Introduction
Gliomas are recognized as highly malignant and
intractable cancers and are the most common type of primary brain
tumors throughout the world (1). The
complex cellular composition, diffuse invasiveness and capacity to
escape conventional therapies have been challenging and have
hampered progress towards an effective treatment (2,3). A major
obstacle limiting the effectiveness of conventional therapies for
gliomas and other types of cancer, is the lack of tumor specificity
(4,5).
Therefore, exploring the therapeutic strategies that specifically
target tumor tissues is critical.
The mesenchymal stem cell (MSC)-mediated strategy
has demonstrated considerable potential for the development of
clinically meaningful patient-tailored anticancer therapies
(6–9).
The tumor-directed migration and incorporation of MSCs have been
demonstrated in a number of preclinical studies in vitro,
using Transwell migration assays, and in vivo, using animal
tumor models (10–12). Directed at the tumor microenvironment,
engineered MSCs are able to express or release anticancer
substances that may constantly act on the adjacent tumor cells
(13). As a direct attacker, an
anticancer gene that was pre-engineered on MSCs plays a critical
role for the action in this system (14). Plasmid DNA transfection and viral
vector transduction are widely used in gene therapy-associated
studies (15–19). These methodologies have the potential
hazards of genomic recombination or insertional mutagenesis, which
is a significant problem when this technology is applied to gene
therapy (20,21). Therefore, an approach that carries
anticancer genes using MSCs, without genetic modification and
without risks for clinical applications, may be desired for
anticancer medicine. Recently, a novel stabilized mRNA construct
has become a more attractive alternative to the most commonly used
DNA-based plasmid (pDNA) (22).
Synthesized mRNA in vitro has been widely used to generate
induced pluripotent stem cells or induce cell reprogramming
(23–26).
Phosphatase and tensin homolog (PTEN) functions as
the important negative regulator of PI3K-AKT-mTOR pathway in
controlling cells apoptosis (27–29). The
activity of PTEN is lost by deletions, mutations or promoter
methylation at a high frequency in numerous human cancers (30). Therefore, restoring PTEN function in
cancer cells may break down the PTEN mutation-dependent cancer cell
growth, termed oncogene addiction, and demonstrates considerable
promise for cancer therapy. The present study describes an
experimental strategy that inhibits malignant glioma U251 cells
using a conditioned medium (CM) from PTEN-engineered MSCs through
the use of in vitro synthesized PTEN mRNA. The strategy has
initiated the application of advantageous PTEN mRNA in the field of
cancer research. The novel synthetic modified PTEN mRNA possesses
great potential for use as an effective therapeutic candidate for
glioblastoma patients.
Materials and methods
Cells and culture conditions
MSCs were isolated from healthy human pancreatic
ductal tissue and expanded ex vivo as previously described
(31). Briefly, human pancreases were
obtained with full informed consent from adult heart-beating
cadaver organ donors through the organ procurement program at the
British Columbia Transplant Society (Vancouver, Canada). A total of
5 pancreatic ductal tissue samples were collected between July 2011
and November 2011 at Vancouver General Hospital (Vancouver,
Canada). The pancreatic ductal tissue taken from the Ricordi®
Chamber (Biorep Technologies, Inc., Miami, FL, USA), which was used
for islet isolation, was utilized as the starting material for MSC
isolation. The human glioma U251 cell line was purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA) to be
used as target cells in the present study. U251 cells were
maintained as suggested by ATCC, and the culture conditions were
kept consistent with those of the MSCs. In order to track the
viability in a timely manner, the U251 cells were pre-labeled with
