Introduction
Impressive headway has been made in chemotherapy
drug development, yet non-small cell lung cancer (NSCLC) types
often turn out to be resistant to the treatment, whether a given
drug is administered repeatedly or not. Furthermore, drug
resistance mechanisms, to a great extent, remain unclear. From the
viewpoint of the transport-obstruction theory, decreased uptake and
increased efflux across tumor cell membranes contribute to such
resistance (1,2). To be specific, certain proteins, such as
ATP-binding cassette (ABC) super-family, transport drug and toxic
materials increase concentration gradients, and this reverse
transportation has much to do with the complex issue of drug
resistance.
Previous studies have detected the expression of
ABC-transporters, such as ABC sub-family B member 1 (ABCB1; also
known as MDR1 or P-gp) in lung cancer, and arrived at the
conclusion that ABCB1 overexpression or polymorphism is a vital
factor behind drug resistance in lung cancer treatment (3,4). For
example, among lung cancer patients, the majority of drug
resistance cases overexpress ABCB1 and ABC subfamily C member 1
(ABCC1) genes, registering an ABCB1 expression level of 25–43% and
an ABCC1 incidence of 80–100% (5–7). Another
previous study has suggested that in NSCLC patients, the ABCB1
2677G>T/A polymorphism and 2677G-3435C haplotype may be used as
treatment response predictors to docetaxel-cisplatin chemotherapy
(8). It has been shown that these
proteins transport chemical compounds from the nucleus to the
cytoplasm, where they are redistributed in order to maintain an
intracellular concentration below the cytotoxic level (9). This unique self-protective mechanism is
closely associated with the multidrug resistance (MDR) phenotype of
cancer cells.
A number of studies have suggested that ABC
transporters impede drug absorption. Such a property predicts the
susceptibility of a patient and therefore highlights the necessity
of individualized chemotherapy. However, it is not yet easy to
convincingly elucidate the molecular mechanism of MDR, as this is
limited by the current level of gene regulation. Recently, nuclear
receptor (NR) families, including pregnane X receptor (PXR) and
constitutive androstane receptor (CAR), have been proven capable of
boosting MDR (10). Together with
xenobiotics, such as rifampin, activated NRs, as a transcriptional
regulator, can play a significant role in modulating drug
transporter genes and drug-metabolizing enzymes (11). A number of xenobiotic transporters are
subject to PXR regulation, including ABCB1, ABCC2, ABCC3, ABCC4,
ABCC5 and breast cancer resistance protein, commonly found in
hepatocytes, the kidneys and the blood brain barrier (9). It has also been reported that with the
presence of the ABCB1 gene enhancer, or upon activation by various
therapeutic agents, such as clotrimazole and nifedipine, PXR is
likely to exercise regulation over ABCB1 gene expression (12).
Under such circumstances, demand arises for a proper
surrogate marker and a simple measuring technique (13,14). Solid
tumor studies in general, however, are beset by the problem of
blood and normal tissue contamination, and often end in
positive-rate, heterogeneous conclusions. Moreover, in the majority
of cases, particularly at the advanced stage, the collection and
inspection of target tissue samples often become difficult.
As is well known, blood cells, functioning as
mediators of the immune response and interacting with all human
tissues, are relevant to the pathogenesis of numerous diseases,
such as renal cell carcinoma or breast cancer. Recent studies have
suggested that the peripheral blood reflects physiological and
pathological changes inside the body, and may be taken as a
substrate for the molecular profiling of human diseases and disease
risks (15). Ban et al, by aid
of flow cytometry, examined the ABCB1 profile of peripheral blood
mononuclear cells (PBMCs) in epilepsy patients and found a higher
basal ABCB1 level in them and in patients on high-dose medications
(16). Valente et al
investigated the downregulation of ABCB1 in the kidneys and PBMCs
of SHR rats, and suggested that for hypertensive humans, assessing
gene activity in PBMCs is a non-invasive, and therefore practical,
test method (17).
With non-invasive, peripheral blood-based lab tests
available, the prospect of PBMCs as an alternative for drug
resistance detection has come to the fore. However, the
practicality of using peripheral blood cells for predicting lung
cancer multidrug resistance requires investigation. With the use of
immunohistochemistry, the present study assesses ABCB1 and PXR
expression in PBMCs and tissue samples obtained from lung cancer
patients. The study examines post-chemotherapy ABCB1 and PXR
expression, and the correlations between the two.
