Introduction
Ascites that does not resolve with standard medical
treatment such a low-sodium diet and diuretics is known as
refractory ascites, and is frequently associated with the
development of hepatorenal syndrome, spontaneous bacterial
peritonitis and dilutional hyponatremia (1). Refractory ascites also causes a loss of
appetite and muscle wasting, and impairs the activities of daily
living (ADL) (1). Thus, refractory
ascites is a life-threatening complication that lowers the quality
of life of cirrhotic patients, and is an independent predictor of
short survival (1,2).
One of the main reasons for cirrhosis-related water
retention is a reduced ability of the kidneys to excrete
electrolyte-free water, due to an increase in the levels of
arginine vasopressin (3). Arginine
vasopressin receptor antagonists, a novel class of diuretics, have
been recently approved for the treatment of cirrhosis-related fluid
retention in Japan (3,4). These diuretics antagonize vasopressin V2
receptors, resulting in the inhibition of electrolyte-free water
reabsorption and an increase in electrolyte-free water excretion
(5). Tolvaptan, a vasopressin V2
receptor antagonist, improves hepatic edema and reduces ascites in
cirrhotic patients (6,7). However, tolvaptan may not always be
effective for treating refractory ascites, since there are various
mechanisms involved in the development of this condition (1,8).
Peritoneovenous shunt was designed to transport
ascites from the peritoneal cavity back into the central venous
circulation, and is used for the treatment of refractory ascites
(9,10). Peritoneovenous shunt is effective in
relieving refractory ascites, and it decreases the required dose of
diuretics and the duration and number of hospitalizations, compared
with paracentesis with intravenous infusion of albumin (11). However, the efficacy of
peritoneovenous shunt on tolvaptan-resistant refractory ascites has
not been reported to date. In addition, the impact of
peritoneovenous shunt on the prognosis of cirrhotic patients with
refractory ascites remains controversial, due to the severe
potential complications of peritoneovenous shunt, including
disseminated intravascular coagulation (11–13).
In the present study, a case of tolvaptan-resistant
refractory ascites associated with liver cirrhosis and portal vein
thrombosis is described. Peritoneovenous shunt markedly reduced the
ascites and improved the ADL score of the patient. Although the
patient succumbed to sepsis at 486 days following implantation of
the shunt, the ascites was under control. Thus, the present case
indicates that peritoneovenous shunt relieves tolvaptan-resistant
ascites, improves ADL and prolongs survival in cirrhotic
patients.
Case report
In June 2014, a 51-year-old Japanese man was
referred to the Kurume University Hospital, affiliated with Kurume
University School of Medicine (Kurume, Japan), for examination of
persistent hepatic dysfunction. The patient was diagnosed with
hepatitis C virus-related liver cirrhosis based on serological
findings (Table I). Although the
patient was treated with peginterferon (Pegintron®; 60
µg once a week; MSD K.K., Tokyo, Japan) and ribavirin
(Rebetol®; 600 mg/day; MSD K.K.) combination therapy for
48 weeks, the hepatitis C virus was not eradicated. Therefore, the
patient was treated with ursodeoxycholic acid (Urso®;
600 mg/day; Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan) and
a glycyrrhizin-containing preparation (Stronger neo-minophagen
C®; 40 ml twice a week; Minophagen Pharmaceutical Co.,
Ltd., Tokyo, Japan) for 5 years.
 | Table I.Biochemical parameters of the patient
on admission. |
Table I.
Biochemical parameters of the patient
on admission.