luciferase using the pGL4.51[luc2/CMV/Neo] vector (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol.
In vitro synthesis of PTEN mRNA and
MSC transfection
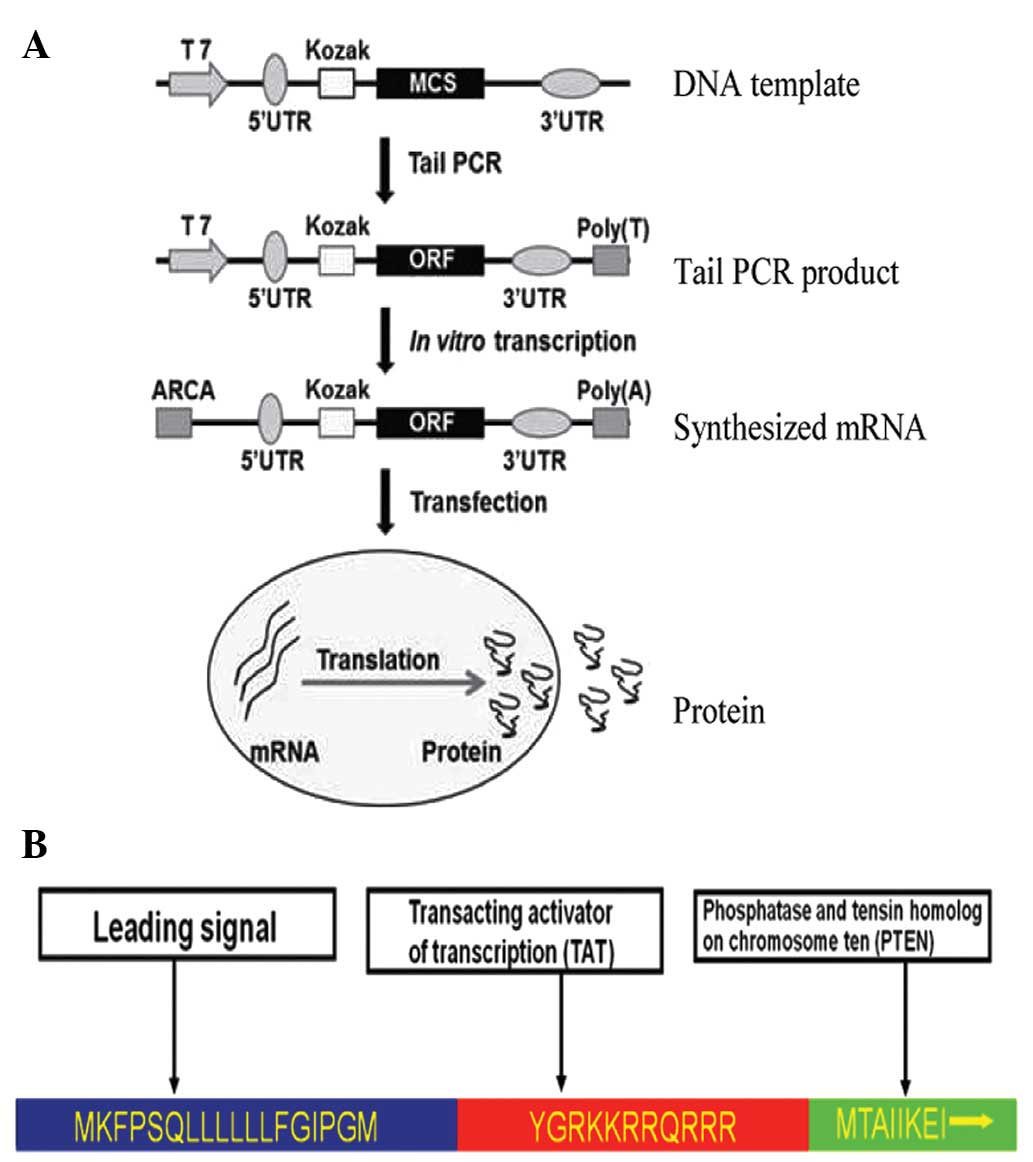
The in vitro transcription template
construction and RNA synthesis is schematized in Fig. 1. The oligonucleotide sequences used in
the construction of in vitro transcription templates are
shown in Table I. The human 5′-UTR
with Kozak and 3′-UTR sequences were synthesized commercially by
Integrated DNA Technologies (Coralville, IA, USA), sub-cloned into
a pcDNA3.3 plasmid and termed the pcDNA 3.3-TOPO TA vector. As
previously described (23), plasmid
inserts were excised using restriction enzyme digestion and used to
template tail polymerase chain reaction (PCR). RNA was synthesized
using the Ambion MEGAscript T7 kit (Thermo Fisher Scientific,
Waltham, MA, USA). The ribonucleoside blend was composed of
anti-reverse cap analogs (New England Biolabs, Inc., Ipswich, MA,
USA) and adenosine triphosphate, guanosine triphosphate,
5-methylcytidine triphosphate and pseudouridine triphosphate
(TriLink Biotechnologies, San Diego, CA, USA). Reactions were
incubated for 5 h at 37°C and followed by DNase treatment,
according to the manufacturer's protocol. Then, the reactions were
treated with Antarctic Phosphatase (New England Biolabs, Inc.) for
2 h at 37°C in order to remove residual 5′-triphosphates. The
synthesized RNA was purified with Ambion MEGAclear spin columns
(Thermo Fisher Scientific) and quantified using NanoDrop (Thermo
Fisher Scientific). RNA transfection was performed using
TransIT-mRNA (Mirus Bio, Madison, WI, USA). RNA was diluted in
Invitrogen Opti-MEM basal media (Thermo Fisher Scientific), and the
Boost reagent (Mirus Bio LLC, Madison, WI, USA) and TransIT-mRNA
were added sequentially according to the manufacturer's protocol.
Subsequent to 2 min incubation at room temperature (RT), the
RNA-lipid complexes were delivered to culture media in the culture
plates. The plates were then returned to the incubator and PTEN
expression was analyzed 12, 24 and 36 h later using western blot
analysis.
 | Table I.Primers for in vitro
synthesized PTEN mRNA. |
Table I.
Primers for in vitro
synthesized PTEN mRNA.
| PTEN mRNA | Forward primer | Reverse primer |
|---|
| PTEN ORF | F1:
ATGAAGTTTCCTTCTCAACTTC | R1:
TCAGACTTTTGTAATTTGTGTATG |
|
| F2:
CGCGGATCCGCCACCATGAAGTTTCC | R2:
CCGCTCGAGTCAGACTTTTGTAATTT |
| 5′ Splint |
GTACTGCTCCTCGCCGTTCATGGTGGC |
|
|
|
TCTTATATTTCTTCTTACTC |
|
| 3′ Splint |
CCCGCAGAAGGCAGCTTACTCATCGTG |
|
|
| GTTCCTGCGGCC |
|
| 5′ UTR |
TTGGACCCTCGTACAGAAGCTAATACG |
|
|
|
ACTCACTATAGGGAAATAAGAGAGAAA |
|
|
|
AGAAGAGTAAGAAGAAATATAAGAGCC |
|
|
| ACC |
|
| 3′ UTR |
GCTGCCTTCTGCGGGGCTTGCCTTCTG |
|
|
|
GCCATGCCCTTCTTCTCTCCCTTGCAC |
|
|
|
CTGTACCTCTTGGTCTTTGAATAAAGC |
|
|
|
CTGAGTAGGAAGTGAGGGTCTAGAACT |
|
|
| AGTGTCGACGC |
|
| Ligation product
PCR |
TTGGACCCTCGTACAGAAGCTAATACG |
GCGTCGACACTAGTTCTAGACCCTCA |
| Tail PCR |
TTGGACCCTCGTACAGAAGCTAATACG |
T120CTTCCTACTCAGGCTTTATTCAAA |
Viability assay of tumor cells in
indirect co-culture
The cytotoxic effects of PTEN-engineered MSCs
(MSCPTEN) on the proliferation of malignant glioma cells were
assessed under indirect co-culture conditions. The luciferase gene
transfected U251 cells were plated at a density of 5,000 cells/well
in a Falcon 96-well plate (BD Biosciences, Franklin Lakes, NJ, USA)
in 100 µl of minimal essential medium (MEM) on day 0. The cells
were cultured using CM that was collected from the cultures of
native MSCs (CMcontrol) and MSCPTEN (CMPTEN)
on day 1. The culture volume was 100 µl with five different ratios
of MSCPTEN to CM from MSCcontrol, as follows: 0, 25, 50,
75 and 100%. On days 0, 3 and 6, 1 µl (0.15 mg/ml) D-luciferin
(Caliper Life Sciences, Waltham, MA, USA) was added into each well
and the cells were incubated in the same culture conditions for an
additional 10 min. Luminescence was measured using the IVIS
Spectrum System (Caliper Life Sciences). The media were replaced
every 36 h and the measurements of every group were repeated three
times.
