Introduction
Skin cancer is the most common malignancy diagnosed
in the United States, with 3.5 million melanomas diagnosed in 2
million individuals annually (1).
Melanoma is a skin cancer characterized by the abnormal
proliferation of melanocytes that invade the basement membrane
(2). Although melanoma accounts for
<5% of skin cancers, it results in the highest number of
mortalities (1–3). The lack of a therapeutic response in
patients that receive the currently available treatments highlights
the importance of an improved understanding of the complex
molecular mechanisms that contribute to melanoma development.
MicroRNAs (miRNAs) are a family of noncoding RNAs
~22 nucleotides in length, which may suppress gene expression by
pairing to the 3′-untranslated region (3′-UTR) of target messenger
(m)RNAs (4–6). The extent of base pairing between miRNA
and mRNA appears to determine the balance between the cleavage and
degradation of mRNA (7). Currently,
it is widely accepted that miRNA alterations are involved in the
initiation and progression of human cancer (4). miRNA-33 (miR-33) is a newly
characterized miRNA located within the intronic sequences of the
sterol regulatory element-binding protein (SREBP) genes, which
regulate cholesterol and fatty acid metabolism in combination with
their host genes (8–11). There are two miR-33 genes in humans,
consisting of miR-33a and miR-33b. miR-33a and miR-33b are
localized in corresponding introns of the SREBP2 and SREBP1 genes,
respectively. Previous studies have focused on the metabolic
function of miR-33. The role of miR-33 in tumor biology has been
identified (12–15), yet its function in melanoma remains
unclear.
PCTAIRE1, also termed cyclin-dependent kinase 16
(CDK16), is a serine/threonine kinase that was originally
identified as a cyclin dependent kinase 1-like kinase (16,17). It
has been reported that PCTAIRE1 is involved in vesicular
exocytosis, protein secretion, neuronal migration, neurite
outgrowth and spermatogenesis (18–21). A
recent study revealed that PCTAIRE1 phosphorylates p27 and promotes
tumorigenesis in liver and breast cancer (22). The present study investigated the
potential role of miR-33a in melanoma, and identified that the
miRNA acts as a tumor suppressor.
Materials and methods
Cell lines
Human melanoma SK-MEL-1 and WM-115 cell lines were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and maintained in Eagle's minimum essential
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). Primary epidermal melanocytes (ATCC; PEMI,
catalog number PCS-200-012™; PEM2, PCS-200-013™) were maintained
following ATCC protocol.
Lentivirus infection
Pre-miR-33a expression and negative control
lentivirus was obtained from GeneChem Co., Ltd. (Shanghai, China).
SK-MEL-1 and WM-115 cells were plated on a 6-well plate (Corning
Incorporated, Corning, NY, USA) and lentivirus was added in medium
with 5 µg/ml polybrene (Solarbio Science & Technology Co.,
Ltd., Beijing, China). After 12 h, the media was replaced with
fresh medium. Stable infection cell strains were selected by
puromycin (Solarbio Science & Technology Co., Ltd.) 48 h
subsequent to infection.
Colony formation assay
SK-MEL-1 and WM-115 cells infected with miR-33a or
negative control lentivirus were plated on a 6-well plate (500
cells/well). Medium was refreshed every 2 days. The cells were
fixed and stained with 0.1% crystal violet in Eagle's minimum
essential medium (Solarbio Science & Technology Co., Ltd.), and
2 weeks later, the colonies containing >50 cells were counted as
a colony. Colonies were counted in 5 fields of view at a
magnification of x40 using a light microscope (BX51; Olympus
Corporation, Tokyo, Japan). Experiments were performed ≥3 times
independently.
Cell proliferation
Cell proliferation was examined using a
bromodeoxyuridine (BrdU) incorporation assay and anaphase analysis.
The BrdU incorporation rate was measured by a BrdU Cell
Proliferation ELISA kit (Abcam, Cambridge, UK), following the
manufacturer's protocol. For anaphase analysis, the number of cells
and the number of cells in anaphase were detected by
4′,6-diamidino-2-phenylindole staining (Solarbio Science &
Technology Co., Ltd.) and were counted in 3 fields of view per well
at a magnification of x20 using an Olympus IX83 microscope (Olympus
Corporation).
Luciferase reporter assay
For the luciferase activity assay, a wild-type
3′-UTR segment of PCTAIRE1 was cloned into a pmirGLO plasmid
(Promega Corporation, Madison, WI, USA). The mutated sequence in
the complementary site for miRNA-33a was generated by site-specific
mutagenesis. All transfections were performed using Invitrogen
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The cells were plated on a 24-well
plate (Corning Incorporated) and co-transfected with either a
wild-type or mutant 3′-UTR segment of PCTAIRE1 and a Renilla
luciferase reporter (Promega Corporation). In total, 48 h
subsequent to transfection, luciferase activity was measured by a
dual-luciferase reporter assay system (Promega Corporation).
