Introduction
Oral squamous cell carcinoma (OSCC) accounts for ~3%
of all malignancies worldwide (1).
Despite this low frequency of occurrence, the 5-year overall
survival rate of patients with OSCC has not exceeded 55% over the
previous decade, due to the local aggressiveness and high
recurrence rate of the disease (2).
Advanced OSCC remains refractory and results in mortality in
>50% of cases (1).
Complete locoregional control is crucial in the
treatment of OSCC, as distant metastases are rarely identified at
the initial presentation (3).
Therefore, multimodal treatment has typically been implemented for
advanced OSCC in order to control locoregional disease, generally
consisting of radical surgery followed by radiotherapy (4). The issue with this combined therapeutic
approach is the high recurrence rate at primary or regional sites
within the first 2 years subsequent to treatment (5). As a result, 5-year survival rates have
been low in patients treated with this therapy (5).
Pre-operative chemoradiotherapy has become an
established component of the clinical management of locoregionally
advanced operable OSCC (6–10). Kirita et al has previously
reported that pre-operative cisplatin (CDDP)-based intravenous
chemotherapy and concurrent radiotherapy (total dose, 40 Gy)
resulted in a clinical tumor response of 92.8% and a good
prognosis, with a 79.3% 5-year overall survival rate, in patients
with resectable advanced OSCC (10).
Several studies have also demonstrated an improved 5-year survival
rate subsequent to using this treatment in patients with advanced
OSCC (7–9). Although the platinum-based
chemotherapeutic regimens used in these protocols significantly
improve local tumor control, adverse events, including nausea,
vomiting, renal damage, anorexia and hematological toxicity, are
severe issues (10).
S-1 is an oral fluoropyrimidine preparation that
consists of tegafur, the dihydropyrimidine dehydrogenase inhibitor
5-chloro-2,4-dihydroxypyridine and potassium oxonate, which
inhibits orotate phosphoribosyl transferase in the gastrointestinal
tract, thereby reducing the gastrointestinal toxicity of
5-fluorouracil (11). A pre-clinical
study using human oral cancer xenograft models has demonstrated
improved responses from the combination of S-1 and fractionated
radiotherapy compared with either treatment alone (12). Furthermore, Harada et al
reported the feasibility and efficacy of S-1 chemotherapy,
performed concomitantly with radiotherapy at a dose of 40 Gy, as a
pre-operative treatment protocol for advanced OSCC in a phase I
trial (13). However, no consensus
has been reached concerning the optimal treatment combination, dose
or timing. In the present study, retrospective clinicopathological
evaluation of pre-operative chemotherapy with S-1 and concurrent
radiotherapy at a total dose of 30 Gy was performed in
locoregionally advanced operable OSCC.
Materials and methods
Patients and staging
In total, 81 patients with advanced resectable
primary OSCC that were treated at the Department of Oral and
Maxillofacial Surgery at Kyushu University Hospital (Fukuoka,
Japan) between January 2004 and December 2010 were evaluated in the
present study. All patients with advanced OSCC in this period
underwent pre-operative chemoradiotherapy, with the exception of
patients that could not undergo S-1 as a chemotherapy regimen due
to serious systemic disease or extreme old age, who were excluded
from the present study. The average patient age was 60.7±12.9 years
(range, 22–81). In total, 65 patients were male and 16 were female.
Informed consent was obtained from all patients for all aspects of
the pre-operative chemoradiotherapy and radical surgery prior to
the initiation of any procedure or treatment and the study was
approved by the ethics committee of Kyushu University Hospital. All
patients demonstrated a performance status of <2, according to
the National Cancer Institute common toxicity criteria, version
4.0. (14), and possessed adequate
hematological, renal and hepatic function for receiving the
treatment regimen with S-1.
Patients were tattooed in at least 4 regions around
the tumor at the time of incisional biopsy, and they underwent
examination by computed tomography (CT), magnetic resonance
imaging, ultrasonography, gastroscopy and thoracic X-ray. Tumor
scintigraphy or F-18 fluorodeoxyglucose positron emission
tomography was performed for the detection of distant metastasis.
Tumor stage was classified according to the tumor-node-metastasis
classification of the International Union Against Cancer (15). In the present study, endophytic tumors
with a maximum size of >30 mm were classified as advanced OSCC,
even if cervical lymph node metastasis was not clinically
identified. Lymph nodes with rim enhancement or heterogeneous
enhancement on CT examination were considered to demonstrate
metastasis, regardless of the length of the short axis diameter.