Migration assay of MSCs with Transwell
co-culture system
A cell migration assay was performed, as previously
described (32). U251 cells were
placed on the plastic surface of 24-well plates (1×105 cells/well).
On the following day, the wild-type MSCs and MSCPTEN in serum-free
media were seeded onto the 8 µm microporous membranes of the
Transwell insert (Corning Incorporated, Corning, NY, USA). MSCs
were incubated for 48 h at 37°C and in a 5% CO2
atmosphere. The inserts were washed with phosphate buffered saline
(PBS) and the upper surface of the membrane was scraped gently
using a cotton swab to remove non-migrated cells. Then, the
membranes were fixed with 95% ethanol (Sangon Biotech Co., Ltd.,
Shanghai, China) and stained using 0.1% crystal violet (Sangon
Biotech Co., Ltd.). The average number of migrated cells was
assessed by counting five randomly selected microscopic fields at
x100 magnification. All experiments were repeated at least three
times. To detect the migration status of the MSCs with normal
cells, U251 glioma cells were replaced with native MSCs on the
plastic surface of the 24-well plates.
Immunoblotting. Immunoblotting was
used to detect the cellular expression of MSCs
Briefly, the MSCs were washed with PBS three times
and collected using cell lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Cell lysates were incubated on ice
for 30 min. The protein concentration was determined using
bicinchoninic acid protein assay reagents (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Equal
amounts of protein (50 µg/sample) were loaded on each lane and
separated by electrophoresis in 12% sodium dodecyl sulfate
polyacrylamide gel and electrotransferred to nitrocellulose
membranes (Beyotime Institute of Biotechnology). The membrane was
incubated in blocking buffer for 1 h at RT and then incubated
overnight at 4°C with monoclonal rabbit anti-mouse PTEN primary
antibodies (cat. no. 217702; dilution, 1:1,000; R&D Systems,
Inc., Minneapolis, MN, USA). The blots were rinsed with
Tris-buffered saline and Tween-20 (EMD Millipore, Billerica, MA,
USA) three times, incubated with horseradish peroxidase-conjugated
goat anti-mouse IgG secondary antibody (cat. no. A0216; dilution,
1:1,000; Beyotime Institute of Biotechnology) for 60 min and
detected by chemiluminescence using ECL Hyperfilm (EMD Millipore).
The CM samples were filtered through a 0.22 µm membrane (EMD
Millipore) and equally concentrated using a 10,000 molecular weight
cut-off (catalog no., 42406; EMD Millipore). Then, PTEN was
detected in the CM.
Fluorescence microscopy
The cell viability was also detected using an
Invitrogen LIVE/DEAD Viability/Cytotoxicity assay kit (Thermo
Fisher Scientific), according to the manufacturer's protocol, but
with a slight modification (10).
Briefly, a total of 1×105 U251 cells were plated onto 24-well