Renilla luciferase activity was used as an internal
reference. Experiments were performed ≥3 times independently.
Extraction of mRNA and miRNA and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA samples were isolated using the RNA Mini
kit (Qiagen China Co., Ltd., Shanghai, China). An Applied
Biosystems Taqman miRNA assay kit and Taqman miRNA assay (Thermo
Fisher Scientific, Inc.) were used to quantify the expression of
mature miRNAs, according to the manufacturer's protocol. Fold
changes were calculated using the ΔΔCq method (23). The Taqman miRNA assay was used to
quantify mature miRNA expression. U6 miRNA was used as an internal
reference for miRNA expression. mRNA expression was measured by
RT-qPCR using the Applied Biosystems Taq Man Universal PCR Master
Mix (Thermo Fisher Scientific, Inc.). Samples were analyzed using
Applied Biosystems StepOnePlus™ Real-Time PCR System (Thermo Fisher
Scientific, Inc.).
Protein extraction and western blot
assay
Whole-cell protein lysates were prepared by removing
the medium, washing the cells with phosphate-buffered saline
(Gibco; Thermo Fisher Scientific, Inc.), scraping the cells from
the plates and pelleting the cells by centrifugation at 700 × g for
10 min (Centrifuge 5418R; Eppendorf North America, Hauppauge, NY,
USA). The cell pellets were resuspended in radioimmunoprecipitation
assay buffer (Solarbio Science & Technology Co., Ltd.), which
contained a protease inhibitor cocktail and a phosphatase inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland). Subsequent to
protein lysis, western blot analysis was performed. Primary
monoclonal rabbit anti-human PCTAIRE1 antibody (dilution, 1:1,000;
catalog no., 4852) and rabbit anti-human p27 antibody (dilution,
1:1,000; catalog no., 3686) were purchased from CST Biological
Reagents Co., Ltd. (Shanghai, China). Polyclonal rabbit anti-human
p27 KIP1 antibody (phospho T187; dilution, 1:1,000; catalog no.,
ab75908) was obtained from Abcam and polyclonal rabbit anti-human
glyceraldehyde 3-phosphate dehydrogenase antibody (dilution,
