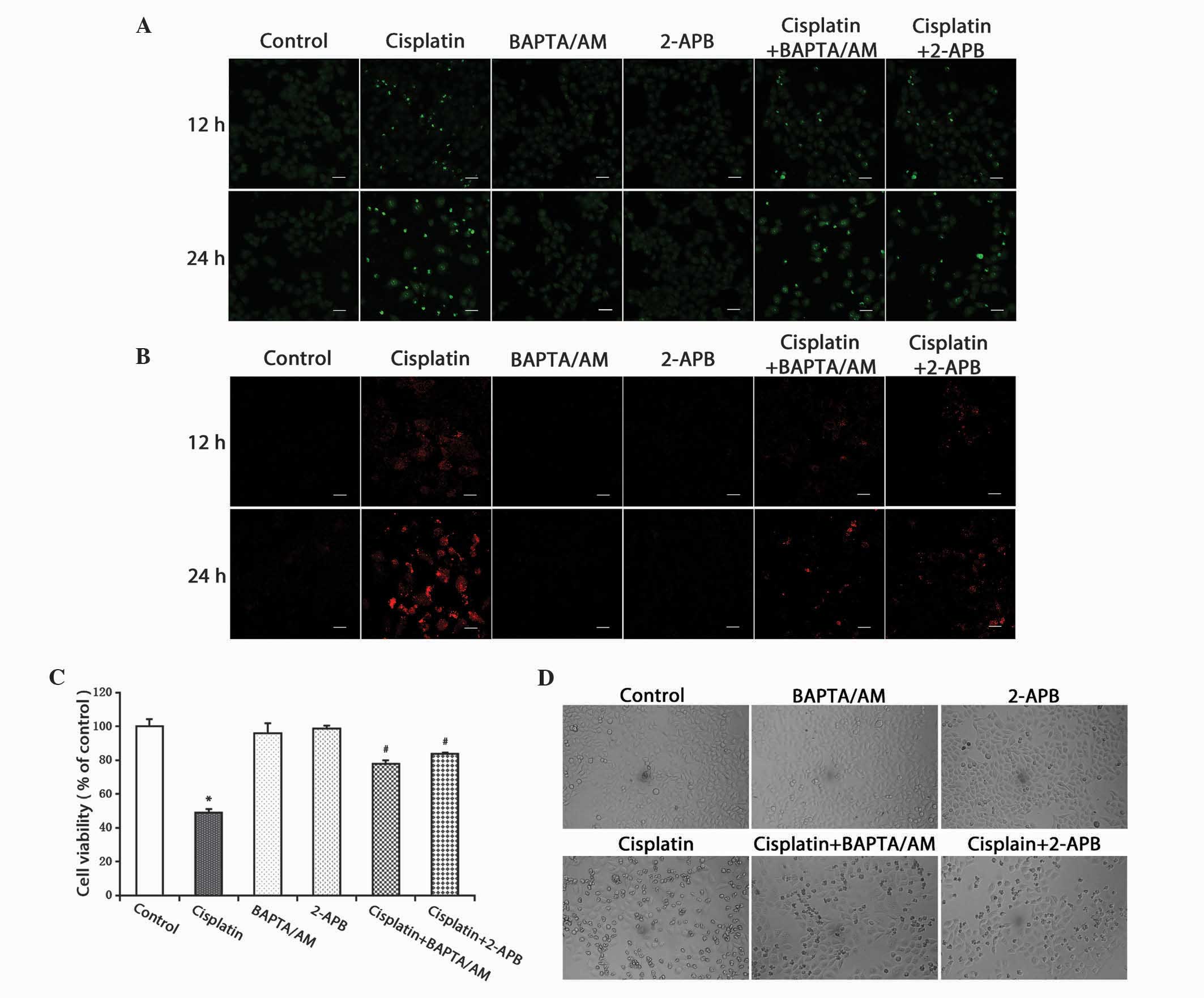 | Figure 2.Inhibition of calcium signaling
decreases the level of free Ca2+ in the cytosol and
mitochondria, and inhibits cell growth. (A) HeLa cells were treated
with cisplatin (5 µg/ml) with or without BAPTA/AM (2.5 µM) and
2-APB (100 µM) for 12 and 24 h. The cells were incubated with the
fluorescent calcium indicator, Fluo-4/AM. Calcium concentrations in
the cytosol were observed by confocal microscopy (scale bar, 40
µm). (B) HeLa cells were treated with cisplatin (5 µg/ml) with or
without BAPTA/AM (2.5 µM) and 2-APB (100 µM) for 12 and 24 h, and
incubated with the fluorescent calcium indicator, Rhod-2. Calcium
concentrations in the mitochondria were observed by confocal
microscopy (scale bar, 30 µm). (C) HeLa cells were treated with
cisplatin (5 µg/ml) with or without BAPTA/AM (2.5 µM) and 2-APB
(100 µM) for 24 h. Cell viability was determined using the
3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Data are presented as the mean ± standard deviation (n=3).