plates in 500 µl of MEM on day 0. The media were replaced with 50
or 100% CM on day 1. On day 4, the cultures were washed twice with
PBS. Freshly prepared working solution containing 1µM calcein
acetoxymethyl and 2 µM ethdium homodimer-1 (Thermo Fisher
Scientific) was then added directly to the cultures, with 250 µl
solution added per well on 24-well plates, and the wells were
incubated at RT for 10 min in the dark. The images were captured
using a fluorescence microscope (IX71; Olympus Corporation, Tokyo,
Japan) and the associated analysis was performed using ImageJ 1.45
k software (National Institute of Health of USA, Bethesda, MD,
USA).
Statistical analysis
Numerical data were expressed as the mean ± standard
error. Statistical differences between the means for the various
groups were evaluated using Prism 4.0 software (GraphPad software,
San Diego, CA, USA) using the Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
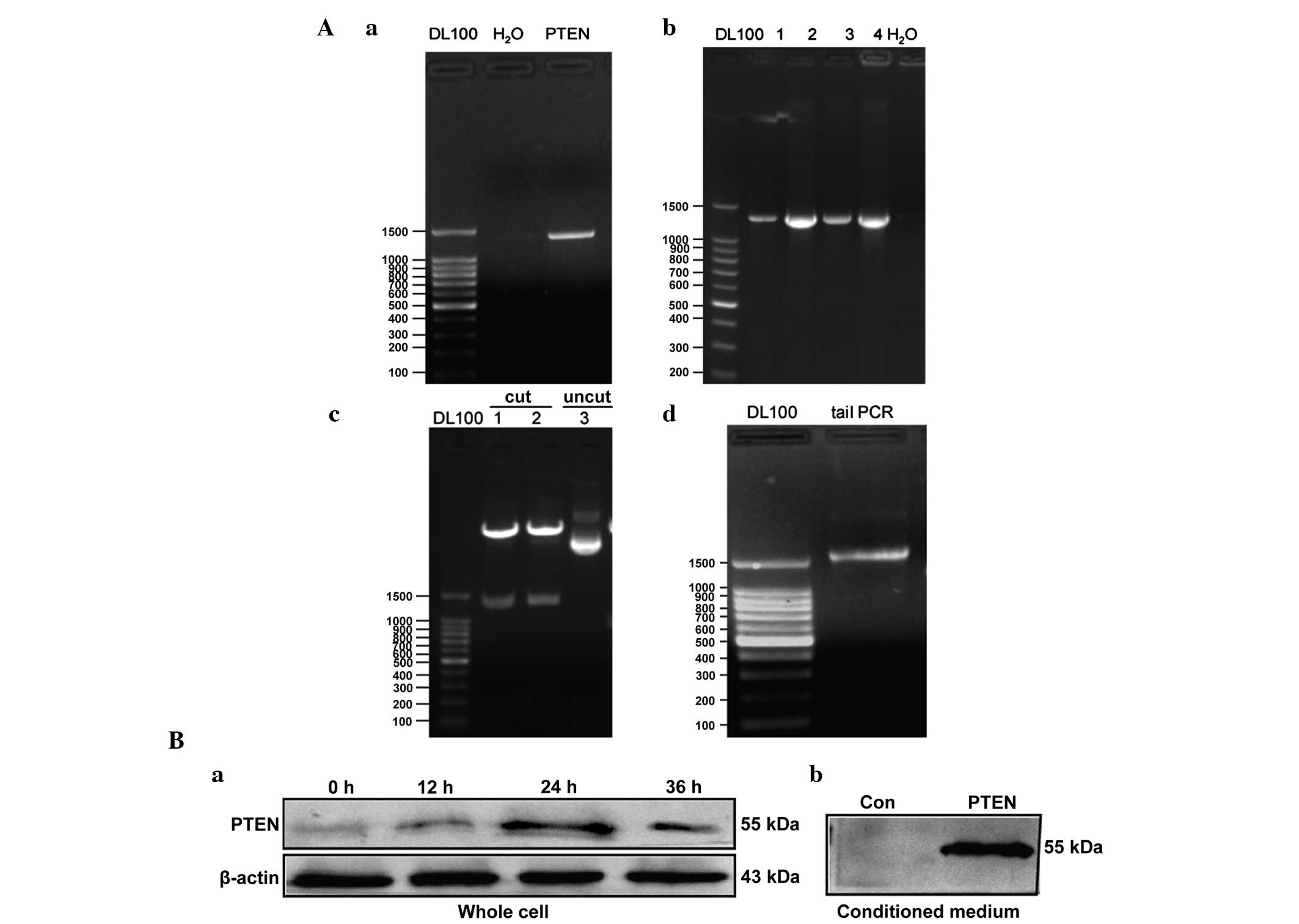
PTEN-bearing mRNA synthesis and
expression in MSCs
Anticancer gene-bearing mRNA was constructed as
illustrated in Fig. 1A. In order to
make the PTEN product secretory (stPTEN), a leading
sequence that corresponded to an 18 amino acid segment (sequence,
MKFPSQLLLLLLFGIPGM) was introduced into the DNA template. A
transacting activator of transcription (TAT; sequence, YGRKKRRQRRR)
was inserted into the PTEN template, to act at the transmembrane
(Fig. 1B). The stPTEN DNA
template was cloned from previously constructed pDsRed1-TAT-PTEN
vectors (Fig. 2Aa) (33) and the PCR product of the
stPTEN sequence was then inserted into the pcDNA
3.3-TOPO TA vector. The inserted DNA sequence was confirmed by an
analysis of the colony using PCR (Fig.
2Ab), restriction enzyme digestion (Fig. 2Ac) and sequencing (data not shown).
The correct plasmid insert was excised by restriction enzyme
digestion (using BAmHI and XhoI; Takara Biotechnology
Co., Ltd., Dalian, China) and used to template tail PCR (Fig. 2Ad). As shown in Fig. 2B, the expression of PTEN was clearly
enhanced in transfected MSCs. The endogenous PTEN was observed in
control MSCs with a slightly varied size, the maximum expression of
transfected PTEN mRNA was observed at 24 h post transfection, and
subsequently decreased at 36 h. The stPTEN was also
detectable in the corresponding CMs.
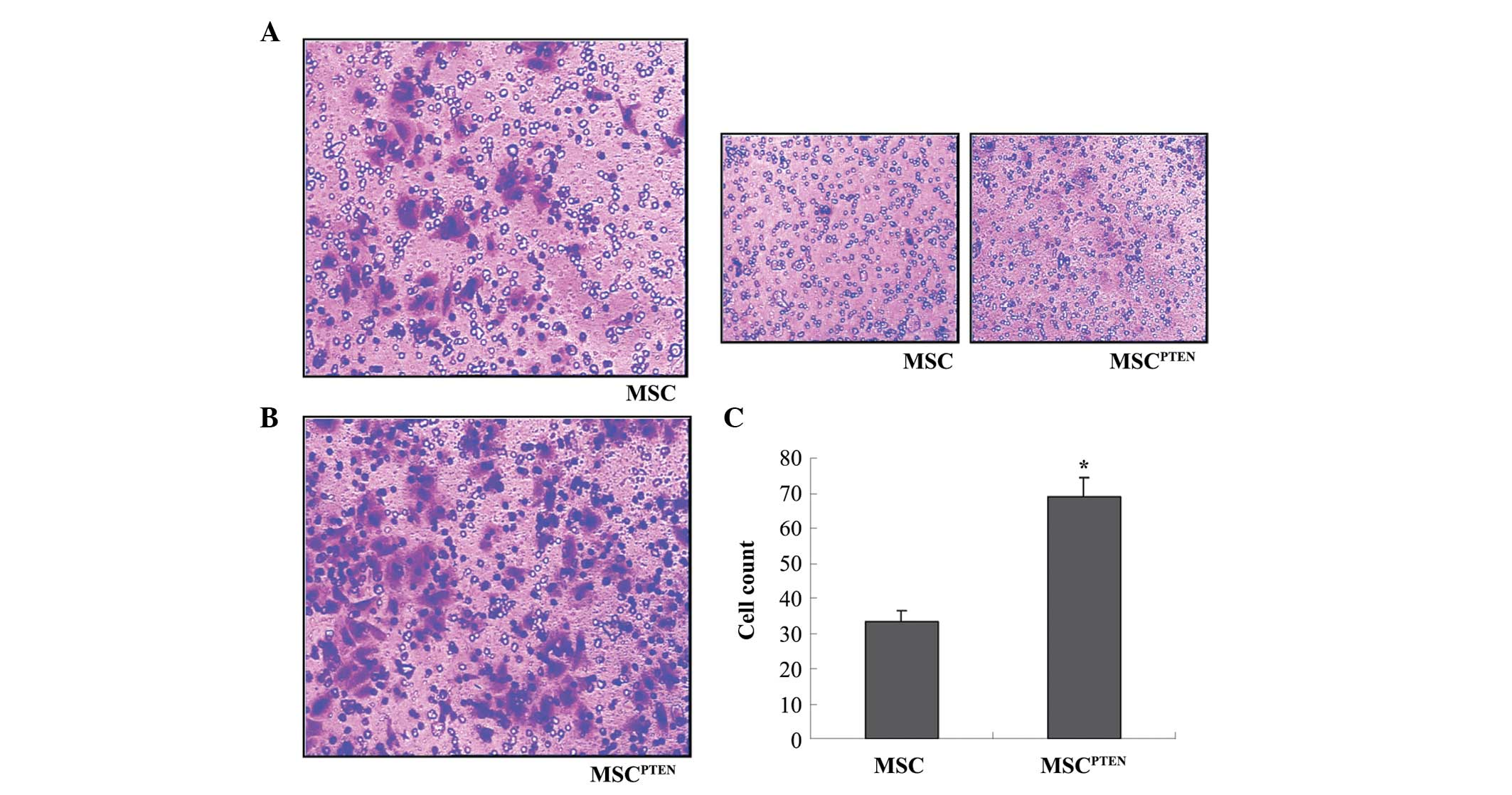
Effect of mRNA transfection on MSC
migration
MSC migration was analyzed using the Transwell
system. Subsequent to 48 h of co-culture, a considerable number of
cells migrated across the microporous membrane towards the U251
cells (Fig. 3A). However, few cells
migrated towards the normal MSC control cells (Fig. 3B). Notably, the transfection of
PTEN-mRNAs significantly enhanced the migration of MSCs towards the
U251 glioma cells compared with control MSCs (Fig. 3C).