1:10,000; catalog no., G9545) was from Sigma-Aldrich (St. Louis,
MO, USA).
Statistical analysis
The significance of differences was analyzed using
two-tailed Student's t-test using Prism 6 software (GraphPad
Software, Inc., La Jolla, CA, USA). All data are presented as the
mean ± standard deviation from ≥3 separate experiments. P<0.05
was considered to indicate a statistically significant
difference.
Results
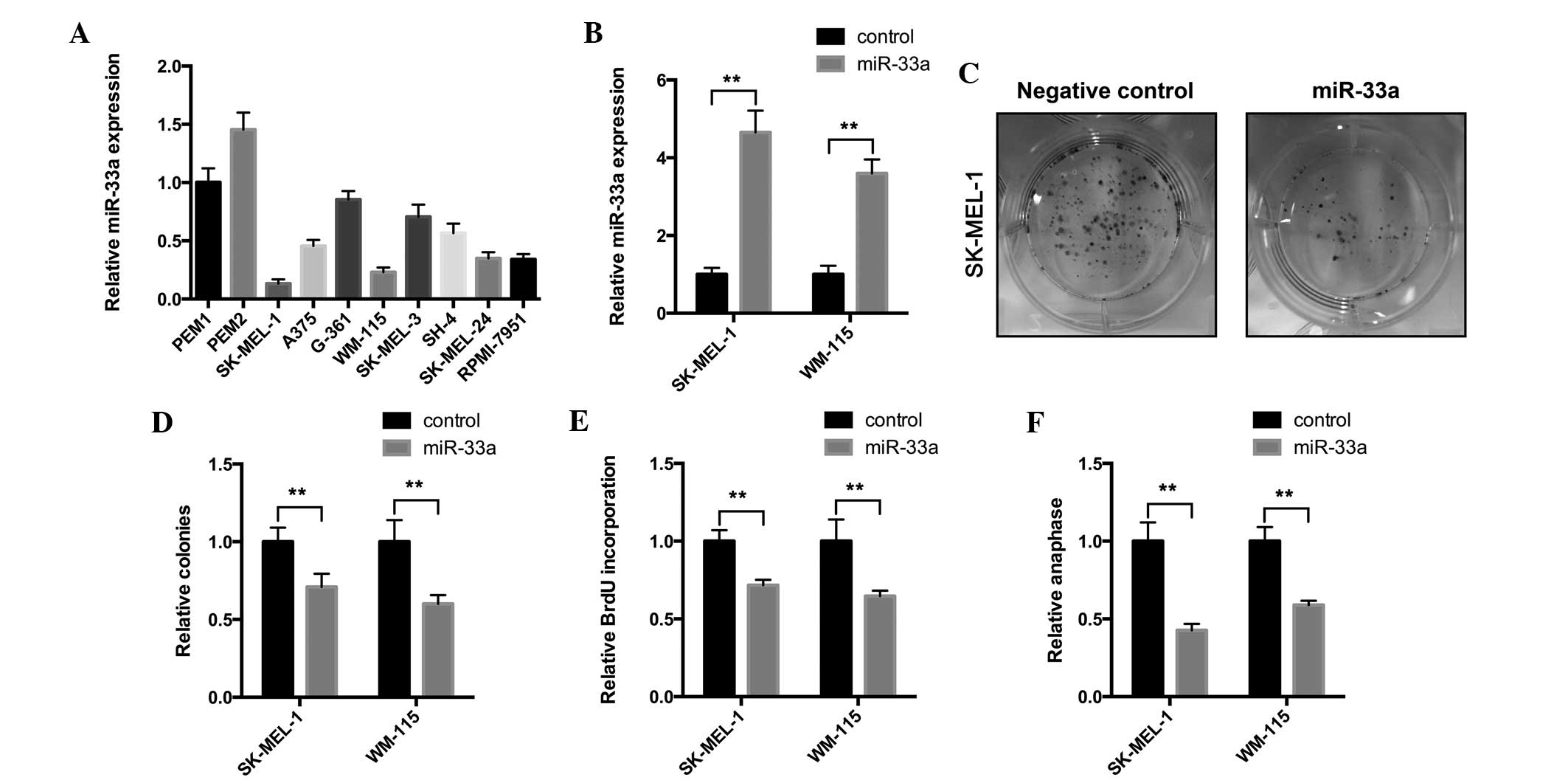
miR-33a is downregulated in melanoma
cells and negatively regulates cell proliferation
To explore the functional role of miR-33a, the
present study first examined the expression of miR-33a in
melanocyte and melanoma cell lines. The RT-qPCR assay results
revealed that miR-33a exhibited decreased expression in melanoma
cell lines, particularly in SK-MEL-1 and WM-115 cells, compared
with melanocyte cells (Fig. 1A).
These results indicate that miR-33a may be lost in melanoma
development. The present study infected SK-MEL-1 and WM-115 cells
with miR-33a-expressing lentivirus. miR-33a overexpression was
confirmed by an RT-qPCR assay (Fig.
1B) and a colony formation assay was performed. As revealed in
Fig. 1C and D, the infection of
miR-33a-expressing lentivirus significantly suppressed colony
numbers and the size of SK-MEL-1 and WM-115 cells. These results
suggest that miR-33a may affect tumorigenesis of melanoma cells.
Furthermore, the present study aimed to investigate the effect of
miR-33a overexpression on the proliferation of melanoma cells. To
investigate the effect of miR-33a on cell proliferation, a BrdU
incorporation assay was performed. In SK-MEL-1 and WM-115 cells,
miR33a overexpression significantly reduced the BrdU incorporation
rate (Fig. 1E), which demonstrated
that cell proliferation was suppressed. Anaphase analysis was also
performed to confirm the suppressive role of miR-33a on
proliferation. Similarly, miR-33a-overexpressing cells exhibited
decreased anaphase cell numbers compared with the negative control
cells (Fig. 1F). Overall, the present
data indicate that miR-33a has a tumor suppressive role in melanoma
cells.