Lymph nodes measuring ≥10 mm in the short axis diameter were also
considered to demonstrate metastasis, as reported in previous
studies (16,17). Additionally, lymph nodes with
peripheral vascularity, aberrant multi-focal vascularity or
non-vascularity on power Doppler ultrasonography were considered to
demonstrate metastasis (16,17). Tumor histological grades were defined
according to the WHO classification (18). The mode of tumor invasion was
determined by hematoxylin-eosin staining of the specimens according
to the criteria reported by Yamamoto et al (19), as follows: Grade 1, well-defined
borderline; grade 2, cords, less-marked borderline; grade 3, groups
of cells, no distinct borderline; grade 4, diffuse invasion; grade
4C, cord-like type; and grade 4D, widespread type. The patient and
tumor characteristics are reported in Table I.
 | Table I.Characteristics of 81 patients with
locally advanced oral squamous cell carcinoma. |
Table I.
Characteristics of 81 patients with
locally advanced oral squamous cell carcinoma.
|
Characteristics | Total, n (%) |
|---|
| Gender |
|
|
Male | 65 (80.2) |
|
Female | 16 (19.8) |
| Age |
|
| ≥65
years | 33 (40.7) |
| <65
years | 48 (59.3) |
| Primary site |
|
|
Tongue | 41 (50.6) |
|
Gingiva | 29 (35.8) |
| Oral
floor | 9
(11.1) |
| Buccal
mucosa | 2 (2.5) |
| Clinical stage |
|
| II | 29 (35.8) |
|
III | 12 (14.8) |
| IV | 40 (49.4) |
| Histological
grade |
|
| Grade
1 | 45 (54.2) |
| Grade
2 | 36 (45.8) |
| Mode of
invasion |
|
|
1/2/3 | 65 (80.2) |
|
4C/4D | 16 (19.8) |
| Pre-operative
treatment |
|
|
Completion | 69 (85.2) |
|
Cessation | 12 (14.8) |
| Local
recurrence |
|
|
Yes | 6 (7.4) |
| No | 75 (92.6) |
| Neck
recurrence |
|
|
Yes | 2 (2.5) |
| No | 79 (97.5) |
Pre-operative chemoradiotherapy
regimen
The patients were prepared for radiotherapy by
undergoing planning CT. The patients received external beam
irradiation to the primary tumor and metastatic lymph nodes in
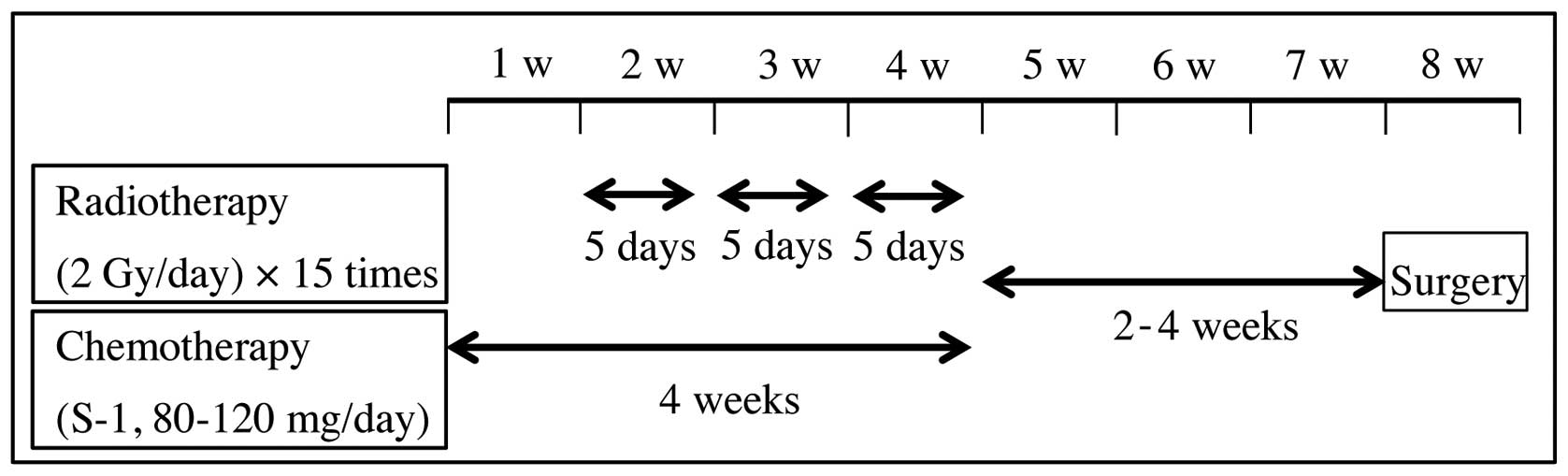
daily fractions of 2 Gy, 5 times weekly, for 3 weeks. Oral
administration of S-1 (TS-1; Taiho Pharmaceutical Co., Ltd., Tokyo,
Japan), twice daily, was commenced 1 week prior to radiotherapy and
was continued throughout the radiotherapy period. Standard
individual doses of S-1 were calculated according to the body
surface area (BSA), as follows: BSA <1.25 m2, 80 mg;
BSA ≥1.25 and <1.5 m2, 100 mg; BSA ≥1.5
m2, 120 mg. However, in patients with reduced renal
function, demonstrated by decreased creatinine clearance values
(normal range, ≥80 ml/min), S-1 was administered at a lower dose,
generally one step lower than the standard dose. Adverse events
were evaluated according to the National Cancer Institute common
toxicity criteria, version 4.0 (14).
The pre-operative chemoradiotherapy regimen is summarized in
Fig. 1.
Surgery
Radical surgery was performed 2–6 weeks (mean,
26.4±5.82 days) subsequent to the end of the pre-operative