Materials and methods
Materials
The study was conducted in strict accordance with
the stipulations of the Helsinki Declaration and was approved by
the Qingdao Municipal Hospital Ethics Committee (Qingdao, China).
All the subjects and their families were informed of the relevant
details and signed consent forms of their own accord prior to the
study. The study duration ranged between November 2011 and October
2012. The subjects consisted of 37 patients with pathologically
proven lung cancer (10 females with a mean age ± standard deviation
(SD) of 51±13 years; and 27 males with a mean age ± SD of 56±18
years). Of the 37 cancer patients, 24 presented with lung
adenocarcinoma and 13 with lung squamous carcinoma. In the control
group (11 females with a mean age ± SD of 41±9 years; and 16 males
with a mean age ± SD of 40±13 years), samples of non-neoplastic
lung tissue were collected from 17 subjects who underwent a lung
wedge resection, either due to bullae (12 cases) or benign tumors
(2 cases of pulmonary hamartoma and 3 of papilloma).
Samples preparation
Tissue sampling was performed through surgical
resection in 31 subjects and through transbronchial fine-needle
aspiration biopsy in 6 subjects. Prior to this, none of the
patients had ever been administered chemotherapy or radiotherapy.
The specimens were fixed in 10% buffered neutral formalin. Directly
prior to and following 1 cycle of first-line chemotherapy
(intravenous vinorelbine, 25 mg/m2 on day 8; intravenous
cisplatin, 75–80 mg/m2 on day 3), a 3-ml blood sample
was collected from each subject and transferred into an
ethylenediaminetetraacetic acid (EDTA)-containing blood collection
tube. PBMCs were immediately isolated by Ficoll-Hypaque (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
density centrifugation at 1,800 × g (5417R; Eppendorf, Hamburg,
Germany).
Immunohistochemical analysis of the
tissue samples
The lung cancer tissue sections and controls were
fixed in 10% neutral formalin, paraffin-embedded and cut into 4-µm
thick slices. The slices were briefly treated with 1 mM EDTA (pH
8.0) prepared by microwave, and then with 3% hydrogen peroxidase,
following 10 min of blocking with endogenous peroxidase. The
polyclonal rabbit anti-PXR (1:100; catalog no. ab85451; Abcam,
Cambridge, UK) and monoclonal rabbit anti-ABCB1 (p170/MDR-1 Ab;
1:200; catalog no. MS-660; Fuzhou Maixin Biotechnology Development
Co., Ltd., Fuzhou, China) primary antibodies were added at 4°C
overnight. Liver carcinoma tissues were used as a positive control,
and normal goat immunoglobulin G (UltraSensitive™ SP kit; catalog
no. KIT-9720; Fuzhou Maixin Biotechnology Development Co., Ltd.)
was used instead of the primary antibody as a negative control.
Secondary antibody (UltraSensitive™ SP kit) was also applied at
room temperature for 30 min. Color development was performed using
3,3′-diaminobenzidine (Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China), and the hemtoxylin (Fuzhou Maixin
Biotechnology Development Co., Ltd.)-stained slices were
coverslipped in permount (Fuzhou Maixin Biotechnology Development
Co., Ltd.).
Immunohistochemistry for PBMCs
Previously centrifuged PMBCs were suspended
carefully in ThinPrep conserved solution (Becton Dickinson,
Franklin Lakes, NJ, USA) for 4–6 h. Afterwards, liquid-based smears
with cytospin (Ningbo Medsun Medical Co., Ltd., Ningbo, China) were
prepared on the principle of the ThinPrep cytological test, and
then fixed in pure acetone solution for 20 min. Following Giemsa
staining (New Biotechnology Development Co., Ltd., Fuzhou, China),
prior to immunostaining, the percentage of lymphocytes was
calculated through microscopic observation. The process yielded a
high lymphocyte purity (96%). Immunohistochemical analysis was
conducted as aforementioned.
PXR and ABCB1 expression in PBMCs and cancerous
tissues was evaluated independently by two pathologists blinded to
the study details. The final score was determined as the staining
intensity plus the percentage of positive cells. The scoring system
was graded as follows: 0–3, negative expression; and 4–10, positive
expression.
Statistical analysis
Statistical analysis was performed by means of a
χ2 test and Fisher's exact test (SPSS software, version
17.0; SPSS Inc., Chicago, IL, USA). P<0.05 was used to denote a
statistically significant difference.