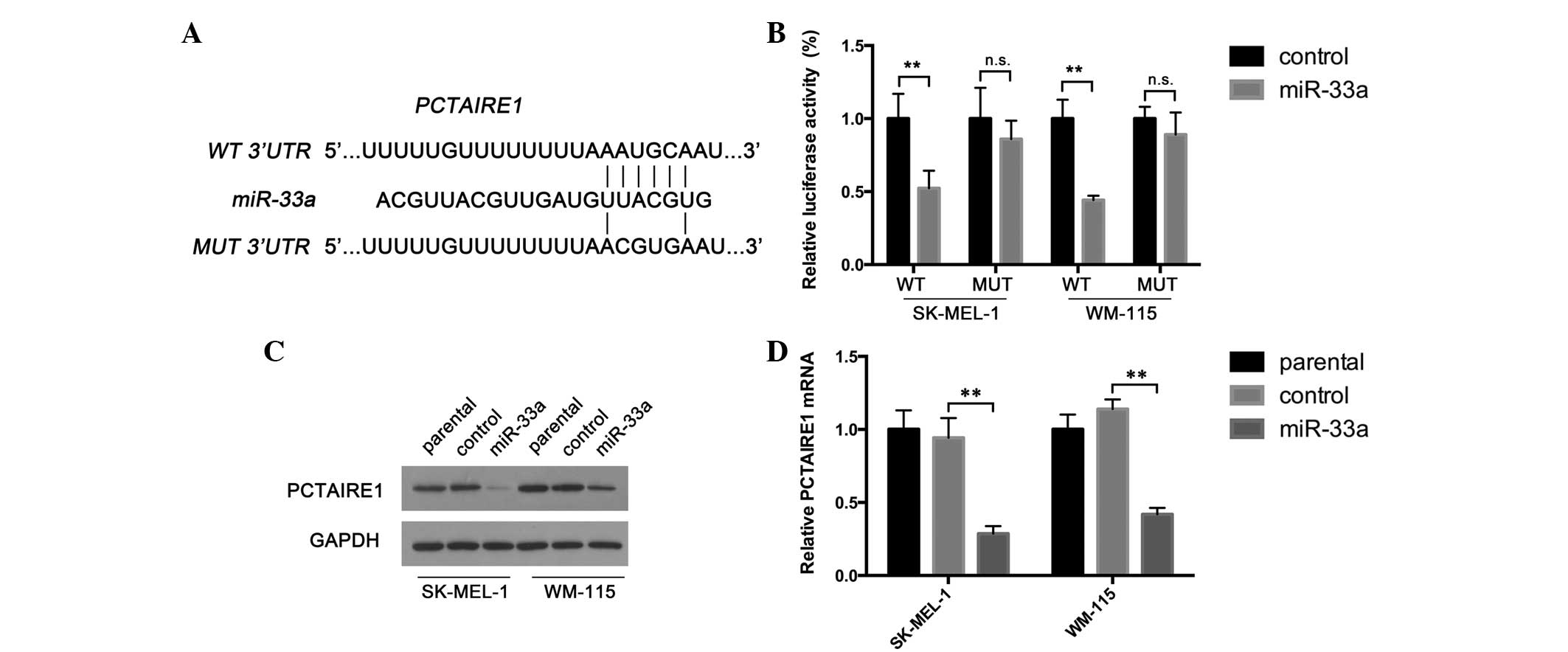
miR-33a targets the 3′-UTR of PCTAIRE1
mRNA and downregulates PCTAIRE1 expression in melanoma cells
miRNAs exert their biological functions by marking
the target transcript for degradation (24). To determine potential miR-33a targets
involved in melanoma proliferation, the present study used a
combination of bioinformatic tools for miRNA target prediction.
TargetScan (Whitehead Institute for Biomedical Research, Cambridge,
MA, USA; available from http://www.targetscan.org/), PicTar (Center for
Comparative Functional Genomics, New York, NY, USA and the Max
Delbruck Centrum, Berlin, Germany; available from http://pictar.mdc-berlin.de/) and miRanda (Memorial
Sloan-Kettering Cancer Center, New York, NY, USA; available from
http://www.microrna.org/microrna/home.do) algorithms
were used to predict miR-33a targets. PCTAIRE1 was revealed as a
potential miR-33a target (Fig. 2A),
and has been reported as a cell cycle regulator in hepatocellular
carcinoma and breast cancer (12).
Therefore, the present study hypothesized that miR-33a may inhibit
melanoma proliferation by directly targeting PCTAIRE1. To determine
whether miR-33a targets this predicted target gene, the present
study generated reporter constructs with the luciferase coding
sequence fused to the wild-type or mutant miR-33a binding sequence
of PCTAIRE1 3′-UTRs (Fig. 2A).
Melanoma cells infected with miR-33a-expressing lentivirus or
negative control lentivirus were transfected with reporter
constructs. The present results demonstrated that miR-33a
overexpression significantly repressed the activity of the
wild-type reporter construct in SK-MEL-1 and WM-115 cells (Fig. 2B). Furthermore, miR-33a had no
repressive effect on reporter vectors carrying the mutated PCTAIRE1
3′-UTR (Fig. 2B). These results
indicate that miR-33a may directly interact with the PCTAIRE1
3′-UTR. To confirm the negative effect that miR-33a exerts on
PCTAIRE1 expression, the present study examined PCTAIRE1 expression
in cells overexpressing miR-33a and negative control cells. As
revealed by Fig. 2C and D, the mRNA
and protein forms of PCTAIRE1 were downregulated following miR-33a
overexpression. Together, the present data suggest that PCTAIRE1 is
a target of miR-33a.