| Parameters | Value | Reference value |
|---|
| Red blood cell count
(x104 cells/mm3) | 346.00 | 380.00–500.00 |
| Hemoglobin levels
(g/dl) | 11.30 | 11.00–15.00 |
| White blood cell
count (cells/mm3) | 4,100.00 |
4,000.00–9,000.00 |
| Platelet count
(x104 cells/mm3) | 3.10 | 13.00–36.00 |
| Aspartate
transaminase levels (U/l) | 49.00 | 13.00–33.00 |
| Alanine
aminotransferase levels (U/l) | 23.00 |
8.00–42.00 |
| Lactate dehydrogenase
levels (U/l) | 303.00 | 119.00–229.00 |
| Alkaline phosphatase
levels (U/l) | 202.00 | 115.00–359.00 |
|
γ-glutamyltranspeptidase levels (U/l) | 14.00 | 10.00–47.00 |
| Total protein levels
(g/dl) | 6.61 | 6.70–8.30 |
| Albumin levels
(g/dl) | 2.96 | 4.00–5.00 |
| Total bilirubin
levels (mg/dl) | 2.54 | 0.30–1.50 |
| C-reactive protein
levels (mg/dl) | 1.39 | <0.40 |
| Total cholesterol
levels (mg/dl) | 125.00 | 128.00–220.00 |
| Fasting blood glucose
levels (mg/dl) | 114.00 |
80.00–109.00 |
| Hemoglobin A1c levels
(%) | 4.50 | 4.30–5.80 |
| Prothrombin activity
(%) | 31.00 |
60.00–130.00 |
| Blood urea nitrogen
levels (mg/dl) | 13.80 |
8.00–22.00 |
| Creatinine levels
(mg/dl) | 0.69 | 0.40–0.70 |
| Blood ammonia levels
(µg/dl) | 69.00 | 12.00–66.00 |
| α-fetoprotein levels
(ng/ml) | 1.90 | <8.70 |
| Carcinoembryonic
antigen levels (ng/ml) | 4.20 | <5.00 |
| Carbohydrate antigen
19–9 levels (U/ml) | 32.20 | <37.00 |
| Child-Pugh score | 12.00 | N/A |
| MELD
scorea | 18.00 | N/A |
| MELD-Na
scorea | 23.00 | N/A |
At the age of 56 years, the patient developed an
infected urachal cyst that was resistant to antibiotic medication.
Following surgical resection of the infected urachal cyst, the
patient developed portal vein thrombosis. Although the patient was
treated with anticoagulant therapy using warfarin potassium
(Warfarin®; 1.5 mg/day; Eisai Co. Ltd., Tokyo, Japan)
for 1 year, the portal vein thrombosis did not improve. Massive
thrombosis developed at the umbilical portion of the portal vein,
as confirmed by magnetic resonance imaging (Signa
HDxt1.5T®; GE Healthcare Japan, Tokyo, Japan) using
gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (EOB
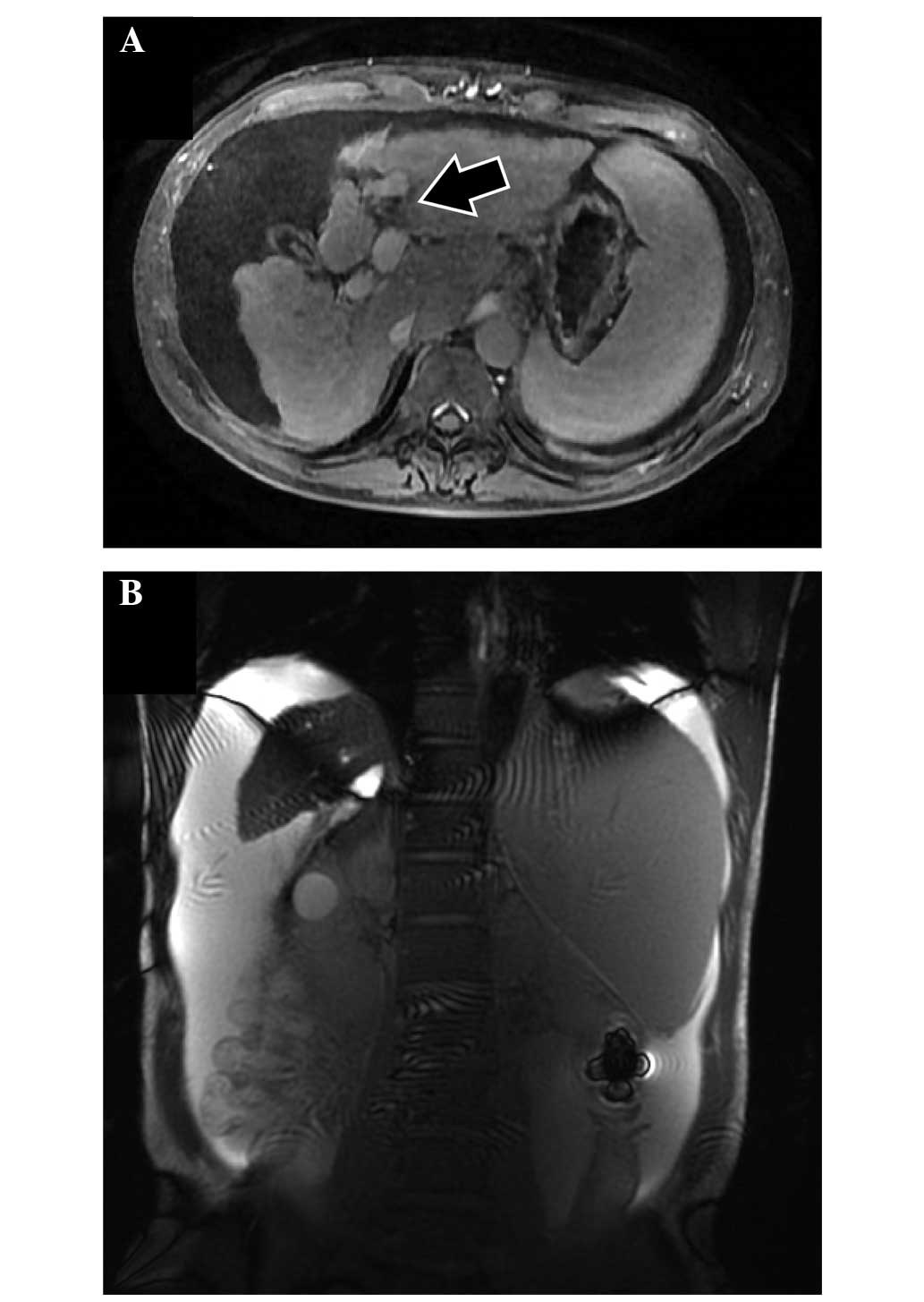
Primovist®; Bayer, Osaka, Japan) (Fig. 1A). In addition, the patient
accumulated a large amount of ascitic fluid (Fig. 1B).