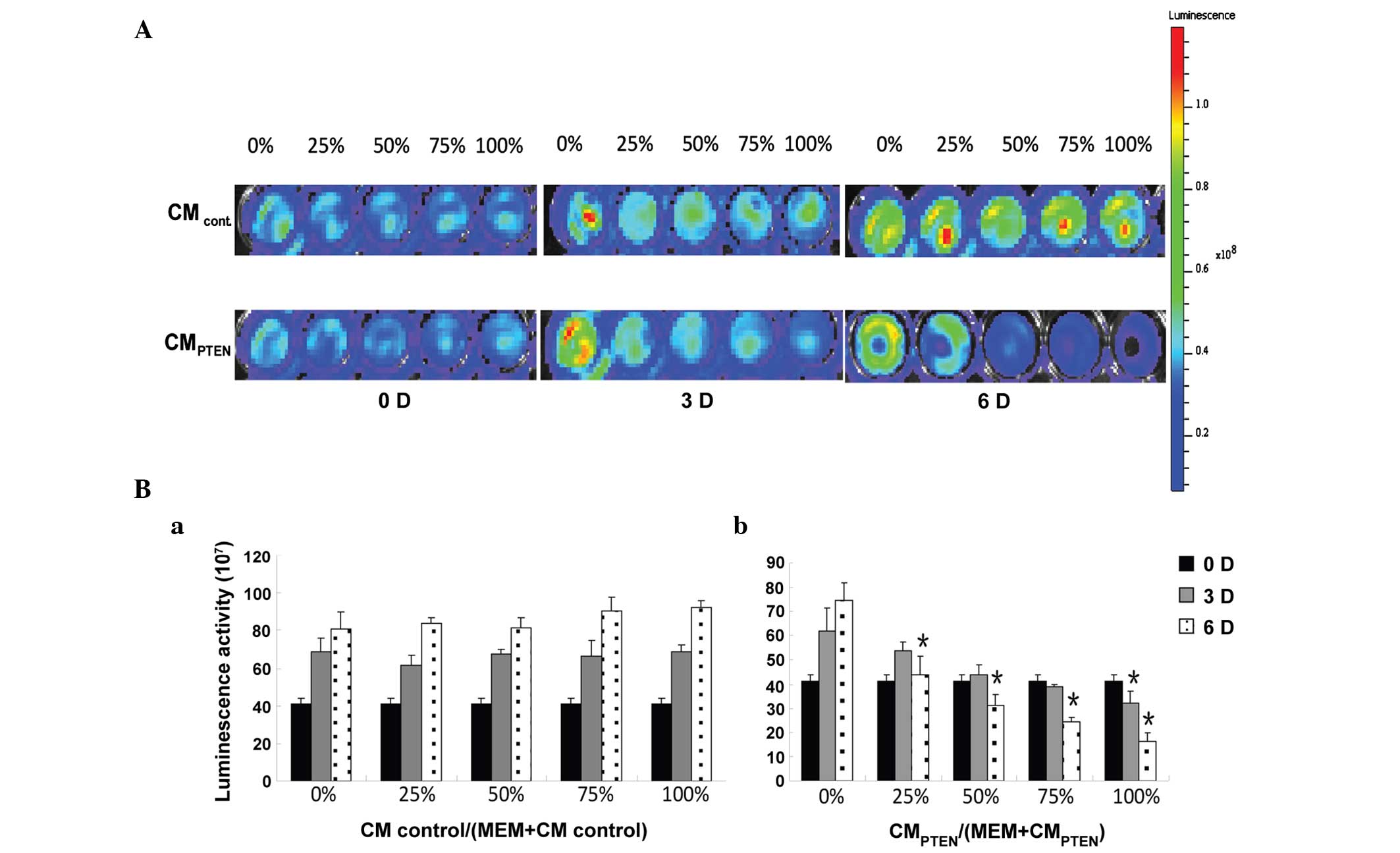
Effect of PTEN-engineered MSCs on the
viability of U251 glioma cells
The indirect co-culture was used to determine the
effects of PTEN-engineered MSCs on the viability of U251 glioma
cells by luminescence. As shown in Fig.
4A, the intensity of luminescence in U251 cells decreased with
the increased incubation time and CM ratio. All test results are
summarized in Fig. 4B. Starting at a
low CM ratio of 25%, the cells incubated with CMPTEN
revealed significant cell death at day 6 (47.7%; P<0.05).
However, significant cell death (P<0.05) started to appear at
day 3, at a CM ratio of 100%. The ratio of
CMPTEN-induced cell death at day 3 was not statistically
different from day 0.
MSCPTEN-mediated U251 cell
death in indirect co-cultures
CM-induced U251 cell death was also examined on day
4 by fluorescence microscopy (IX71; Olympus Corporation) subsequent
to LIVE/DEAD staining. Two CM ratios, 50 and 100%, were used in
this component of the study. As shown in Fig. 5, the cell death was proportionally
associated with CMPTEN. The dose-dependent cell death
indicates that MSCPTEN-derived PTEN is an important mediator
responsible for U251 cell death during indirect co-culture. Marked
cell death was not detected in the CM obtained from the native MSCs
in the current experimental conditions.