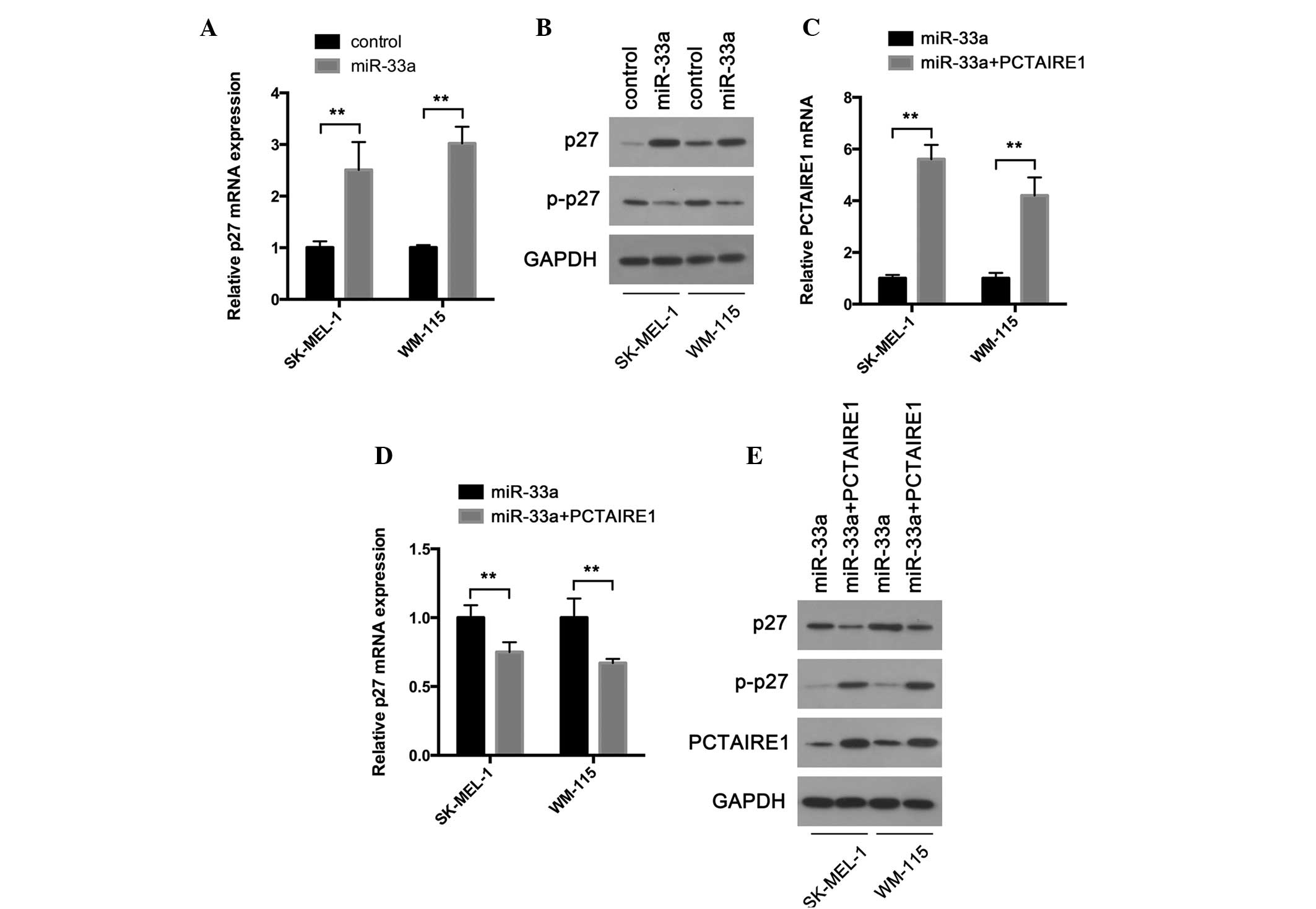
miR-33a suppresses p27 phosphorylation
and upregulates p27 expression in melanoma cells by targeting
PCTAIRE1
p27 is a tumor suppressor that regulates the
proliferation, motility and apoptosis of cells (25). A recent study demonstrated that
PCTAIRE1 phosphorylates p27 and enhances the degradation of p27,
therefore promoting cell proliferation (22). The present study hypothesized that
miR-33a may exert its tumor suppressive function by preventing
PCTAIRE1-induced phosphorylation of p27. To test this hypothesis,
the present study first examined whether p27 expression was
controlled by miR-33a. As expected, RT-qPCR revealed that p27
transcripts were highly upregulated in melanoma cells infected with
miR-33a-expressing lentivirus compared with cells infected with
negative control lentivirus (Fig.