Results
Pre-chemotherapy PXR and ABCB1 protein
expression in PBMCs
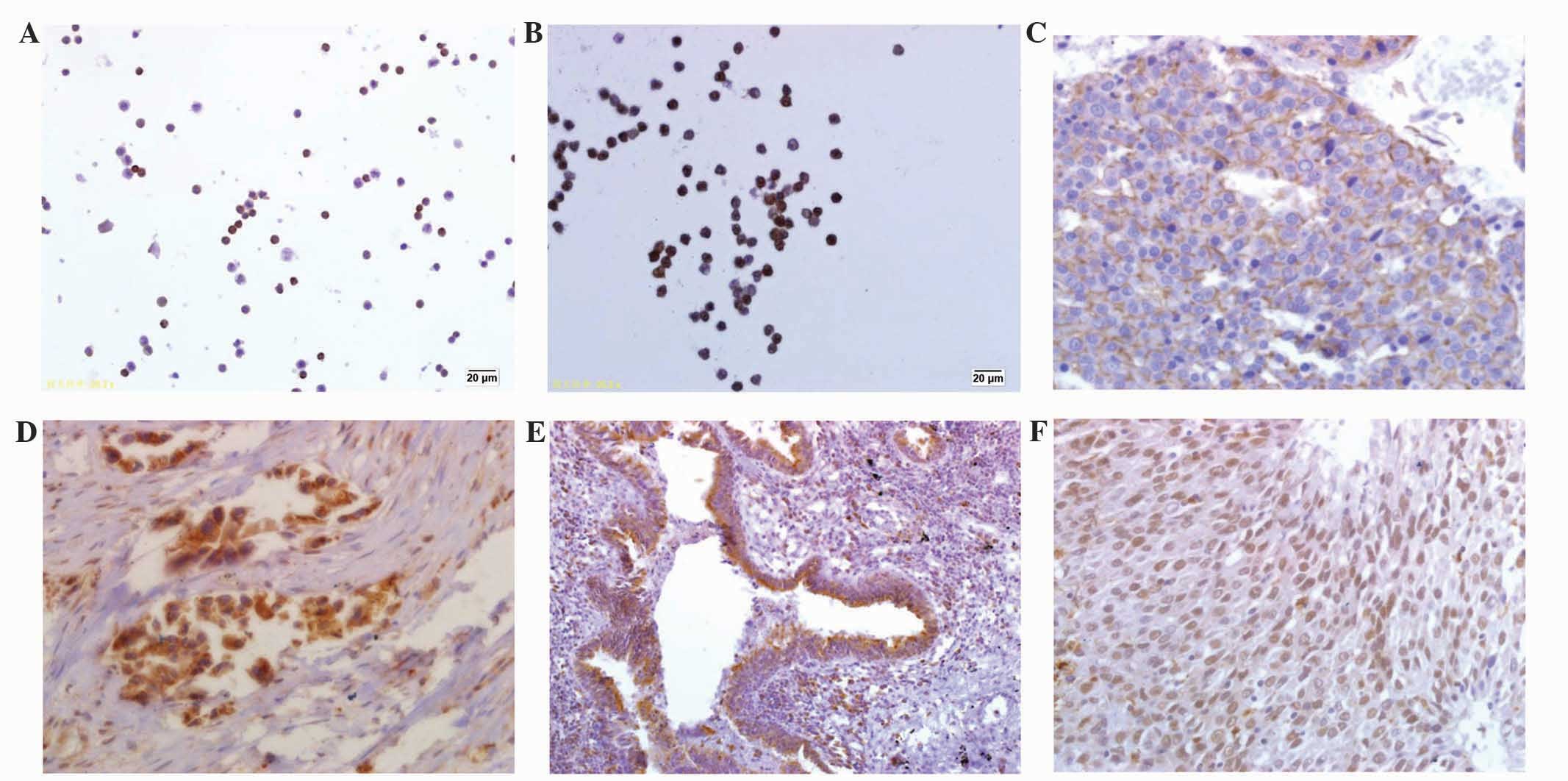
Results from the immunostaining demonstrated
widespread PXR and ABCB1 expression in the PBMCs from the lung
cancer patients (prior to and following chemotherapy) and the
control group. Generally, conspicuous PXR expression was mostly
found in the nucleus of the lymphocytes, while high ABCB1
expression was found on the lymphocyte membrane (Fig. 1A and B). In the control group, the
positive rate of ABCB1 and PXR expression reached 23.5% (4/17) and
64.7% (11/17), respectively. In the lung cancer patients, positive
ABCB1 and PXR protein expression increased to 59.5% (22/37) and
62.2% (23/37), respectively (Table
I). PBMCs from the lung cancer patients exhibited a significant
increase in ABCB1 expression. This agreed with the outcomes from
previous studies. Unexpectedly, statistical analysis indicated that
significant correlations between PXR and ABCB1 expression could
only be found in the PBMCs from the lung cancer patients.
 | Table I.Comparison of ABCB1 and PXR protein
expression in the PBMCs of the NSCLC patients and control group
prior to chemotherapy. |
Table I.