chemoradiotherapy. The primary tumors were resected with safety
margins of ≥10 mm from the tattoos around the tumor, regardless of
the clinical response. Neck dissection was required for the
treatment of patients with clinically involved lymph nodes and for
patients that required an extraoral approach or the transfer of
vascularized flaps. Immediate surgical reconstruction was
undertaken using local flaps or vascularized free flaps.
Clinicopathological evaluation of
treatment
The clinical response to pre-operative
chemoradiotherapy was determined at 2–3 weeks subsequent to the end
of chemotherapy administration, according to the response
evaluation criteria in solid tumors guidelines, version 1.1
(20). Complete response (CR) was
defined as the disappearance of all target lesions, with reduction
in the short axis of any pathological lymph nodes to <10 mm.
Partial response (PR) was defined as a minimum decrease of 30% in
the sum of the diameters of the target lesions, using the baseline
sum of the diameters as a reference. Progressive disease (PD) was
defined as a minimum increase of 20% in the sum of the diameters of
the target lesions, using the smallest sum recorded during the
study as a reference, and with an absolute increase of ≥5 mm in the
sum of the diameters. Stable disease (SD) was indicated by
insufficient shrinkage to qualify for PR and insufficient increase
to qualify for PD, using the smallest sum diameter recorded during
the study as a reference. The same diagnostic modalities as those
initially applied were used to evaluate the clinical response.
The classification of therapeutic efficacy
established by Shimosato et al (21) was used to evaluate the
histopathological response of primary site tumors, as follows:
Grade 0, no noticeable change; grade I, minimal cellular changes,
but the majority of tumor cells appear viable; grade IIa, despite
the presence of cellular changes and partial destruction of the
tumors, the tumor remains readily recognizable and numerous tumor
cells appear viable; grade IIb, tumor destruction is extensive, but
viable cell nests are present in small regions of the tumor (up to
one-quarter of the tumor mass, excluding areas of coagulative
necrosis); grade III, only a small number of scattered, markedly
altered, and presumably non-viable tumor cells are present, singly
or in small clusters, and a small number or no viable cells are
observed; and grade IV, no tumor cells remaining in any section.
The slicing of the resected specimens was performed using a
step-section method at intervals of 5 mm.
Statistical analysis
All statistical analyses in the present study were
performed using JMP software version 8 (SAS Institute Japan, Ltd.,
Tokyo, Japan). Associations between the incidence of locoregional
recurrence and clinical or histopathological response were assessed
using Fisher's exact test. The survival time was measured from the
first day of treatment until mortality or the last patient contact.