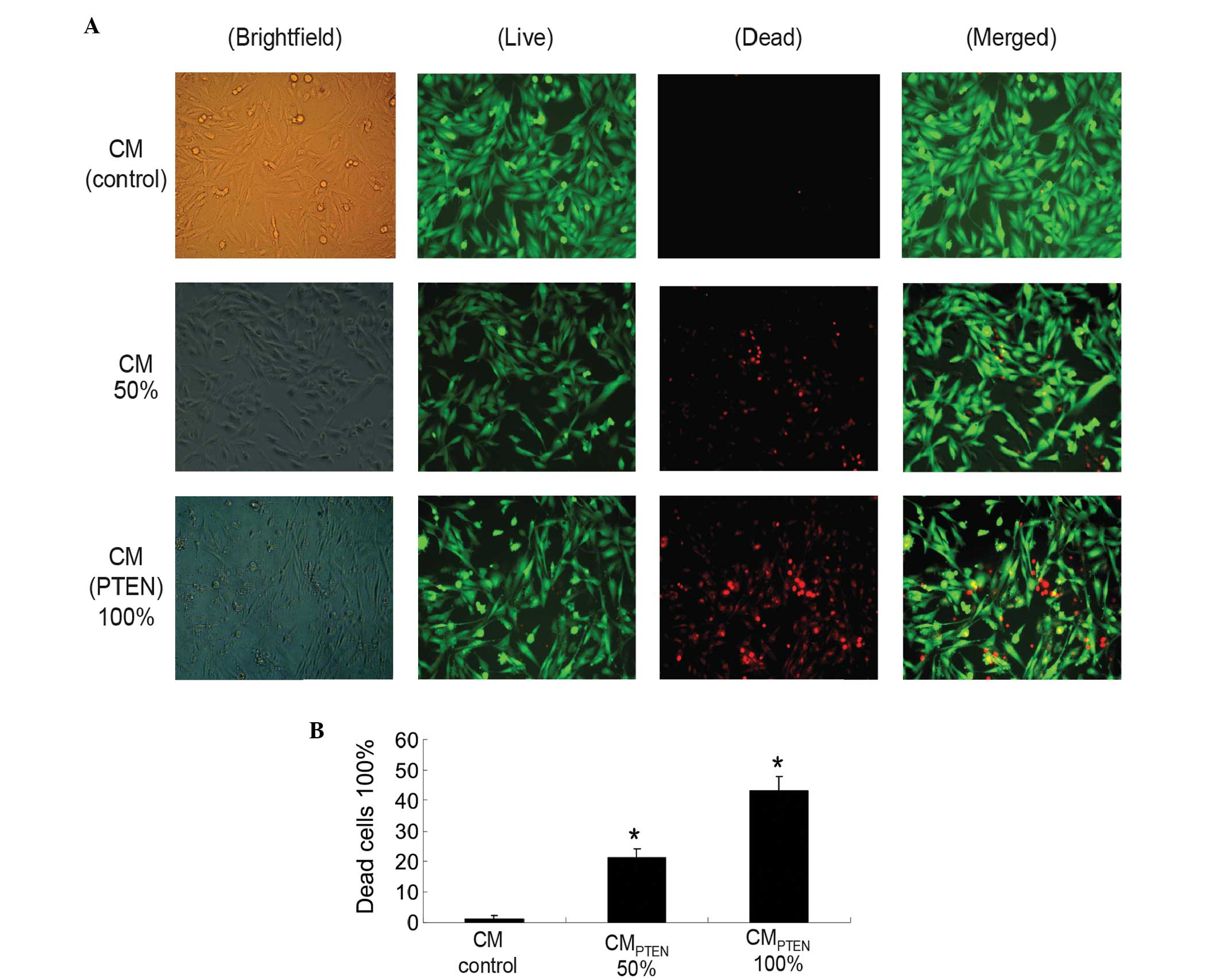 | Figure 5.U251 cell viability in indirect
co-cultures. (A) Cells were incubated in various CMs (indicated on
the left side of the graph) at various ratios (indicated at the
top). LIVE/DEAD staining was performed on day 4 following the
initiation of the indirect co-culture (orginal magnification,
x400). (B) Summary of cell viability of indirect co-cultures. Data
are expressed as the mean ± standard error of the mean for three
independent experiments. *P<0.05 vs. control. Brightfield, whole
population of cells attached to the culture surface; Live, live
cells stained with calcein in green; Dead, dead cells stained with
ethdium homodimer-1 in red; Merged, merged images; CM, conditioned
media, PTEN, phophatase and tensin homolog; CMCONTROL,
CM collected from the cultures of native MSCs; CMPTEN,
CM collected fom the cultures of PTEN-engineered MSCs. |
Discussion
Malignant glioma is one of the most challenging
cancers to treat successfully, mainly due to the particularity of
its location and biological characteristics, including the
infiltrative nature, resistance to apoptosis, propensity for
recurrence and resistance to conventional therapies (3). Since the identification of the
tumor-oriented migration capacity of MSCs, the application of
specific anticancer gene-engineered MSCs has demonstrated potential
for the development of targeted therapy of malignant gliomas
(34–36). While MSCs may carry anticancer genes
with various viral or plasmid vectors, these methods may not be
used in clinical applications (37).
Synthetic mRNA in vitro is a novel technique
that presents a safe, efficient strategy for gene therapy (25). The use of synthetic mRNA completely
removes the risk of modifying the host genome (38–39), and
the safety of synthetic mRNA has been demonstrated in clinical
studies (40). Therefore, the
synthetic mRNA-based strategy is a simple and non-integrating
approach for the application of specific anticancer gene-engineered
MSCs.
The loss of PTEN expression in a wide range of
cancer cells, including gliomas, reflects the importance of PTEN in
the maintenance of cancer cell survival, with >50% of human
glioblastoma cases exhibiting loss of expression (41). PTEN function restoration may inhibit
cancer cell growth and may induce cell death under certain
circumstances, such as loss of PTEN expression (28–30). In
consideration of the potential clinical applications,
stPTEN mRNA was synthesized in vitro and used in
the present MSC-mediated anticancer study. As demonstrated in
Fig. 2B, the synthesized PTEN mRNA
was expressed in MSCs with the peak activity at 24 h following
transfection. The expression is consistent with a previous study,
which used a DNA-based vector in the same cell model (33).