The biochemical parameters on admission indicated
decompensated liver cirrhosis (Table
I), in addition to the following ascitic findings:
Yellow-tinged appearance; serum-ascites albumin gradient of 2.39;
fluid-serum total protein ratio of 0.163; α-fetoprotein levels of
0.6 ng/ml; carcinoembryonic antigen levels of 0.5 ng/ml;
carbohydrate antigen 19–9 levels of 6.8 U/ml; and cell number of
neutrophils 35 cells/µl, suggesting transudate ascites. The patient
was treated with a low-sodium diet (salt, 7 g/day), diuretics
[furosemide (Lasix®; Sanofi K.K., Tokyo, Japan), 60
mg/day, and spironolactone (Aldactone-A®; Pfizer Japan
Inc., Tokyo, Japan), 100 mg/day] for 1 year and an albumin
preparation (Albuminar®; 12.5 g, three times a month;
CSL Behring K.K., Tokyo, Japan) for 4 months; however, the amount
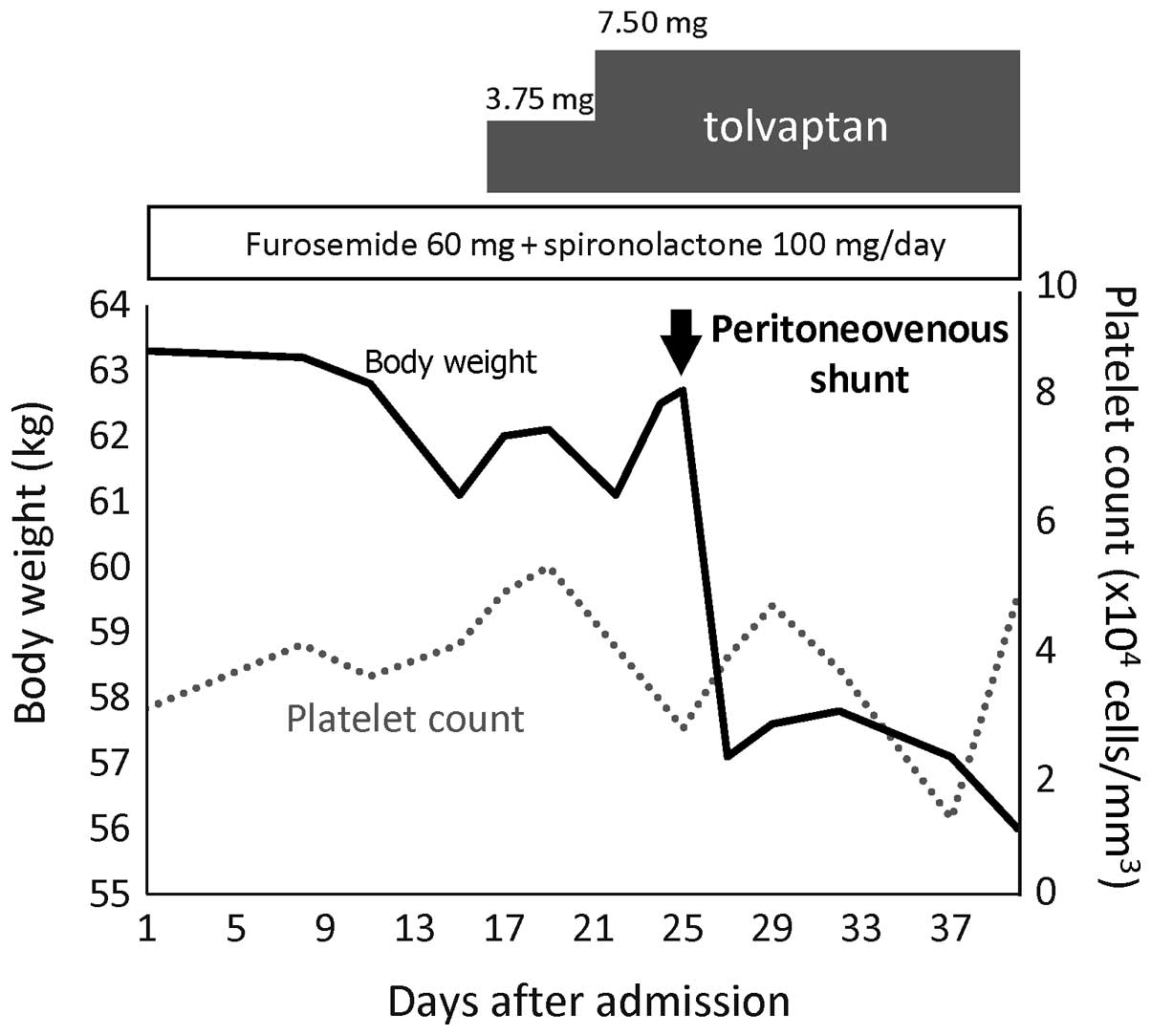
of ascitic fluid was not reduced (Fig.
2). Tolvaptan (Samusuca®; 7.5 mg/day; Otsuka
Pharmaceutical Co., Ltd., Tokyo, Japan) was added to the treatment
on day 16, and was continued for 4 months, but the ascites remained
resistant to treatment (Fig. 2).
Thus, the refractory ascites did not improve by diet or medication,
including tolvaptan, and the patient gradually lost appetite.
The patient presented a Child-Pugh score of 12