3A). In accordance with these results, a western blot assay
confirmed the upregulated protein expression of PCTAIRE1 following
miR-33a overexpression (Fig. 3B).
Phosphorylation of p27 leads to its degradation and promotes the
activation of assembled cyclin D-cyclin dependent kinases (25). Therefore, the present study examined
the phosphorylation state of p27. The present results demonstrated
that cells overexpressing miR-33a exhibited less phosphorylated p27
compared with negative control cells (Fig. 3B). Furthermore, the present study
examined whether miR-33a modulates p27 phosphorylation and
accumulation by targeting the PCTAIRE1 gene. The present study
overexpressed PCTAIRE1 in cells overexpressing miR-33a by
transfecting the miR-33a cells with a PCTAIRE1 expression plasmid.
RT-qPCR and a western blot assay were performed to confirm PCTAIRE1
overexpression (Fig. 3C and E). As
expected, enforced PCTAIRE1 expression reversed miR-33a induced p27
accumulation (Fig. 3D and E). p27
phosphorylation was also upregulated (Fig. 3E). Overall, the present data indicate
that miR-33a may regulate p27 by targeting PCTAIRE1, and therefore
may inhibit cell proliferation.
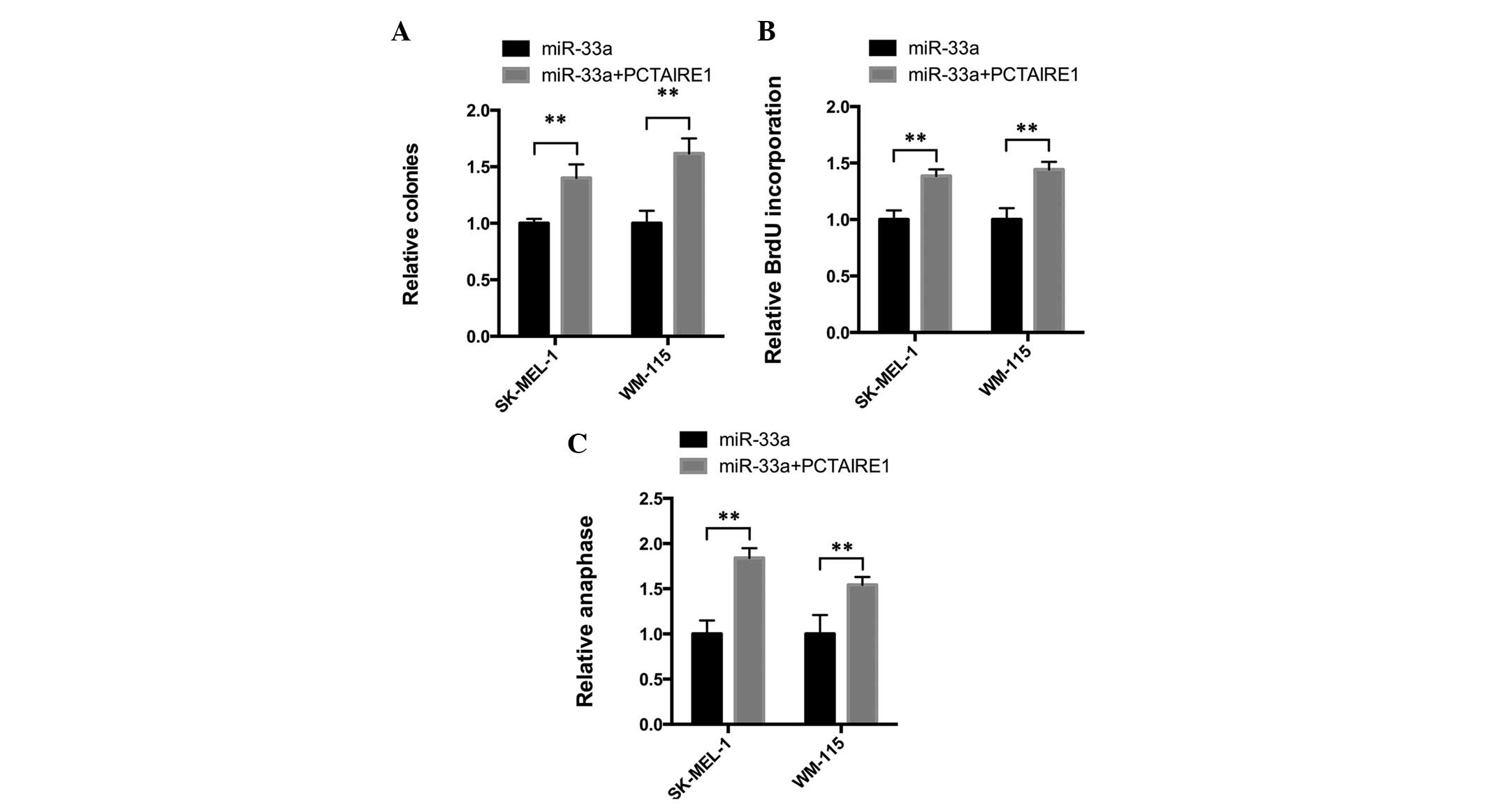
PCTAIRE1 overexpression reverses
miR-33a-induced colony formation and cell proliferation
inhibition
The aforementioned results indicate that miR-33a may
demonstrate tumor suppressor activity by downregulating PCTAIRE1
and upregulating p27. To investigate this hypothesis, the present
study overexpressed PCTAIRE1 in cells overexpressing miR-33a
(Fig. 3C and E) and a colony
formation assay was performed. As expected, in SK-MEL-1 and WM-115
cells, PCTAIRE1 overexpression significantly reversed the
miR-33a-induced tumor suppressive effects (Fig. 4A). Furthermore, the BrdU incorporation
rate was increased following PCTAIRE1 overexpression (Fig. 4B). In addition, the number of cells in
anaphase was also increased in PCTAIRE1-overexpressing cells
(Fig. 4). Overall, the present
results confirmed that miR-33a demonstrates tumor suppressive
activity by targeting PCTAIRE1.
Discussion
miR-33a has been previously identified as a lipid
regulator that controls the cellular balance of cholesterol and
fatty acid metabolism (8–11). The present study demonstrated that
miR-33a is downregulated in melanoma cells compared with
melanocytes. Knockdown of miR-33a enhanced colony formation and