*P<0.05 vs. control; #P<0.05 vs. cisplatin. (D)
HeLa cells were treated with cisplatin (5 µg/ml) with or without
BAPTA/AM (2.5 µM) and 2-APB (100 µM) for 24 h. Cell morphology was
observed using an inverted phase contrast microscope at x100
magnification. BAPTA/AM,
bis-(o-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid
acetoxymethyl ester; 2-APB, 2-aminoethyl diphenylborinate. |
The MTT assay was used to detect cell viability. The
results demonstrated that cisplatin inhibited cell viability in the
HeLa cells. Treatment combined with BAPTA/AM or 2-APB decreased the
cytotoxic effects of cisplatin (P<0.001), while treatment with
BAPTA/AM or 2-APB alone had no significant effect on cell viability
(Fig. 2C). At the same time, an
optical microscope was used to examine cellular morphological
changes. Compared with the controls, the cells treated with
cisplatin became round and fragmented. The numbers of rounded and
fragmented cells in the cultures treated with cisplatin combined
with BAPTA/AM or 2-APB were less than that in the group treated
with only cisplatin (Fig. 2D).
Based on the MTT results, flow cytometry was used to
detect the ratio of cell apoptosis induced by cisplatin in the HeLa
cells. Consistent with the MTT results, the rate of apoptosis in
the cells treated with cisplatin combined with BAPTA/AM or 2-APB
(37.8 and 36.1%, respectively) was lower than that of the cells
treated only with cisplatin (55.3%) (Fig.
3A). Using confocal microscopy, apoptotic chromatin
condensation was examined with Hoechst 33342 staining. Treatment
with cisplatin alone induced significant apoptotic chromatin
condensation in the HeLa cells, while the cells treated with
cisplatin combined with BAPTA/AM or 2-APB showed a lower ratio of
apoptotic chromatin condensation (Fig.
3B).
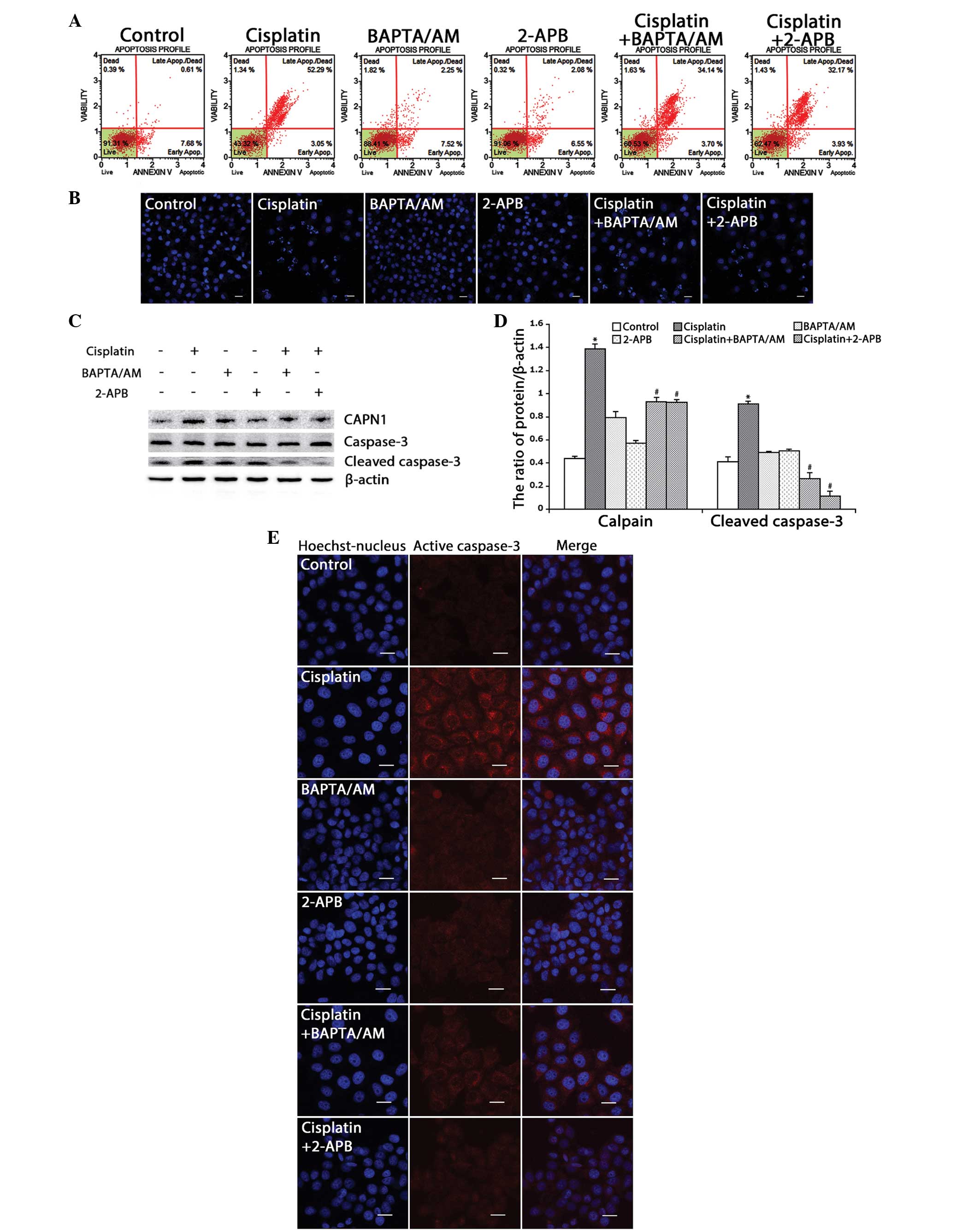 | Figure 3.Inhibition of calcium signaling