Survival rates were calculated using the Kaplan-Meier method, and
the P-value was calculated using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The primary site was the tongue in 41 patients
(50.6%), the gingiva in 29 patients (35.4%) and the oral floor in 9
patients (11.1%). Of the OSCC patients, 29 patients demonstrated
stage II disease (35.8%), 12 demonstrated stage III disease
(14.8%), and 40 demonstrated stage IV disease (49.4%). Out of the
81 patients that received pre-operative chemoradiotherapy, 69
patients (85.2%) completed treatment according to the planned
schedule. In the remaining 12 patients (14.8%), treatment was
stopped to prevent the side-effects from becoming severe, as the
satisfactory response of the primary tumor was obtained. The local
and neck recurrence rates were 7.4 and 2.5%, respectively.
Reconstruction was performed using a microvascular flap in 73
patients, a cervical island flap in 4 patients, a split-thickness
skin graft in 3 patients and primary closure in 1 patient (data not
shown).
Toxicity
Patients that experienced toxicities during
treatment or within 2 weeks subsequent to chemoradiotherapy are
reported in Table II. With regard to
hematological toxicity, leukocytopenia of grades 1 and 2 developed
in 28 patients (34.6%), and 3 patients experienced grade 3
toxicity. Neutropenia of grades 1 and 2 was observed in 23 patients
(28.4%) and grade 3 was observed in 3 patients. Anemia or
thrombocytopenia of grades 1 and 2 occurred in 42 patients (51.9%)
and 18 patients (22.2%), respectively. No patients in the present
study experienced grade 4 hematological toxicities. With regard to
non-hematological toxicities, grade 1–3 oropharyngeal mucositis was
observed in all patients, of which 15 patients (18.5%) experienced
grade 3 mucositis. Dry mouth was the second most common adverse
event and occurred in 48 patients (59.3%). Dermatitis developed in
9 patients (11.1%), nausea in 9 patients (11.1%) and diarrhea in 4
patients (4.9%). The complications or late adverse events,
including radiation osteomyelitis, that are associated with
pre-operative chemoradiotherapy were not identified during radical
surgery or post-operatively.
 | Table II.Incidence of adverse events in 81
patients with locally advanced oral squamous cell carcinoma. |
Table II.
Incidence of adverse events in 81
patients with locally advanced oral squamous cell carcinoma.
|
| Grade, n |
|---|
|
|
|
|---|
| Adverse event | 1 | 2 | 3 |
|---|
| Hematological
toxicity |
|
|
|
|
Leukocytopenia | 12 | 14 | 3 |
|
Neutropenia | 8 | 15 | 3 |
|
Anemia | 38 | 4 | 0 |
|
Thronbocytopenia | 16 | 2 | 0 |
| Non-hematological
toxicity |
|
|
|
|
Dermatitis | 9 | 0 | 0 |
|
Oropharyngeal mucositis | 8 | 58 | 15 |
|
Nausea | 9 | 0 | 0 |
|
Diarrhea | 4 | 0 | 0 |
| Dry
mouth | 48 | 0 | 0 |
Efficacy
The clinical responses of the primary tumors are
reported in Table III. CR was
achieved by 6 patients (7.4%), and partial response was observed in
51 patients (63.0%). The clinical response rate, calculated as the
sum of the patients that achieved complete and partial response,
was 84.6% for T2 tumors, 66.7% for T3 tumors and 55.6% for T4
tumors. The histopathological effect achieved following
pre-operative chemoradiotherapy was grade IV in 19 patients, grade
III in 3 patients, grade IIb in 39 patients, grade IIa in 14
patients and grade I in 6 patients. The histopathological response
rate, defined as grades IIb-IV, was 75.3% (Table IV). The association between the
clinical and histopathological responses was further examined. The
results revealed that the patients with good clinical response
demonstrated good histopathological response, indicating that the
clinical response correlated positively with histopathological
response (Table V).
 | Table III.Clinical response of primary tumors
subsequent to pre-operative chemoradiotherapy in 81 patients with
locally advanced oral squamous cell carcinoma. |
Table III.
Clinical response of primary tumors
subsequent to pre-operative chemoradiotherapy in 81 patients with
locally advanced oral squamous cell carcinoma.
|
| Clinical response,
n |
|
|---|
|
|
|
|
|---|
| T
classification | CR | PR | SD | PD | Response rate,
% |
|---|
| T2 | 5 | 28 | 6 | 0 | 84.6 |
| T3 | 0 | 4 | 2 | 0 | 66.7 |
| T4 | 1 | 19 | 12 | 4 | 55.6 |
| Total | 6 | 51 | 20 | 4 | 70.4 |
 | Table IV.Histopathological evaluation of the
response rate of primary tumors in 81 patients with locally
advanced oral squamous cell carcinoma subsequent to pre-operative
chemoradiotherapy. |
Table IV.