cell proliferation in melanoma cells. Therefore, the present study
reveals that miR-33a acts as a novel tumor suppressor in
melanoma.
PCTAIRE1 is widely expressed in human tissue and
certain studies suggest that PCTAIRE1 participates in various
biological processes, including insulin secretion, secretory cargo
transport and dendrite development (19,20,26). The
role that PCTAIRE1 plays in tumor progression has been recently
revealed; PCTAIRE1 phosphorylates p27 and regulates mitosis in
cancer cells (22). However, the
mechanism by which PCTAIRE1 is regulated is unclear. Although
certain miR-33a targets have been identified, its direct target in
melanoma and the mechanism by which miR-33a exerts its tumor
suppressive behavior remains unknown. The present study
demonstrated that PCTAIRE1 is negatively regulated by the novel
miRNA miR-33a. By performing a luciferase reporter assay and
western blot assays, the present study demonstrated that miR-33a
directly targets PCTAIRE1 in human melanoma SK-MEL-1 and WM-115
cells. These results provide novel information concerning the
mechanisms by which miR-33a and PCTAIRE1 act.
p27, also termed KIP1 or p27kip, is a one
of the most widely studied tumor suppressors (25). It has been reported that p27 regulates
cell proliferation, motility and apoptosis (25,27–29). The
present study revealed that p27 is upregulated by miR-33a, and this
may be due to miR-33a reducing the level of phosphorylated p27.
Furthermore, the present study confirmed that PCTAIRE1 negatively
regulates p27 expression in melanoma cells and the effects of
miR-33a on p27 may be dependent on miR-33a directly targeting
PCTAIRE1.
In summary, the present analysis reveals that
miR-33a is an important negative regulator of cell proliferation.
In addition, the present study demonstrated that PCTAIRE1 is a
target of miR-33a in melanoma cells. Furthermore, miR-33a maintains
p27 expression by targeting PCTAIRE1. Overexpression of PCTAIRE1
reversed the miR-33a-induced suppression of cell proliferation.
Overall, the present findings indicate that miR-33a acts as a tumor
suppressor in melanoma cells.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikawa S, Ishii H, Haraguchi N, Kano Y,
Fukusumi T, Ohta K, Ozaki M, Dewi DL, Sakai D, Satoh T, et al:
microRNA-based cancer cell reprogramming technology. Exp Ther Med.