points (Table I). In consequence, the
patient was informed about his predicted poor prognosis, and was
communicated that liver transplantation was the only possible
therapeutic strategy associated with long-term survival. However,
the patient did not consent to liver transplantation; therefore, a
peritoneovenous shunt was implanted upon obtaining consent to
relieve the ascites-associated symptoms on day 25. Following
implantation of the shunt, the volume of urine immediately
increased and the ascites markedly decreased. The patient's body
weight decreased from 62.7 to 57.1 kg in 2 days (Fig. 2). Although the patient exhibited
disseminated intravascular coagulation on day 37, this condition
improved with the use of thrombomodulin alfa
(Recomoduin®; 265,000 U/day; Pfizer Japan Inc.) for 3
days and antibiotic treatment [piperacillin
(Pentcillin®; 2 g/day for 7 days; Taisho Toyama
Pharmaceutical Co., Ltd., Tokyo, Japan) and levofloxacin (Cravit;
500 mg/day for 10 days; Daiichi Sankyo Company, Ltd., Tokyo,
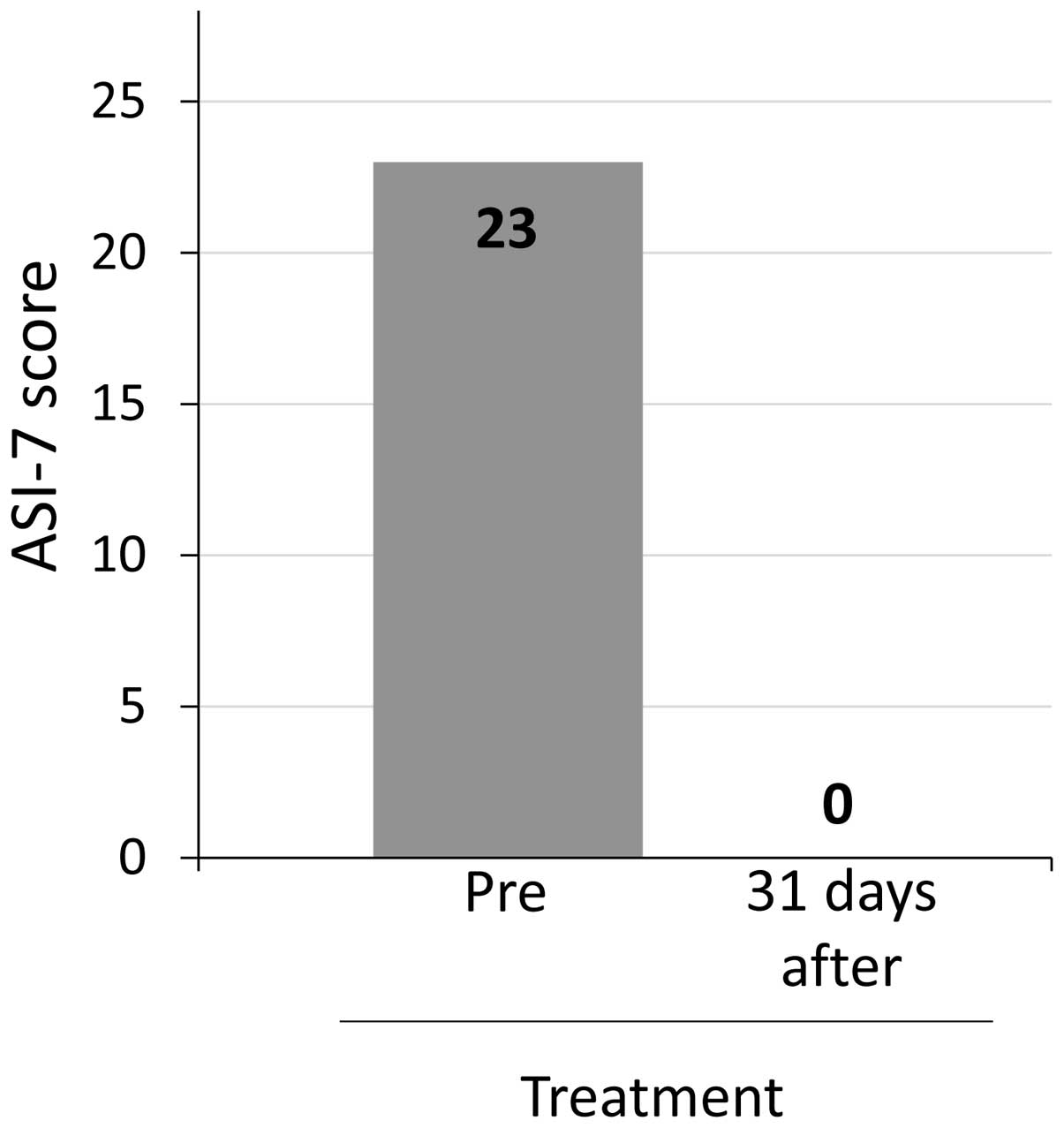
Japan)]. The patient's ascites symptom inventory-7 (ASI-7) score,
an ascites-specific symptom scale (14), decreased from 23 to 0 points on day 56
(Fig. 3). Along with the reduction of
the ascites, the patient's appetite improved, and therefore the