Histopathological evaluation of the
response rate of primary tumors in 81 patients with locally
advanced oral squamous cell carcinoma subsequent to pre-operative
chemoradiotherapy.
|
| Histopathological
response, n |
|
|---|
|
|
|
|
|---|
| T
classification | I | IIa | IIb | III | IV | Response rate,
% |
|---|
| T2 | 1 | 3 | 21 | 2 | 12 | 89.7 |
| T3 | 0 | 3 | 2 | 0 | 1 | 50.0 |
| T4 | 5 | 8 | 16 | 1 | 6 | 63.9 |
| Total | 6 | 14 | 39 | 3 | 19 | 75.3 |
 | Table V.Association between the clinical
response and histopathological response of primary tumors in 81
patients with locally advanced oral squamous cell carcinoma. |
Table V.
Association between the clinical
response and histopathological response of primary tumors in 81
patients with locally advanced oral squamous cell carcinoma.
|
| Histopathological
response, n |
|
|---|
|
|
|
|
|---|
| Clinical
response | I | IIa | IIb | III | IV | Total |
|---|
| PD | 2 | 1 | 1 | 0 | 0 | 4 |
| SD | 4 | 9 | 7 | 0 | 0 | 20 |
| PR | 0 | 4 | 31 | 2 | 14 | 51 |
| CR | 0 | 0 | 0 | 1 | 5 | 6 |
| Total | 6 | 14 | 39 | 3 | 19 | 81 |
Post-operative chemoradiotherapy was performed in 10
patients that possessed >3 involved lymph nodes, extracapsular
spread or nodal metastases over multiple neck levels. These
patients received external beam irradiation to the cervical region
in daily fractions of 2 Gy, 5 times a week, for 4 weeks (total
dose, 60–70 Gy) in conjunction with the oral administration of S-1.
Post-operatively, local failure developed in 6 patients (7.4%) and
neck failure developed in 2 patients (2.5%). Distant metastases
were observed in 7 patients (8.6%). The median duration of
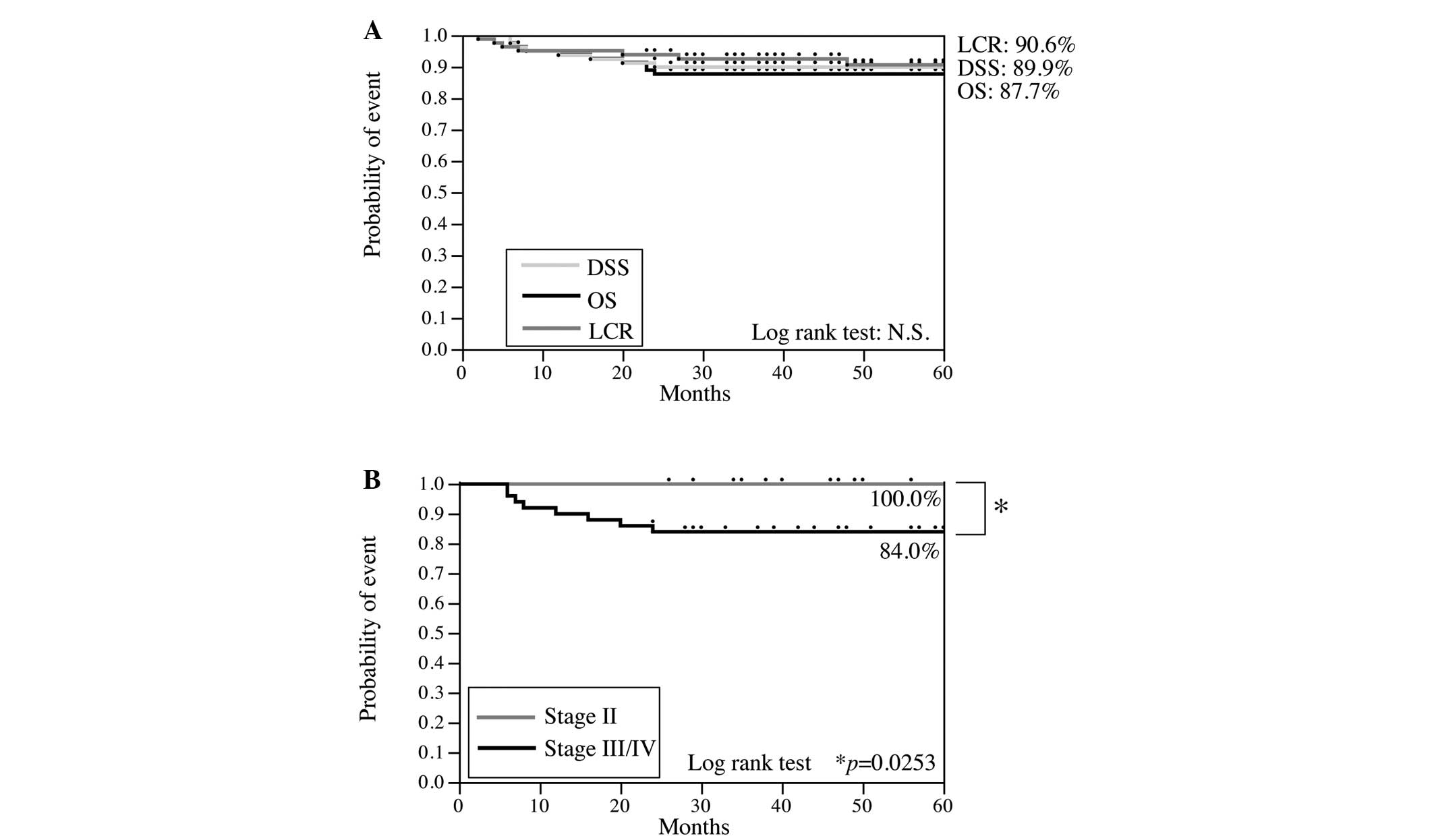
follow-up was 59.0 months (range, 24–108 months). The overall
survival (OS), disease-specific survival (DSS) and locoregional
control (LRC) rates at 5 years were 87.7, 89.9%, and 90.6,
respectively (Fig. 2).
Risk factors for locoregional
recurrence
In order to further improve the outcome of treatment
for patients with advanced OSCC, the risk factors for locoregional
recurrence were examined in the present study (Table VI). The incidence of locoregional
recurrence was positively associated with the progression of the
clinical stage (P<0.05). Furthermore, locoregional recurrence
occurred more frequently in patients that demonstrated a poor
histopathological response (grades I and IIa) compared with
patients that demonstrated a good response (grades IIb-IV)
(P<0.01). No locoregional recurrence was observed in patients
with grade IIb-IV histopathological responses. The residual tumor