Comparison of ABCB1 and PXR protein
expression in the PBMCs of the NSCLC patients and control group
prior to chemotherapy.
|
| NSCLC group | Control group |
|---|
|
|
|
|
|---|
| Protein |
ABCB1+ |
ABCB1− | Sum | r | P-value |
ABCB1+ |
ABCB1− | Sum | r | P-value |
|---|
| PXR+,
n | 18 | 5 | 23 (62.2) | 0.49 | 0.03 | 3 | 8 | 11 (64.7) | 0.12 | 0.62 |
| PXR−,
n | 4 | 10 | 14 (37.8) |
|
| 1 | 5 | 6
(35.3) |
| Sum, n (%) | 22
(59.5)a | 15 (40.5) | 37
(100.0) |
|
| 4 (23.5) | 13 (76.5) | 17
(100.0) |
The results showed that no associations were
identified between PXR and ABCB1 expression in the PBMCs of the
control group. It may be inferred that the similarity in PXR
expression in the two groups is attributable to the
multi-biological functions of PXR. As a ligand-activated
transcription factor, PXR plays a vital role in drug transportation
regulation, as well as in endobiotic metabolism, including glucose
metabolism, androgen metabolism, vitamin metabolism and bone
mineral homeostasis (18). PXR, once
activated, even participates in the immune response and
inflammation. Thus, broad biological and physiological implications
of PXR activation may underlie its unstable expression in PBMCs,
particularly when the study is based on a limited sample size.
Pre-chemotherapy PXR and ABCB1 protein
expression in cancerous tissues
In the tissue samples collected, PXR expression was
mainly located in the nucleus, perinuclear regions and cytoplasm,
while ABCB1 was located in the cytoplasm and on the cell membrane
(Fig. 1C and D). For normal lung
tissues, PXR protein was mainly expressed in the bronchial
epithelium and alveolar macrophages (Fig.
1E), while ABCB1 protein was expressed on alveolar epithelial
cells. Each protein was expressed on the tumor cells in the
cancerous tissues. In the lung cancer patients, the ABCB1 and PXR
proteins were positively expressed in 64.9% (24/37) and 56.8%
(21/37) of tissues, respectively, compared with 41.2% (7/17) and
35.3% (6/17) in the normal lung samples (Table II). Increased ABCB1 and MDR1 protein
expression was also observed in the lung cancer tissues, although
its significance could not be confirmed yet, since the results were
not statistically significant. Significant correlations between PXR
and ABCB1 was observed in normal lung tissues and tumor specimens.
Unlike in squamous carcinoma, strong positive PXR expression could
be observed in the adenocarcinoma, with a significant association
with differential classification (Table
III). By contrast, ABCB1 expression in cancerous tissues was
not associated with cancer classification and differentiation.
 | Table II.Comparison of ABCB1 and PXR protein
expression in non-small cell cancer tissue samples and non-neoplasm
samples. |
Table II.
Comparison of ABCB1 and PXR protein
expression in non-small cell cancer tissue samples and non-neoplasm
samples.
|
| Cancer tissues | Non-neoplasm
samples |
|---|
|
|
|
|
|---|
| Protein |
ABCB1+ |
ABCB1− | Sum | r | P-value |
ABCB1+ |
ABCB1− | Sum | r | P-value |
|---|
| PXR+,
n | 17 | 4 | 21 (56.8) | 0.38 | 0.02 | 5 | 1 | 6
(35.3) | 0.63 | 0.03 |
| PXR−,
n | 7 | 9 | 16 (43.2) |
|
| 2 | 9 | 11 (64.7) |
|
| Sum, n (%) | 24 (64.9) | 13 (35.1) | 37
(100.0) |
|
| 7 (41.2) | 10 (58.8) | 17
(100.0) |
|
 | Table III.Analysis of PXR protein expression
associated with differentiation in non-small cell lung cancer
patients. |
Table III.