patient was discharged from the hospital.
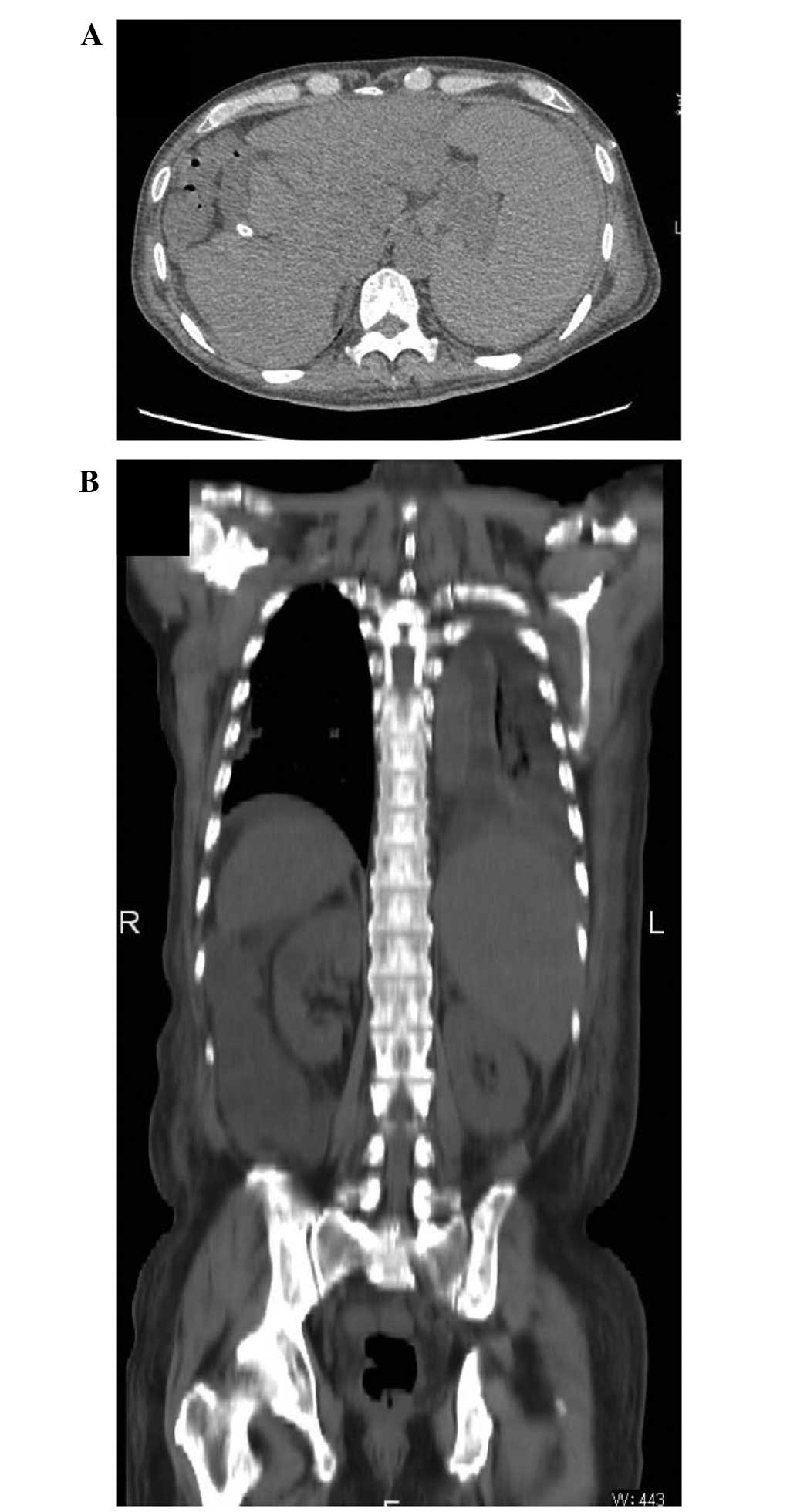
A computed tomography scan (Discovery CT750
HD®; GE Healthcare Japan) revealed that the patient's
ascites remained well controlled at >450 days following
implantation of the shunt, and his liver function did not worsen,
despite lowering the doses of diuretics administered to the patient
(furosemide, 20 mg/day and spironolactone, 50 mg/day) and
withdrawing tolvaptan (Fig. 4;
Table II). The patient developed
infectious diseases, including pulmonary cryptococcosis, and
short-term admission was required in four occasions during the
458-day period subsequent to the implantation of the shunt. Despite
the infections, the patient experienced a normal daily life with
his family. The patient succumbed to sepsis, probably due to
bacterial translocation from the intestine, on day 486 following
implantation of the shunt. However, his ADL was preserved until 8
days prior to mortality.
 | Table II.Body weight and biochemical parameters
prior to and on day 456 following implantation of the
peritoneovenous shunt. |
Table II.
Body weight and biochemical parameters
prior to and on day 456 following implantation of the
peritoneovenous shunt.
| Characteristics | Prior to shunt | 456 days following
shunt |
|---|