In the present study, the tumor cell-oriented
migration of MSCs, which were engineered with DNA-based anticancer
gene expression vectors, was investigated using the xCELLigence
system in pancreatic cancer cells and glioma DBTRG cells (33,42). In
order to make the strategy clinically practical, the effects of
synthesized mRNA transfection on the migration ability of MSCs was
detected. As shown in Fig. 3, the
migration of MSCs was not obstructed by PTEN mRNA transfection.
Notably, the migration of MSCs towards the U251 cells was
significantly increased by transfected PTEN mRNA. The underlying
mechanisms require additional studies in the future. The results of
the present study suggest that synthesized mRNA may be applied to
the MSC-mediated anticancer strategy.
To the best of our knowledge, the present study is
the first demonstration that synthetic modified PTEN mRNA
engineered MSC-mediated cytotoxic effects on glioma cells. In the
present study, the cytotoxic effects of CM from MSCPTEN
on U251 cells were examined using a luminescence technique and
fluorescence microscopy. Under the current indirect co-culture
conditions, U251 cells were sensitive to CMPTEN
(Fig. 4). The significant
cytotoxicity was observed on day 6 and at a CM ratio of 50%. The
LIVE/DEAD assay possessed the advantage of being straightforward,
and reflected the intact status of the detected cells at any time
point. As shown in Fig. 5,
dose-responsible death of U251 glioma cells revealed the anticancer
effect of MSCs that were transfected with PTEN-mRNAs. However, the
drawback of the LIVE/DEAD assay is that it only applies to the
cells that remain on the culture surface during staining. The
detached cells, the majority of which are dead cells, are not
included in the assessment. Multiple assessments and in vivo
experiments are required in additional studies.
In the present study, a safe and efficient strategy
of mRNA-based gene transfer was successfully applied in the
MSCs-mediated cytotoxicity of tumor cells. The approach of
MSC-mediated gene therapy caused a dual-targeted killing effect to
the tumor, mainly due to the tropism characteristics of MCSs and
engineered anticancer agents. mRNA-based gene transfer may also
kill tumor cells locally and consistently. The methodology used in
the present study also holds the potential to use the MSCs of the
patients and to switch the tumor attackers corresponding to the
individual condition. In conclusion, the present study provides an
essential foundation for additional in vivo and potentially
clinical anticancer studies using synthesized mRNAs.
Acknowledgments
The present study was supported by the Ministry of
Science and Technology (grant no. 2013ZX10001004-002-005), the
Environmental Protection Department of Hubei Province (grant no.
2013HB13), the Science and Technology Department of Hubei Province
(grant no. 2014CFA068) and the Public Health and Family Planning
Department of Hubei Province (grant no. WJ2015MB223).
References
|
1
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Westphal M and Lamszus K: The neurobiology
of gliomas: From cell biology to the development of therapeutic
approaches. Nat Rev Neurosci. 12:495–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson SD: Malignant gliomas: Diagnosis
and treatment. Dis Mon. 57:558–569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun XY, Nong J, Qin K, Warnock GL and Dai
LJ: Mesenchymal stem cell-mediated cancer therapy: A dual-targeted
strategy of personalized medicine. World J Stem Cells. 3:96–103.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabatabai G, Wick W and Weller M: Stem
cell-mediated gene therapies for malignant gliomas: A promising
targeted therapeutic approach? Discov Med. 11:529–536.
2011.PubMed/NCBI
|
|
6
|
Loebinger MR and Janes SM: Stem cells as
vectors for antitumour therapy. Thorax. 65:362–369. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai LJ, Moniri MR, Zeng ZR, Zhou JX, Rayat
J and Warnock GL: Potential implications of mesenchymal stem cells
in cancer therapy. Cancer Lett. 305:8–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Zhang L, Xu W, Qian H, Ye S, Zhu
W, Cao H, Yan Y, Li W, Wang M, et al: Experimental therapy for lung
cancer: Umbilical cord-derived mesenchymal stem cell-mediated
interleukin-24 delivery. Curr Cancer Drug Targets. 13:92–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Zhang L, Xu W, Qian H, Ye S, Zhu
W, Cao H, Yan Y, Li W, Wang M, et al: Experimental therapy for lung
cancer: umbilical cord-derived mesenchymal stem cell-mediated
interleukin-24 delivery. Curr Cancer Drug Targets. 13:92–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun XY, Nong J, Qin K, Lu H, Moniri MR,
Dai LJ and Warnock GL: MSC(TRAIL)-mediated HepG2 cell death in
direct and indirect co-cultures. Anticancer Res. 31:3705–3712.
2011.PubMed/NCBI
|
|
11
|
Rodríguez R, García-Castro J, Trigueros C,
Arranz García M and Menéndez P: Multipotent mesenchymal stromal
cells: clinical applications and cancer modeling. Adv Exp Med Biol.
741:187–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fritz V and Jorgensen C: Mesenchymal stem
cells: An emerging tool for cancer targeting and therapy. Curr Stem
Cell Res Ther. 3:32–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagi H, Soto-Gutierrez A, Parekkadan B,
Kitagawa Y, Tompkins RG, Kobayashi N and Yarmush ML: Mesenchymal
stem cells: Mechanisms of immunomodulation and homing. Cell
Transplant. 19:667–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amara I, Touati W, Beaune P and de Waziers
I: Mesenchymal stem cells as cellular vehicles for prodrug gene
therapy against tumors. Biochimie. 105:4–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
17
|
Li X, Lu Y, Huang W, Xu H, Chen X, Geng Q,
Fan H, Tan Y, Xue G and Jiang X: In vitro effect of
adenovirus-mediated human Gamma Interferon gene transfer into human
mesenchymal stem cells for chronic myelogenous leukemia. Hematol
Oncol. 24:151–158. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang XJ, Lu JT, Tu HJ, Huang KM, Fu R, Cao
G, Huang M, Cheng LH, Dai LJ and Zhang L: TRAIL-engineered bone
marrow-derived mesenchymal stem cells: TRAIL expression and
cytotoxic effects on C6 glioma cells. Anticancer Res. 34:729–734.