in the patients with locoregional recurrence was more frequently
identified in the muscular layers, though no significant difference
was found. However, no significant associations were identified
between the locoregional incidence and age, histological grade,
mode of invasion, waiting period prior to surgery or depth of the
residual tumor.
 | Table VI.Association between locoregional
recurrence and the clinicopathological features of 81 patients with
locally advanced oral squamous cell carcinoma. |
Table VI.
Association between locoregional
recurrence and the clinicopathological features of 81 patients with
locally advanced oral squamous cell carcinoma.
|
| Locoregional
recurrence |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | Yes | No | P-value |
|---|
| Age |
|
|
1.000 |
| ≥65
years | 4 | 44 |
|
| <65
years | 4 | 29 |
|
| Clinical stage |
|
|
0.046 |
| II | 0 | 29 |
|
|
III/IV | 8 | 44 |
|
| Histological
grade |
|
|
0.456 |
| 1 | 3 | 42 |
|
| 2 | 5 | 31 |
|
| Mode of
invasion |
|
|
0.189 |
|
1–3 | 5 | 60 |
|
| 4C or
D | 3 | 13 |
|
| Wait prior to
procedure |
|
|
0.456 |
| ≥28
days | 5 | 31 |
|
| <28
days | 3 | 42 |
|
| Histopathological
tumor response |
|
| <0.001 |
| I or
IIa | 8 | 12 |
|
|
IIb-IV | 0 | 61 |
|
| Depth of residual
tumor |
|
|
0.336 |
|
Superficial | 1 | 14 |
|
|
Deep | 7 | 37 |
|
Discussion
Neoadjuvant induction chemoradiotherapy followed by
radical surgery has become an established treatment for the
clinical management of locally advanced OSCC over the previous 20
years. Several studies have demonstrated that this treatment
results in a higher overall survival rate in patients with OSCC
(6–10,22–24). The
inclusion of pre-operative chemoradiotherapy is credited with this
improvement in the overall survival rate. The beneficial effects of
neoadjuvant induction chemoradiotherapy followed by radical surgery
include downstaging of the primary tumor, an increased
resectability rate and the elimination of micrometastases. However,
protocols have varied widely between institutions. Kirita et
al treated advanced oral cancer with pre-operative cisplatin-
(15 mg/m2, days 1–3) or carboplatin-based intravenous
chemotherapy (70–100 mg/m2 carboplatin, days 1–3; 5
mg/m2 peplomycin or 500 mg, 5-FU, days 4–7) administered
concurrently with radiotherapy at a total dose of 40 Gy (6). Mücke et al also demonstrated the
efficacy of low-dose radiotherapy (total dose, 20 Gy) combined with
concurrent low-dose cisplatin (12.5 mg/m2) for 5 days,
as pre-operative therapy (24).