| Body weight (kg) | 62.70 | 58.90 |
| Platelet count (x104
cells/mm3) | 3.10 | 2.20 |
| Aspartate
transaminase levels (U/l) | 49.00 | 39.00 |
| Alanine
aminotransferase levels (U/l) | 23.00 | 26.00 |
|
γ-glutamyltranspeptidase levels (U/l) | 14.00 | 14.00 |
| Total bilirubin
levels (mg/dl) | 2.54 | 3.56 |
| Albumin levels
(g/dl) | 2.96 | 2.43 |
| Prothrombin activity
(%) | 34.00 | 59.00 |
| Cholinesterase
levels (U/l) | 49.00 | 48.00 |
| Creatinine levels
(mg/dl) | 0.69 | 0.48 |
| Sodium levels
(mEq/l) | 131.00 | 133.00 |
| Child-Pugh
score | 12.00 | 12.00 |
| MELD
scorea | 18.00 | 14.00 |
| MELD-Na
scorea | 23.00 | 23.00 |
Discussion
In the present report, a case of tolvaptan-resistant
refractory ascites associated with liver cirrhosis and portal vein
thrombosis is described. Peritoneovenous shunt markedly reduced the
ascites and ASI-7 score. Although the patient experienced recurrent
infectious diseases and succumbed to sepsis on day 486 following
implantation of the shunt, the ascites was under control. Thus, the
present case supports the efficacy of peritoneovenous shunt in
treating tolvaptan-resistant refractory ascites in cirrhotic
patients with portal vein thrombosis. In addition, peritoneovenous
shunt may prolong the survival of cirrhotic patents with refractory
ascites.