Analysis of PXR protein expression
associated with differentiation in non-small cell lung cancer
patients.
| Cancer type | Cases |
PXR+ |
ABCB1+ |
|---|
| SCC, n |
|
|
|
| High
differentiation | 5 | 1 | 3 |
|
Moderate to low
differentiation | 8 | 3 | 5 |
| AC, n |
|
|
|
| High
differentiation | 6 | 4 | 5 |
|
Moderate to low
differentiation | 18 | 13a | 11 |
Recently, further evidence has been provided in
support of the theory concerning PXR translocation in the
biological process, although conflicting views persist (19). In the 3 adenocarcinoma cases (from
moderate to low differentiation) in the present study, PXR protein
expression was located in the nucleus and perinuclear regions; in
the remaining cases, expression was located in the cell neoplasm
(Fig. 1F). This phenomenon indicates
that the incidence of the nuclear translocation of PXR protein may
be associated with cancer classification and differentiation in
NSCLC.
Correlations between PXR and ABCB1
protein expression in PBMCs and tissue samples
All the lung cancer patients underwent a surgical
resection prior to chemotherapy. Logically, this makes it
impossible to study changes in MDR1 and PXR expression triggered by
chemotherapeutic agents. This poses a challenge for clinicians to
investigate dynamic drug resistance in the middle of a therapeutic
scheme. On the other hand, due to currently available techniques,
the easy access to peripheral blood provides a solution. The
present study first examined how the protein expression of PXR in
PBMCs and tissue specimens was associated with that of ABCB1 prior
to chemotherapy, as aforementioned. Following this, possible
correlations between each prospective biomarker in the PBMCs and
the counterpart tissue samples were also scrutinized (Table IV). However, no significant
correlations were observed.
 | Table IV.Correlation analysis of ABCB1 and PXR
in PBMCs and tissue samples. |
Table IV.
Correlation analysis of ABCB1 and PXR
in PBMCs and tissue samples.
| Correlation
between | r | P-value |
|---|
| ABCB1 in PBMCs and
tissues (N) | 0.765 | 1.000 |
| ABCB1 in PBMCs and
tissues (C) | 0.633 | 0.077 |
| PXR in PBMCs and
tissues (N) | 0.859 | 1.000 |
| PXR in PBMCs and
tissues (C) | 0.537 | 0.057 |
Post-chemotherapy PXR and ABCB1
expression and tumor recurrence
Upregulation of PXR and ABCB1 expression could be
observed in the PBMCs of the lung cancer patients who had been
administered chemotherapeutic treatment following the resection.
Subsequent to 1 cycle of first-line chemotherapy, the positive PXR
expression rate rose as high as 100%. Post-treatment ABCB1
expression was positive in 31 cases (83.8%) in comparison with 22
cases (59.5%) prior to treatment. Tumor recurrence due to drug
resistance signifies a setback for first-line chemotherapy
treatment in cases where the recurrence occurs within 3–6 months of
chemotherapy. In the present study, follow-up visits found a
recurrence time of <6 months in 5 of the adenocarcinoma cases
with moderate to poor differentiation. For 3 of these cases, lab
results denoted uniquely nuclear expression of PXR protein. In
addition, all 5 cases had previous experience of pre- and
post-chemotherapy ABCB1 and PXR co-expression in the PBMCs.
Although the underlying mechanisms of PXR
translocation remain undetermined, the results obtained indicate
that for the prediction of drug resistance in chemotherapy, nuclear
PXR expression may be an ideal indicator, and ABCB1 and PXR
co-expression may be better than a single biomarker in PBMCs of
NSCLC, in terms of sensitivity.
Discussion
The objective of the present study was to find a
useful technical method, based on biomarker expression detection in
PBMCs, for monitoring drug resistance in lung cancer chemotherapy.
Previous studies have reported the correlations between PXR and ABC
superfamily members in PBMCs and normal tissues in organs such as
the liver and intestine. The present study is the first to
investigate the correlations between PXR and ABCB1 in lung cancer
cases, and to probe into the relevance of these possible biomarkers
to tumor recurrence. It was found that the co-expression of ABCB1
and PXR protein in the PBMCs, as well as nuclear PXR expression,
indicated the high incidence of tumor recurrence in NSCLC.
Additionally, a liquid-based cytology technique, combined with IHC,
was used to study protein expression in the peripheral blood
cells.