decreases cisplatin-induced mitochondria-mediated apoptosis in HeLa
cells. (A) HeLa cells were treated with cisplatin (5 µg/ml) with or
without BAPTA/AM (2.5 µM) and 2-APB (100 µM) for 24 h and then
stained with Annexin-V. Data are presented as the mean ± SD (n=3).
(B) HeLa cells were treated with cisplatin (5 µg/ml) with or
without BAPTA/AM (2.5 µM) and 2-APB (100 µM) for 24 h, and stained
with Hoechst 33342. Cell morphology was observed by confocal
microscopy (scale bar, 30 µm). (C) The expression of calpain,
caspase-3 and cleaved caspase-3 in HeLa cells treated with
cisplatin (5 µg/ml) with or without BAPTA/AM (2.5 µM) and 2-APB
(100 µM) for 12 h by western blotting. (D) Quantitation of calpain
and cleaved caspase-3 protein levels. Data are presented as the
mean ± SD (n=3). *P<0.05 vs. control; #P<0.05 vs.
cisplatin. (E) The expression of cleaved caspase-3 was detected by
confocal microscopy following the various treatments for 12 h
(scale bar, 20 µm). BAPTA/AM,
bis-(o-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid
acetoxymethyl ester; 2-APB, 2-aminoethyl diphenylborinate; SD,
standard deviation. |
CAPN1 (also known as µ-calpain) is a
Ca2+-dependent cysteine protease, and its activation
requires high levels of free calcium in the cytosol (20,21). Thus,
the present study assessed calcium levels and apoptosis in the HeLa
cells by monitoring the expression of CAPN1 and cleaved caspase-3
by western blotting. As shown in Fig. 3C
and D, cisplatin enhanced the expression of CAPN1 and cleaved
caspase-3 (P<0.001). Meanwhile, treatment with cisplatin
combined with BAPTA/AM or 2-APB decreased the level of CAPN1 and
cleaved caspase-3 induced by cisplatin. Consistent with the western
blotting results, immunofluorescence against caspase-3 revealed a
similar effect (Fig. 3E). These
results indicated that cisplatin upregulates intracellular calcium
mobilization and initiates apoptotic events.