2014.PubMed/NCBI
|
|
19
|
Sasportas LS, Kasmieh R, Wakimoto H,
Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza
RL, Weissleder R and Shah K: Assessment of therapeutic efficacy and
fate of engineered human mesenchymal stem cells for cancer therapy.
Proc Natl Acad Sci USA. 106:4822–4827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenecker J, Huth S and Rudolph C: Gene
therapy for cystic fibrosis lung disease: Current status and future
perspectives. Curr Opin Mol Ther. 8:439–445. 2006.PubMed/NCBI
|
|
21
|
Hacein-Bey-Abina S, Hauer J, Lim A, Picard
C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S,
Belohradsky BH, et al: Efficacy of gene therapy for X-linked severe
combined immunodeficiency. N Engl J Med. 363:355–364. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leonhardt C, Schwake G, Stögbauer TR,
Rappl S, Kuhr JT, Ligon TS and Rädler JO: Single-cell mRNA
transfection studies: Delivery, kinetics and statistics by numbers.
Nanomedicine (Lond). 10:679–688. 2014.
|
|
23
|
Wang XL, Hu P, Guo XR, Yan D, Yuan Y, Yan
SR and Li DS: Reprogramming human umbilical cord mesenchymal
stromal cells to islet-like cells with the use of in
vitro-synthesized pancreatic-duodenal homebox 1 messenger RNA.
Cytotherapy. 16:1519–1527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo XR, Wang XL, Li MC, Yuan YH, Chen Y,
Zou DD, Bian LJ and Li DS: PDX-1 mRNA-induced reprogramming of
mouse pancreas-derived mesenchymal stem cells into
insulin-producing cells in vitro. Clin Exp Med. 10:152–160.
2014.
|
|
25
|
Warren L, Manos PD, Ahfeldt T, Loh YH, Li
H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al: Highly
efficient reprogramming to pluripotency and directed
differentiation of human cells with synthetic modified mRNA. Cell
Stem Cell. 7:618–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zangi L, Lui KO, von Gise A, Ma Q, Ebina
W, Ptaszek LM, Später D, Xu H, Tabebordbar M, Gorbatov R, et al:
Modified mRNA directs the fate of heart progenitor cells and
induces vascular regeneration after myocardial infarction. Nat
Biotechnol. 31:898–907. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Zhou Y, Reske SN and Shen C: PTEN
mutation: Many birds with one stone in tumorigenesis. Anticancer
Res. 28:3613–3619. 2008.PubMed/NCBI
|
|
28
|
Ciuffreda L, Falcone I, Incani UC, Del
Curatolo A, Conciatori F, Matteoni S, Vari S, Vaccaro V, Cognetti F
and Milella M: PTEN expression and function in adult cancer stem
cells and prospects for therapeutic targeting. Adv Biol Regul.
56:66–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muniyan S, Ingersoll MA, Batra SK and Lin
MF: Cellular prostatic acid phosphatase, a PTEN-functional
homologue in prostate epithelia, functions as a prostate-specific
tumor suppressor. Biochim Biophys Acta. 1846:88–98. 2014.PubMed/NCBI
|
|
30
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moniri MR, Sun XY, Rayat J, Dai D, Ao Z,
He Z, Verchere CB, Dai LJ and Warnock GL: TRAIL-engineered
pancreas-derived mesenchymal stem cells: Characterization and
cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther.
19:652–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamizo A, Marini F, Amano T, Khan A,
Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et
al: Human bone marrow-derived mesenchymal stem cells in the
treatment of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
33
|
Yang ZS, Tang XJ, Guo XR, Zou DD, Sun XY,
Feng JB, Luo J, Dai LJ and Warnock GL: Cancer cell-oriented
migration of mesenchymal stem cells engineered with an anticancer
gene (PTEN): An imaging demonstration. Onco Targets Ther.
7:441–446. 2014.PubMed/NCBI
|
|
34
|
Menon LG, Kelly K, Yang HW, Kim SK, Black
PM and Carroll RS: Human bone marrow-derived mesenchymal stromal
cells expressing S-TRAIL as a cellular delivery vehicle for human
glioma therapy. Stem Cells. 27:2320–2330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dwyer RM, Khan S, Barry FP, O'Brien T and
Kerin MJ: Advances in mesenchymal stem cell-mediated gene therapy
for cancer. Stem Cell Res Ther. 1:252010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knoop K, Kolokythas M, Klutz K, Willhauck
MJ, Wunderlich N, Draganovici D, Zach C, Gildehaus FJ, Böning G,
Göke B, et al: Image-guided, tumor stroma-targeted 131I therapy of
hepatocellular cancer after systemic mesenchymal stem cell-mediated
NIS gene delivery. Mol Ther. 19:1704–1713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez-Quintanilla JI, Bhere D, Heidari
P, He D, Mahmood U and Shah K: Therapeutic efficacy and fate of
bimodal engineered stem cells in malignant brain tumors. Stem
Cells. 31:1706–1714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li M, Sancho-Martinez I and Belmonte
Izpisua JC: Cell fate conversion by mRNA. Stem Cell Res Ther.
2:52011. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kormann MS, Hasenpusch G, Aneja MK, Nica
G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M,
Schams A, et al: Expression of therapeutic proteins after delivery
of chemically modified mRNA in mice. Nat Biotechnol. 29:154–157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van Nuffel AM, Corthals J, Neyns B,
Heirman C, Thielemans K and Bonehill A: Immunotherapy of cancer
with dendritic cells loaded with tumor antigens and activated
through mRNA electroporation. Methods Mol Biol. 629:405–452.
2010.PubMed/NCBI
|
|
41
|
Leslie NR and Downes CP: PTEN function:
How normal cells control it and tumour cells lose it. Biochem J.
382:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moniri Roshan M, Young A, Reinheimer K,
Rayat J, Dai LJ and Warnock GL: Dynamic assessment of cell
viability, proliferation and migration using real time cell
analyzer system (RTCA). Cytotechnology. 67:379–386. 2015.
View Article : Google Scholar : PubMed/NCBI
|