Chemotherapy is a well-established treatment for
lung cancer. However, while the solution is being distributed and
metabolized in tumor cells, decreased intracellular drug
concentration subtracts from the therapeutic effect. MDR is
partially responsible for this predicament. Multi-biological
processes and bundles of genes (ABCB1, ABCC1, lung resistance
protein and excision repair cross-complementation group 1) are
involved in chemotherapeutic drug resistance. Elevated expression
of P-glycoprotein, encoded by the ABCB1 gene, has been found in
lung, breast, colon and prostate cancers. The ABCB1 expression
level in PBMCs could rise rapidly within 24 h post-administration
(20) and ABCB1 mRNA expression tends
to increase ~7.5-fold, induced by paclitaxel in primary NSCLC cell
lines, such as the A549 cell line (20,21).
Although these findings touch upon possible
functions of ABCB1 in lung cancer chemotherapy drug resistance, in
clinical practice, blockage or reversal of MDR-associated genes is
rarely achieved (12,22). Recent findings have revealed that
certain NRs, i.e., those from the ligand-dependent NR (androgen
receptor and glucocorticoid receptor) and orphan receptor
(peroxisome proliferator-activated receptor, PXR and CAR)
subclasses, give rise to the promoter region activation of target
genes through binding to specific response elements mediated by a
conserved DNA-binding domain (DBD). This provides fresh insights
into the transcriptional regulation of MDR. Several previous
studies, based on findings in cancer cell lines and solid tumors,
showed that PXR can act as a modulator to stimulate associated drug
resistance markers; ABCB1 and anion transporter polypeptide 1A2
have possible roles in tumor proliferation and apoptosis (16,17,23–25).
Certain studies have indicated that PXR functions as a pivotal
regulator of small molecule tyrosine kinase inhibitors, which
mediate ABC-transporter protein induction in the process of drug
resistance (26). Previous studies
have found that various breast cancer cell lines (such as MCF-7)
that are resistant to chemotherapeutic agents enhance PXR
expression (25). Certain researchers
believe that PXR expression is low or simply non-existent in the
lung, stomach or pancreas (27).
However, in the course of the present study, PXR expression was
noted in the bronchial epithelium and alveolar macrophages in
immunostained normal lung tissues. Furthermore, expression in the
adenocarcinoma samples was prevalent, which was indicative of
selective and changeable expression patterns of PXR in the process
of tumorigenesis. The upregulation of PXR in adenocarcinoma,
suggestive of drug resistance in clinical therapy, also pointed to
the role of PXR in the mechanism of drug resistance.
Although peripheral blood is most accessible in
clinical examination, gene expression in PBMCs is not the same as
that in tissues. To judge whether PBMCs can be used as a suitable
surrogate for the measurement of PXR and ABCB1 protein expression,
parallel investigations were conducted into the expression in PBMCs
and lung cancer tissues. Considering the multiple cell types in
PBMCs, the ThinPrep cytological test was used to preserve the
PBMCs, which were used as cytospin-processed, liquid-based smears
to avoid expression heterogeneity that could be created by
quantitative polymerase chain reaction (qPCR). Prior to the study,
this procedure had only been applied in cervical, hydrothorax and
ascites cytology (28). To the best
of our knowledge, the present study is the first practical use of
the procedure in peripheral blood protein expression detection,
combined with immunohistochemical technology. It has been shown
that the procedure can leave PBMC surface antigens intact. As a
transcriptional factor, PXR is correlated with the ABCB1 gene in
the majority of cases. However, the present study uncovered
unstable PXR expression in PBMCs themselves, and came to the
conclusion that PXR expression in peripheral blood is not reliable
as a drug resistance predictor. In the PBMCs and tissue samples,
evidence of correlations between ABCB1 and PXR was clear, whereas
in the PBMCs of normal controls, there was no significant
correlation. We hypothesize that the diverse biological functions
of PBMCs in vivo may, to a certain extent, contribute to the
discrepancies in PXR expression, as indicated by research findings
in vitro. We suggest that the co-expression of ABCB1 and PXR
in PBMCs and tissue samples, instead of the expression of either as
a single marker, is more sensitive and therefore of diagnostic
value to drug resistance.
A larger sample size is required to confirm this, as
the supposition is only based on initial studies. At present, the
role of PXR in inducing tumor cell proliferation, apoptosis and
drug resistance remains controversial. According to the associated
literature, PXR activation, namely higher nuclear expression of
PXR, has been indicated to have an impact upon tumor prognosis,
although opposing views exist (14,29). The
present results revealed that in the majority of cases of NSCLC,
PXR expression is located in the cytoplasm and perinuclear regions,
while nucleus expression is only present in adenocarcinoma cases
with moderate to low differentiation. The mechanism of this viable
expression patterns deserves further study. Based on the results
available, we suggest that PXR expression translocation is
associated with its biological function in drug resistance.