Cisplatin-induced pro-apoptosis
calcium signaling is derived from ER stress
Cisplatin has been reported to induce ER stress and
downstream processes associated with apoptosis (7,10,22). To further verify if the resultant
apoptosis was partially induced by ER stress, the present study
assessed the occurrence of ER stress and ER-associated apoptosis by
examining the expression levels of ER stress-associated
proteins.
The expression of GRP78, an ER molecular chaperone
that induces ER stress upon accumulation, was detected. Western
blotting revealed that cisplatin upregulated the expression of
GRP78, and that combined treatment with BAPTA/AM or 2-APB
downregulated the expression of GRP78 (Fig. 4A and B; P<0.001). Using confocal
microscopy, it was observed that the accumulation of GRP78
increased in cells treated with cisplatin only, while combined
treatment with BAPTA/AM or 2-APB markedly decreased the
cisplatin-induced GRP78 accumulation (Fig. 4C).
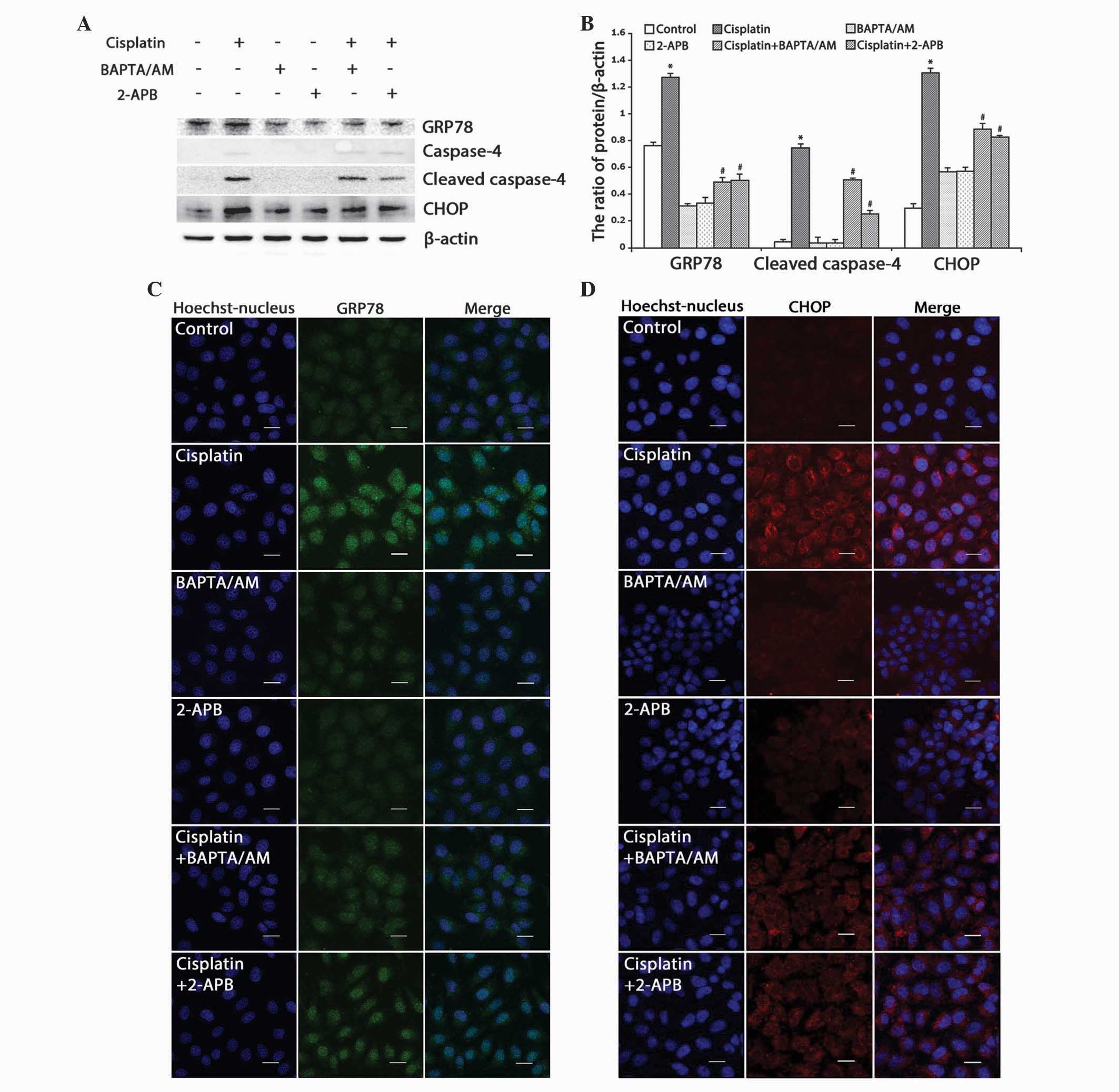 | Figure 4.Inhibition of calcium signaling
decreases cisplatin-induced ER stress-mediated apoptosis in HeLa
cells. (A) Western blotting detection of ER stress proteins in HeLa
cells treated with cisplatin (5 µg/ml) with or without BAPTA/AM
(2.5 µM) and 2-APB (100 µM) for 12 h. (B) Quantitation of ER stress
protein levels. Data are presented as the mean ± standard deviation
(n=3). *P<0.05 vs. control; #P<0.05 vs. cisplatin.