The refractory ascites in the present patient was
initially treated with tolvaptan in combination with conventional
diuretic therapy. However, the patient did not respond to this
treatment. At 31 days following the implantation of the
peritoneovenous shunt, the tolvaptan-resistant refractory ascites
was markedly reduced, and the patient's symptoms, as evaluated by
ASI-7 score, disappeared. Although it is unclear why his ascites
did not respond to tolvaptan treatment, a possible reason is that
the ascites was not caused by a reduced ability to excrete
solute-free-water through an increase in arginine vasopressin
levels, which is the most common cause of cirrhosis-related water
retention (3). In the present case,
the ascites became intractable following the development of portal
vein thrombosis; therefore, severe portal hypertension appears to
be the main pathogenesis of the refractory ascites exhibited by the
patient. Thus, etiological differences in refractory ascites may
account for a lack of response to tolvaptan treatment. To the best
of our knowledge, the present case report is the first to
demonstrate the efficacy of peritoneovenous shunt for the treatment
of tolvaptan-resistant refractory ascites in a cirrhotic patient
with portal vein thrombosis.
Although peritoneovenous shunt is known to be
effective in relieving refractory ascites, Nitta et al
(12) reported that peritoneovenous
shunt should not be considered in cirrhotic patients with
refractory ascites and prolonged prothrombin time, due to an
increase in the risk of disseminated intravascular coagulation.
Ginès et al (11) performed a
randomized controlled trial and demonstrated that peritoneovenous
shunt did not prolong the survival of cirrhotic patients with
refractory ascites, compared with patients treated with
large-volume paracentesis plus intravenous albumin. In the present
case, the patient exhibited hepatitis C virus-related decompensated
liver cirrhosis with portal vein thrombosis, and his Child-Pugh
score was 12 points. Although it is unclear whether peritoneovenous
shunt improved the prognosis, Heuman et al (15) reported a 180-day survival rate of
58.6% in cirrhotic patients with persistent ascites and low levels
of sodium in serum. Girleanu et al (16) examined the natural course of
nonmalignant partial portal vein thrombosis in cirrhotic patients,
and reported a 6-month survival rate of 66.66% and a median
survival time of 8.6 months in patients with worsening portal vein
thrombosis. These findings support a prolonged survival with
implantation of peritoneovenous shunt in the present patient. In
addition, Miyaaki et al (13)
reported two patients who experienced an increase in liver volume
following implantation of a peritoneovenous shunt, further
supporting the possibility that survival is prolonged with
peritoneovenous shunt.
The ASI-7 score of the present patient markedly
improved with the disappearance of the refractory ascites following
implantation of the shunt. However, the patient experienced
recurrent infectious diseases and succumbed to sepsis on day 486
following implantation of the shunt. Recent advances in the
treatment of malnourishment, ascites, esophageal varices and
hepatocellular carcinoma indicate that infectious diseases may be
one of the major causes of mortality in patients with liver
cirrhosis in the future (17,18). Arvaniti et al (19) reported that infections increased
mortality by 4-fold in cirrhotic patients, and that 30% of
cirrhotic patients succumbed to disease within 1 month following
the infection, and an additional 30% succumbed to disease within 1
year. Therefore, further studies in patients with advanced liver
cirrhosis should be focused on the management of infectious
diseases.
In conclusion, the present study describes a case of
tolvaptan-resistant refractory ascites related to liver cirrhosis
with portal vein thrombosis. The patient was subjected to
peritoneovenous shunt, which markedly reduced the ascites and the
ASI-7 score. Although the patient succumbed to sepsis on day 486
subsequent to the implantation of the shunt, his ascites was under
control. Thus, the present case indicates that peritoneovenous
shunt relieves tolvaptan-resistant ascites and improves ADL. In
addition, the findings of the present case indicate that
peritoneovenous shunt may prolong the survival of cirrhotic patents
with refractory ascites.