In summary, the present study demonstrated that the
ABCB1 and PXR proteins are expressed in PBMCs of NSCLC patients and
normal controls. This is a prerequisite factor for discussions on
peripheral blood application as a surrogate material for drug
resistance prediction. Furthermore, to avoid heterogeneity of the
cell population in PBMCs and cancerous tissues, immunohistochemical
techniques were employed to evaluate protein expression instead of
qPCR. For the first time in a pathobiological examination, ThinPrep
liquid-based smears were introduced for PBMC immunostaining.
Unexpectedly, PXR expression in cancerous tissues appeared
ubiquitous compared with the selective distribution in the normal
lung tissues.
As a result of these findings, we hypothesize that
the co-expression of PXR and ABCB1 in PBMCs is an ideal indicator
for tumor recurrence, and that despite the initial results which
require further examination, PXR translocation with tumor
classification and differentiation is indicative of a specific role
in drug resistance.
Acknowledgements
The present study was supported by grants from the
Science and Development Foundation of Qingdao, Shandong, China (no.
11-2-Technology 3-2-(3)-nsh).
Glossary
Abbreviations
Abbreviations:
|
PXR
|
pregnane X receptor
|
|
ABCB1
|
ATP-binding cassette sub-family B
member 1
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
NSCLC
|
non-small cell lung cancer
|
|
ABCC1
|
ATP-binding cassette subfamily C
member 1
|
|
NRs
|
nuclear receptor families
|
|
CAR
|
constitutive androstane receptor
|
|
MDR
|
multidrug resistance
|
References
|
1
|
Nishio K, Nakamura T, Koh Y, Suzuki T,
Fukumoto H and Saijo N: Drug resistance in lung cancer. Curr Opin
Oncol. 11:109–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikuta K, Takemura K, Sasaki K, Kihara M,
Nishimura M, Ueda N, Naito S, Lee E, Shimizu E and Yamauchi A:
Expression of multidrug resistance proteins and accumulation of
cisplatin in human non-small cell lung cancer cells. Biol Pharm
Bull. 28:707–712. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triller N, Korosec P, Kern I, Kosnik M and
Debeljak A: Multidrug resistance in small cell lung cancer:
Expression of P-glycoprotein, multidrug resistance protein 1 and
lung resistance protein in chemo-naive patients and in relapsed
disease. Lung Cancer. 54:235–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young LC, Campling BG, Voskoglou-Nomikos
T, Cole SP, Deeley RG and Gerlach JH: Expression of multidrug
resistance protein-related genes in lung cancer: Correlation with
drug response. Clin Cancer Res. 5:673–680. 1999.PubMed/NCBI
|
|
6
|
Lario Paredes A, Blanco García C, Elizondo
Echenique M and Lobo C: Expression of proteins associated with
multidrug resistance and resistance to chemotherapy in lung cancer.
Arch Bronconeumol. 43:479–484. 2007.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galimberti S, Marchetti A, Buttitta F,
Carnicelli V, Pellegrini S, Bevilacqua G and Petrini M: Multidrug
resistance related genes and p53 expression in human non small cell
lung cancer. Anticancer Res. 18:2973–2976. 1998.PubMed/NCBI
|
|
8
|
Pan JH, Han JX, Wu JM, Sheng LJ, Huang HN
and Yu QZ: MDR1 single nucleotide polymorphisms predict response to
vinorelbine-based chemotherapy in patients with non-small cell lung
cancer. Respiration. 75:380–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Tang Y, Guo C, Wang J, Boral D and
Nie D: Nuclear receptors in the multidrug resistance through the
regulation of drug-metabolizing enzymes and transporters.
Biochemical Pharmacology. 83:1112–1126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harmsen S, Meijerman I, Beijnen JH and
Schellens JH: The role of nuclear receptors in pharmacokinetic
drug-drug interactions in oncology. Cancer Treat Rev. 33:369–380.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Omiecinski CJ, Vanden Heuvel JP, Perdew GH
and Peters JM: Xenobiotic metabolism, disposition and regulation by
receptors: From biochemical phenomenon to predictors of major
toxicities. Toxicol Sci. 120(Suppl 1): S49–S75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geick A, Eichelbaum M and Burk O: Nuclear
receptor response elements mediate induction of intestinal MDR1 by
rifampin. J Biol Chem. 276:14581–14587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernandez-Yanez M, Heymach JV and Zurita
AJ: Circulating biomarkers in advanced renal cell carcinoma:
Clinical applications. Curr Oncol Rep. 14:221–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pondugula SR and Mani S: Pregnane
xenobiotic receptor in cancer pathogenesis and therapeutic
response. Cancer Lett. 328:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohr S and Liew CC: The peripheral-blood
transcriptome: New insights into disease and risk assessment.