(C) The expression of GRP78 was detected by confocal microscopy
following various treatments for 12 h (scale bar, 20 µm). (D) The
expression of CHOP was detected by confocal microscopy following
various treatments for 12 h (scale bar, 20 µm). BAPTA/AM,
bis-(o-aminophenoxy)ethane-N,N,N',N'-tetra-acetic acid
acetoxymethyl ester; 2-APB, 2-aminoethyl diphenylborinate; CHOP,
C/EBP homologous protein; GRP78, glucose-regulated protein, 78 kDa;
ER, endoplasmic reticulum. |
Next, the ER stress-associated apoptotic proteins,
caspase-4 and CHOP, were monitored. Western blotting revealed that
cisplatin enhanced the expression of CHOP and cleaved caspase-4,
while combined treatment with BAPTA/AM or 2-APB inhibited these
effects (Fig. 4A and B; P<0.001).
Similarly, confocal microscopy revealed marked accumulation of CHOP
in cells treated with cisplatin alone, and low-level CHOP
accumulation in groups treated with cisplatin combined with
BAPTA/AM or 2-APB (Fig. 4D).
These results demonstrated that cisplatin induced
pro-apoptotic calcium signaling, which resulted in ER
stress-mediated apoptotic events in the HeLa cells.
Discussion
Cisplatin is one of several effective antitumor
drugs widely used against multiple solid tumors in the clinic; it
acts by damaging DNA, inhibiting DNA synthesis and inducing
apoptosis in cancer cells (5,23). Previous studies have reported that
chemotherapy drugs, including cisplatin, cause mitochondrial
dysfunction, the release of cytochrome c, the activation of
the caspase-mediated cascade and apoptotic cell death (24–27). In
the present study, it was found that cisplatin inhibited cell
growth, upregulated active caspase-3 and induced the
mitochondria-mediated apoptosis pathway in human HeLa cells
(Figs. 2 and 3). An increasing amount of evidence has
indicated that cisplatin induces cell apoptosis through ER stress
(9,10,22). The
ER is an important organelle in eukaryotic cells, and is involved
in several critical processes. Under stressful conditions, numerous
unfolded and incompletely folded proteins accumulate in the ER
lumen, which stimulates the UPR, leading to ER stress (11,12). It is
generally considered that the accumulation of the calcium-dependent
molecular chaperone, GRP78, initiates ER stress (10,28). The
results of the present study indicated that treatment with
cisplatin induces the accumulation of GRP78 in HeLa cells and
results in ER stress (Fig. 4A and C).
The transcription factor CHOP (also known as DNA damage-inducible
transcript 3) is an ER stress-associated apoptosis protein that
integrates the apoptotic endoplasmic reticulum to nucleus signaling
1, eukaryotic translation initiation factor 2α kinase 3 and
activating transcription factor 6 pathways in the UPR (29–32).
Caspase-4 is a calcium-dependent caspase; its overexpression
stimulates the downstream cascade reaction and leads to cell
apoptosis, which is caused by destruction of the ER and calcium
pool emptying (33–35). Thus, the present study examined the
expression of these two ER stress-associated apoptotic proteins.