4:8–14. 2012.PubMed/NCBI
|
|
7
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerin I, Clerbaux LA, Haumont O, Lanthier
N, Das AK, Burant CF, Leclercq IA, MacDougald OA and Bommer GT:
Expression of miR-33 from an SREBP2 intron inhibits cholesterol
export and fatty acid oxidation. J Biol Chem. 285:33652–33661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marquart TJ, Allen RM, Ory DS and Baldán
A: miR-33 links SREBP-2 induction to repression of sterol
transporters. Proc Natl Acad Sci USA. 107:12228–12232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Najafi-Shoushtari SH, Kristo F, Li Y,
Shioda T, Cohen DE, Gerszten RE and Näär AM: MicroRNA-33 and the
SREBP host genes cooperate to control cholesterol homeostasis.
Science. 328:1566–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dávalos A, Goedeke L, Smibert P, Ramírez
CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U,
Pastor-Pareja JC, et al: miR-33a/b contribute to the regulation of
fatty acid metabolism and insulin signaling. Proc Natl Acad Sci
USA. 108:9232–9237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cirera-Salinas D, Pauta M, Allen RM,
Salerno AG, Ramírez CM, Chamorro-Jorganes A, Wanschel AC, Lasuncion
MA, Morales-Ruiz M, Suarez Y, et al: Mir-33 regulates cell
proliferation and cell cycle progression. Cell Cycle. 11:922–933.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takwi AA and Li Y, Buscaglia Becker LE,
Zhang J, Choudhury S, Park AK, Liu M, Young KH, Park WY, Martin RC
and Li Y: A statin-regulated microRNA represses human c-Myc
expression and function. EMBO Mol Med. 4:896–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas M, Lange-Grünweller K, Weirauch U,
Gutsch D, Aigner A, Grünweller A and Hartmann RK: The
proto-oncogene Pim-1 is a target of miR-33a. Oncogene. 31:918–928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rice SJ, Lai SC, Wood LW, Helsley KR,
Runkle EA, Winslow MM and Mu D: MicroRNA-33a mediates the
regulation of high mobility group AT-hook 2 gene (HMGA2) by thyroid
transcription factor 1 (TTF-1/NKX2-1). J Biol Chem.
288:16348–16360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyerson M, Enders GH, Wu CL, Su LK, Gorka
C, Nelson C, Harlow E and Tsai LH: A family of human cdc2-related
protein kinases. EMBO J. 11:2909–2917. 1992.PubMed/NCBI
|
|
17
|
Okuda T, Cleveland JL and Downing JR:
PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related
protein kinase gene family. Oncogene. 7:2249–2258. 1992.PubMed/NCBI
|
|
18
|
Yeung ML, Houzet L, Yedavalli VS and Jeang
KT: A genome-wide short hairpin RNA screening of jurkat T-cells for
human proteins contributing to productive HIV-1 replication. J Biol
Chem. 284:19463–19473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu WY, Cheng K, Fu AK and Ip NY:
Cyclin-dependent kinase 5-dependent phosphorylation of Pctaire1
regulates dendrite development. Neuroscience. 180:353–359. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XY, Gu XT, Saiyin H, Wan B, Zhang YJ,
Li J, Wang YL, Gao R, Wang YF, Dong WP, et al: Brain-selective
kinase 2 (BRSK2) phosphorylation on PCTAIRE1 negatively regulates
glucose-stimulated insulin secretion in pancreatic β-cells. J Biol
Chem. 287:30368–30375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hill SJ, Rolland T, Adelmant G, Xia X,
Owen MS, Dricot A, Zack TI, Sahni N, Jacob Y, Hao T, et al:
Systematic screening reveals a role for BRCA1 in the response to
transcription-associated DNA damage. Genes Dev. 28:1957–1975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yanagi T, Krajewska M, Matsuzawa S and
Reed JC: PCTAIRE1 phosphorylates p27 and regulates mitosis in
cancer cells. Cancer Res. 74:5795–5807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palmer KJ, Konkel JE and Stephens DJ:
PCTAIRE protein kinases interact directly with the COPII complex
and modulate secretory cargo transport. J Cell Sci. 118:3839–3847.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vervoorts J and Lüscher B:
Post-translational regulation of the tumor suppressor p27(KIP1).
Cell Mol Life Sci. 65:3255–3264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SR, Shin JW, Kim HO, Son BH, Yoo CH
and Shin JH: Determining the effect of transforming growth
factor-β1 on cdk4 and p27 in gastric cancer and cholangiocarcinoma.
Oncol Lett. 5:694–698. 2013.PubMed/NCBI
|
|
29
|
Zhang M, Li J, Wang L, Tian Z, Zhang P, Xu
Q, Zhang C, Wei F and Chen W: Prognostic significance of p21, p27
and survivin protein expression in patients with oral squamous cell
carcinoma. Oncol Lett. 6:381–386. 2013.PubMed/NCBI
|