Trends Mol Med. 13:422–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ban JJ, Jung KH, Chu K, Lee ST, Jeon D,
Park KI, Moon HJ, Kim H, Kim S, Lee SK and Roh JK: Profiles of
multidrug resistance protein-1 in the peripheral blood mononuclear
cells of patients with refractory epilepsy. PLoS One. 7:e369852012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valente RC, Capella LS, Nascimento CR,
Braga F, Echevarria-Lima J, Lopes AG and Capella MA: ABCB1
(P-glycoprotein) but not ABCC1 (MRP1) is downregulated in
peripheral blood mononuclear cells of spontaneously hypertensive
rats. Pflugers Arch. 456:359–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helsley RN, Sui Y, Ai N, Park SH, Welsh WJ
and Zhou C: Pregnane X eeceptor mediates dyslipidemia induced by
the HIV protease inhibitor amprenavir in mice. Mol Pharmacol.
83:1190–1199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Godoy P, Hewitt NJ, Albrecht U, Andersen
ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger
J, et al: Recent advances in 2D and 3D in vitro systems using
primary hepatocytes, alternative hepatocyte sources and
non-parenchymal liver cells and their use in investigating
mechanisms of hepatotoxicity, cell signaling and ADME. Arch
Toxicol. 87:1315–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Melguizo C, Prados J, Luque R, Ortiz R,
Rama AR, Caba O, Rodríguez-Serrano F, Álvarez PJ and Aránega A:
Modulation of multidrug resistance gene expression in peripheral
blood mononuclear cells of lung cancer patients and evaluation of
their clinical significance. Cancer Chemother Pharmacol.
71:537–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melguizo C, Prados J, Luque R, Ortiz R,
Caba O, Alvarez PJ, Gonzalez B and Aranega A: Modulation of MDR1
and MRP3 gene expression in lung cancer cells after paclitaxel and
carboplatin exposure. Int J Mol Sci. 13:16624–16635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Synold TW, Dussalt I and Forman BM: The
orphan nuclear SXR coordinately regulates drug metabolism and
efflux. Nat Med. 7:584–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prados J, Melguizo C, Ortiz R, Perazzoli
G, Cabeza L, Alvarez PJ, Rodriguez-Serrano F and Aranega A: Colon
cancer therapy: Recent developments in nanomedicine to improve the
efficacy of conventional chemotherapeutic drugs. Anticancer Agents
Med Chem. 13:1204–1216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prados J, Melguizo C, Roldan H, Alvarez
PJ, Ortiz R, Arias JL and Aranega A: RNA interference in the
treatment of colon cancer. BioDrugs. 27:317–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clares B, Biedma-Ortiz RA, Sáez-Fernández
E, Prados JC, Melguizo C, Cabeza L, Ortiz R and Arias JL:
Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for
combined hyperthermia and chemotherapy against colon cancer. Eur J
Pharm Biopharm. 85:329–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harmsen S, Meijerman I, Maas-Bakker RF,
Beijnen JH and Schellens JH: PXR-mediated P-glycoprotein induction
by small molecule tyrosine kinase inhibitors. Eur J Pharm Sci.
48:644–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao E, Ji M, Wu J, Ma R, Zhang X, He Y,
Zha Q, Song X, Zhu LW and Tang J: Expression of the PXR gene in
various types of cancer and drug resistance. Oncol Lett.
5:1093–1100. 2013.PubMed/NCBI
|
|
28
|
Hoda RS: Non-gynecologic cytology on
liquid-based preparations: A morphologic review of facts and
artifacts. Diagn Cytopathol. 35:621–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martín-Banderas L, Sáez-Fernández E,
Holgado MÁ, Durán-Lobato MM, Prados JC, Melguizo C and Arias JL:
Biocompatible gemcitabine-based nanomedicine engineered by flow
focusing for efficient antitumor activity. Int J Pharm.
443:103–109. 2013. View Article : Google Scholar : PubMed/NCBI
|