The results demonstrated that cisplatin markedly increased the
expression levels of these two proteins (Fig. 4A and B). These data suggested that in
addition to the mitochondria-mediated apoptotic pathway, cisplatin
could also induce cell apoptosis via ER stress. Notably,
concurrently with the occurrence of apoptosis, the cytosolic and
mitochondrial free Ca2+ levels increased, and calpain
expression was upregulated. The modulation of calcium concentration
in the cytoplasm affected the activation of the calpains, which are
evolutionarily conserved Ca2+-dependent cysteine
proteases (36,37). It is reported that the overexpression
of calpains promotes the cleavage of Bid, which in turn modulates
Bax and activates caspase-3. There is also evidence to suggest that
activation of caspase-4 requires calpains (38–40). The
aforementioned results suggest that calcium signaling involves two
apoptotic pathways: i) Calcium ion entry into the mitochondria,
which induces calcium disorder in he mitochondria and leads to
mitochondria-mediated apoptosis; and ii) calcium signaling in the
cytosol activates calcium-dependent proteases and results in the
expression of ER stress-associated apoptotic proteins.
To investigate this hypothesis, the calcium
chelating agent, BAPTA/AM, was used to block free Ca2+
in the cytoplasm. Won et al reported that apoptosis induced
by 3α,23-isopropylidenedioxyolean-12-en-27-oic acid was
substantially reduced following combined treatment with BAPTA/AM in
HeLa cells (40). In the present
study, HeLa cells were treated with cisplatin combined with
BAPTA/AM. The data demonstrated that calcium concentrations in the
cytoplasm and expression levels of calpain were reduced in the
presence of BAPTA/AM. Moreover, in the group treated with cisplatin
combined with BAPTA/AM, the ER-stress proteins CHOP and active
caspase-4 were downregulated (Fig. 4A and
B). These results indicated that endogenous calcium efflux
participates in ER stress-mediated apoptosis induced by cisplatin
in HeLa cells. Intercellular calcium homeostasis is controlled by
calcium ion channels on the ER membrane, which include IP3R,
ryanodine receptors and sarcoendoplasmic reticular
Ca2+-ATPase pumps (41,42). To
further confirm the source of calcium signaling, the IP3R
inhibitor, 2-APB, was used to inhibit calcium release from the ER.
Yoon et al reported that 2-APB potently inhibited calcium
release from the ER and suppressed celastrol-induced apoptosis in
breast cancer cell lines (MDA-MB 435S and MCF-7) (43). In the present study, it was found that
combined treatment with 2-APB decreased calcium release from the ER
and inhibited cisplatin-induced apoptosis in the HeLa cells. The
data revealed that these inhibitory effects mainly manifested via
two mechanisms: i) 2-APB inhibits calcium flux from the ER to the
mitochondria and decreases the expression of cleaved caspase-3,
which attenuates mitochondria-mediated apoptosis; and ii) 2-APB
blocks calcium flux from the ER to the cytosol, inhibits the
activation of calpains, and downregulates the expression of the ER
stress proteins, CHOP and caspase-4. These results suggested that
blocking calcium release from the ER effectively mitigates the ER
stress-associated apoptotic pathway. Previous studies have revealed
that cisplatin initiates ER stress and interferes with calcium
homeostasis (44,45). The present study found that blocking
calcium efflux decreased the level of ER stress correspondingly.
This phenomenon suggests that calcium signaling may act as a
positive feedback mechanism by modulating ER stress. However,
further investigations are required.
In summary, the present study demonstrated that
cisplatin induced ER stress and led to apoptosis in human HeLa
cells. Notably, treatment with cisplatin combined with BAPTA/AM or
2-APB markedly decreased the inhibition of cell growth and the rate
of apoptosis. The results demonstrate that calcium efflux from the
ER regulates cell apoptosis triggered by cisplatin in HeLa cells.
In addition, the study provided further mechanistic insights into
the tumor cell-killing effect of cisplatin and potential
therapeutic strategies to improve cisplatin chemotherapy.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (grant nos. 81272876 and 81372793) and
the Department of Education of Jilin Province Project (grant no.
2013361).
References
|
1
|
Reedijk J: New clues for platinum
antitumor chemistry: Kinetically controlled metal binding to DNA.
Proc Natl Acad Sci USA. 100:3611–3616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wozniak K and Blasiak J: Recognition and
repair of DNA-cisplatin adducts. Acta Biochim Pol. 49:583–596.