Glossary
Abbreviations
Abbreviations:
|
ADL
|
activities of daily living
|
|
ASI-7
|
ascites symptom inventory-7
|
References
|
1
|
Salerno F, Guevara M, Bernardi M, Moreau
R, Wong F, Angeli P, Garcia-Tsao G and Lee SS: Refractory ascites:
Pathogenesis, definition and therapy of a severe complication in
patients with cirrhosis. Liver Int. 30:937–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arroyo V, Ginès P, Gerbes AL, Dudley FJ,
Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H and Schölmerich J:
Definition and diagnostic criteria of refractory ascites and
hepatorenal syndrome in cirrhosis. International Ascites Club.
Hepatology. 23:164–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyer TD: Tolvaptan and hyponatremia in a
patient with cirrhosis. Hepatology. 51:699–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okita K, Kawazoe S, Hasebe C, Kajimura K,
Kaneko A, Okada M and Sakaida I: ASCITES Dose-Finding Trial Group:
Dose-finding trial of tolvaptan in liver cirrhosis patients with
hepatic edema: A randomized, double-blind, placebo-controlled
trial. Hepatol Res. 44:83–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schrier RW, Gross P, Gheorghiade M, Berl
T, Verbalis JG, Czerwiec FS and Orlandi C: SALT Investigators:
Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for
hyponatremia. N Engl J Med. 355:2099–2112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okita K, Sakaida I, Okada M, Kaneko A,
Chayama K, Kato M, Sata M, Yoshihara H, Ono N and Murawaki Y: A
multicenter, open-label, dose-ranging study to exploratively
evaluate the efficacy, safety and dose-response of tolvaptan in
patients with decompensated liver cirrhosis. J Gastroenterol.
45:979–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaida I, Kawazoe S, Kajimura K, Saito T,
Okuse C, Takaguchi K, Okada M and Okita K: ASCITES-DOUBLEBLIND
Study Group: Tolvaptan for improvement of hepatic edema: A phase 3,
multicenter, randomized, double-blind, placebo-controlled trial.
Hepatol Res. 44:73–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dahl E, Gluud LL, Kimer N and Krag A:
Meta-analysis: The safety and efficacy of vaptans (tolvaptan,
satavaptan and lixivaptan) in cirrhosis with ascites or
hyponatraemia. Aliment Pharmacol Ther. 36:619–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leveen HH, Christoudias G, Ip M, Luft R,
Falk G and Grosberg S: Peritoneo-venous shunting for ascites. Ann
Surg. 180:580–591. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin LG: Percutaneous placement and
management of peritoneovenous shunts. Semin Intervent Radiol.
29:129–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ginès P, Arroyo V, Vargas V, et al:
Paracentesis with intravenous infusion of albumin as compared with
peritoneovenous shunting in cirrhosis with refractory ascites. N
Engl J Med. 325:829–835. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nitta H, Okamura S, Mizumoto T, et al:
Prognosis assessment of patients with refractory ascites treated
with a peritoneovenous shunt. Hepatogastroenterology. 60:1607–1610.
2013.PubMed/NCBI
|
|
13
|
Miyaaki H, Murakami E, Ichikawa T, et al:
Long-term increase in liver volume after Denver peritoneovenous
shunt: Report of two cases. Liver Int. 29:774–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onishi Y, Wakita T, Fukuhara S, et al:
Development and validation of a symptom scale specific for ascites
accompanied with cirrhosis: The ASI-7. Clin Transl Gastroenterol.
5:e482014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heuman DM, Abou-Assi SG, Habib A, Williams
LM, Stravitz RT, Sanyal AJ, Fisher RA and Mihas AA: Persistent
ascites and low serum sodium identify patients with cirrhosis and
low MELD scores who are at high risk for early death. Hepatology.
40:802–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Girleanu I, Stanciu C, Cojocariu C, et al:
Natural course of nonmalignant partial portal vein thrombosis in
cirrhotic patients. Saudi J Gastroenterol. 20:288–292. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi T, Shiraishi K, Ito T, et al:
Branched-chain amino acids prevent hepatocarcinogenesis and prolong
survival of patients with cirrhosis. Clin Gastroenterol Hepatol.
12:1012–1018.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nahon P, Lescat M, Layese R, et al:
Bacterial infection in compensated viral cirrhosis impairs 5-year
survival (ANRS CO12 CirVir prospective cohort). Gut:
gutjnl-2015-310275. 2015.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Arvaniti V, D'Amico G, Fede G, Manousou P,
Tsochatzis E, Pleguezuelo M and Burroughs AK: Infections in
patients with cirrhosis increase mortality four-fold and should be
used in determining prognosis. Gastroenterology. 139:1246–1256.
2010. View Article : Google Scholar : PubMed/NCBI
|