2002.PubMed/NCBI
|
|
3
|
Koberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
4
|
Basu A and Krishnamurthy S: Cellular
responses to Cisplatin-induced DNA damage. J Nucleic Acids.
2010:pii; 2013672010. View Article : Google Scholar
|
|
5
|
Terada Y, Inoue K, Matsumoto T, Ishihara
M, Hamada K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T,
et al: 5-Aminolevulinic acid protects against cisplatin-induced
nephrotoxicity without compromising the anticancer efficiency of
cisplatin in rats in vitro and in vivo. PloS One.
8:e808502013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maimaitiyiming H, Li Y, Cui W, Tong X,
Norman H, Qi X and Wang S: Increasing cGMP-dependent protein kinase
I activity attenuates cisplatin-induced kidney injury through
protection of mitochondria function. Am J Physiol Renal Physiol.
305:F881–F890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang M, Wang CY, Huang S, Yang T and Dong
Z: Cisplatin-induced apoptosis in p53-deficient renal cells via the
intrinsic mitochondrial pathway. Am J Physiol Renal Physiol.
296:F983–F993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Wang C and Li Z: A new strategy of
promoting cisplatin chemotherapeutic efficiency by targeting
endoplasmic reticulum stress. Mol Clin Oncol. 2:3–7.
2014.PubMed/NCBI
|
|
9
|
Xu Y, Yu H, Qin H, et al: Inhibition of
autophagy enhances cisplatin cytotoxicity through endoplasmic
reticulum stress in human cervical cancer cells. Cancer Lett.
314:232–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nature reviews. Nat Rev Drug Discov. 7:1013–1030.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schröder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smedler E and Uhlén P: Frequency decoding
of calcium oscillations. Biochim Biophys Acta. 1840:964–969. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torres M, Encina G, Soto C and Hetz C:
Abnormal calcium homeostasis and protein folding stress at the ER:
A common factor in familial and infectious prion disorders. Commun
Integr Biol. 4:258–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mekahli D, Bultynck G, Parys JB, De Smedt
H and Missiaen L: Endoplasmic-reticulum calcium depletion and
disease. Cold Spring Harb Perspect Biol. 3:pii, a0043172011.
View Article : Google Scholar
|
|
15
|
Rharass T, Lemcke H, Lantow M, Kuznetsov
SA, Weiss DG and Panáková D: Ca2+-mediated mitochondrial
reactive oxygen species metabolism augments Wnt/β-catenin pathway
activation to facilitate cell differentiation. J Biol Chem.
289:27937–27951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tusskorn O, Senggunprai L, Prawan A,
Kukongviriyapan U and Kukongviriyapan V: Phenethyl isothiocyanate
induces calcium mobilization and mitochondrial cell death pathway
in cholangiocarcinoma KKU-M214 cells. BMC Cancer. 13:5712013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy SS and Hajnóczky G: Calcium,
mitochondria and apoptosis studied by fluorescence measurements.
Methods. 46:213–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burkeen JF, Womac AD, Earnest DJ and Zoran
MJ: Mitochondrial calcium signaling mediates rhythmic extracellular
ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci.
31:8432–8440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neuhof C and Neuhof H: Calpain system and
its involvement in myocardial ischemia and reperfusion injury.
World J Cardiol. 6:638–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki M, Endo M, Shinohara F, Echigo S
and Rikiishi H: Enhancement of cisplatin cytotoxicity by SAHA
involves endoplasmic reticulum stress-mediated apoptosis in oral
squamous cell carcinoma cells. Cancer Chemother Pharmacol.
64:1115–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
23
|
Strasser A, O'Connor L and Dixit VM:
Apoptosis signaling. Annu Rev Biochem. 69:217–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Kamdar O, Le W, Rosen GD and
Upadhyay D: Nicotine induces resistance to chemotherapy by
modulating mitochondrial signaling in lung cancer. Am J Respir Cell
Mol Biol. 40:135–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin L, Tabe Y, Kojima K, Zhou Y, Pittaluga
S, Konopleva M, Miida T and Raffeld M: MDM2 antagonist Nutlin-3
enhances bortezomib-mediated mitochondrial apoptosis in
TP53-mutated mantle cell lymphoma. Cancer Lett. 299:161–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cullen KJ, Yang Z, Schumaker L and Guo Z:
Mitochondria as a critical target of the chemotherapeutic agent
cisplatin in head and neck cancer. J Bioenerg Biomembr. 39:43–50.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong JT, Xu Y, Yi HW, Su J, Yu HM, Xiang
XY, Li XN, Zhang ZC and Sun LK: The BH3 mimetic S1 induces
autophagy through ER stress and disruption of Bcl-2/Beclin 1
interaction in human glioma U251 cells. Cancer Lett. 323:180–187.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scheuner D, Song B, McEwen E, Liu C,
Laybutt R, Gillespie P, Saunders T, Bonner-Weir S and Kaufman RJ:
Translational control is required for the unfolded protein response
and in vivo glucose homeostasis. Mol Cell. 7:1165–1176.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatsuta T, Hosono M, Miura Y, Sugawara S,
Kariya Y, Hakomori S and Nitta K: Involvement of ER stress in
apoptosis induced by sialic acid-binding lectin (leczyme) from
bullfrog eggs. Int J Oncol. 43:1799–1808. 2013.PubMed/NCBI
|
|
33
|
Li C, Wei J, Li Y, He X, Zhou Q, Yan J,
Zhang J, Liu Y, Liu Y and Shu HB: Transmembrane Protein 214
(TMEM214) mediates endoplasmic reticulum stress-induced caspase 4
enzyme activation and apoptosis. J Biol Chem. 288:17908–17917.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hitomi J, Katayama T, Eguchi Y, Kudo T,
Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K,
et al: Involvement of caspase-4 in endoplasmic reticulum
stress-induced apoptosis and Abeta-induced cell death. J Cell Biol.
165:347–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tompa P, Töth-Boconádi R and Friedrich P:
Frequency decoding of fast calcium oscillations by calpain. Cell
Calcium. 29:161–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Angka L, Lee EA, Rota SG, Hanlon T, Sukhai
M, Minden M, McMillan EM, Quadrilatero J and Spagnuolo PA:
Glucopsychosine increases cytosolic calcium to induce
calpain-mediated apoptosis of acute myeloid leukemia cells. Cancer
Lett. 348:29–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma AK and Rohrer B: Calcium-induced
calpain mediates apoptosis via caspase-3 in a mouse photoreceptor
cell line. J Biol Chem. 279:35564–35572. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mandic A, Viktorsson K, Strandberg L,
Heiden T, Hansson J, Linder S and Shoshan MC: Calpain-mediated Bid
cleavage and calpain-independent Bak modulation: Two separate
pathways in cisplatin-induced apoptosis. Mol Cell Biol.
22:3003–3013. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuzaki S, Hiratsuka T, Kuwahara R,
Katayama T and Tohyama M: Caspase-4 is partially cleaved by calpain
via the impairment of Ca2+ homeostasis under the ER
stress. Neurochem Int. 56:352–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Won SJ, Ki YS, Chung KS, Choi JH, Bae KH
and Lee KT: 3α,23-isopropylidenedioxyolean-12-en-27-oic acid, a
triterpene isolated from Aceriphyllum rossii, induces
apoptosis in human cervical cancer HeLa cells through mitochondrial
dysfunction and endoplasmic reticulum stress. Biol Pharm Bull.
33:1620–1626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gerasimenko JV, Gerasimenko OV and
Petersen OH: The role of Ca2+ in the pathophysiology of
pancreatitis. J Physiol. 592:269–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoon MJ, Lee AR, Jeong SA, Kim YS, Kim JY,
Kwon YJ and Choi KS: Release of Ca2+ from the
endoplasmic reticulum and its subsequent influx into mitochondria
trigger celastrol-induced paraptosis in cancer cells. Oncotarget.
5:6816–6831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian F, Schrödl K, Kiefl R, Huber RM and
Bergner A: The hedgehog pathway inhibitor GDC-0449 alters
intracellular Ca2+ homeostasis and inhibits cell growth
in cisplatin-resistant lung cancer cells. Anticancer Res. 32:89–94.
2012.PubMed/NCBI
|
|
45
|
Chen Y, Tsai YH and Tseng SH: RECK
regulated endoplasmic reticulum stress response and enhanced
cisplatin-induced cell death in neuroblastoma cells. Surgery.
154:968–979. 2013. View Article : Google Scholar : PubMed/NCBI
|


