However, Iguchi et al reported the use of combined
intra-arterial pirarubicin and continuous intravenous
5-fluorouracil (5-FU) with a radiation dose of 40 Gy (25). This study concluded that these
regimens were effective as a pre-operative treatment, with a
notably high response rate and an acceptable incidence of adverse
events. However, it is widely known that CDDP, a radiosensitizing
agent, requires excess hydration and antidotes due to the tendency
of this agent to cause renal dysfunction (26). Furthermore, the intra-arterial
infusion of anticancer drugs is associated with technical
difficulty and is occasionally accompanied by serious
complications, such as permanent neurological deficits. Therefore,
these treatments are feasible only in a limited number of patients
and institutions.
Previous studies have demonstrated that the oral
administration of S-1 with concurrent radiotherapy is a feasible
and effective treatment for patients with advanced OSCC (13,27–29).
However, only a small number of studies have assessed the use of
S-1 with pre-operative chemoradiotherapy. In the present study, the
feasibility and efficacy of pre-operative S-1 chemotherapy
administered concurrently with radiotherapy at a total of 30 Gy was
retrospectively evaluated.
Harada et al previously demonstrated that all
adverse events associated with pre-operative S-1 chemotherapy and
concurrent radiotherapy were tolerable and controllable (27). In the present study, no severe grade 4
hematological, gastrointestinal or skin toxicities were
encountered. Oropharyngeal mucositis was the most common adverse
event, with grades 2 and 3 mucositis occurring in 71.6 and 18.5% of
patients, respectively. The mucositis was transient and tolerable
in all cases. In the study by Harada et al (27), grade 3 mucositis was observed in 84.6%
of the patients that received pre-operative S-1 chemotherapy with
concurrent radiotherapy at a total dose of 40 Gy. This difference
in the incidence of grade 3 mucositis may be due to the difference
in the total radiation dose. In addition, using a lower total
radiation dose in the pre-operative treatment enables the use of a
higher dose of chemoradiotherapy post-operatively, prevents
osteoradionecrosis of the jaw, prevents severe late toxicity and
shortens the tumor-bearing period. It is therefore suggested that
this regimen has more advantages than those observed at a total
dose of >40 Gy.
In the present study, the overall histological
response rate (grades IIb-IV) was 75.3%. Notably, pathological CR
(grade IV) was obtained in 23.5% of the patients. The outcome of
this regimen was an LRC rate of 90.6%, DSS rate of 89.9% and OS
rate of 87.7%. In phase II trials of pre-operative S-1 chemotherapy
combined with radiotherapy at a total dose of 40 Gy for stage
III/IV OSCC, the histopathological response rate of the primary
tumor was 78.4% and the LRC, DSS and OS rates in this study were
91.5, 83.8 and 83.8%, respectively (27). However, a direct comparison between
these previous results and the present data cannot be made due to
including stage II OSCC in the present study. In the patients with
stage III/IV OSCC in the present study, the histopathological
response rate was 63.5% (data not shown), which was decreased
compared with the response rate of previous studies. However, the
OS rate of the patients with stage III/IV OSCC in the present study
was 84.0%, which yielded a similar survival curve to those obtained
in previous studies (6–9,23,24). Miyawaki et al demonstrated that
the administration of S-1 chemoradiotherapy at 30 Gy to treat OSCC
at stages II–IV, which was similar to the present regimen, was
histopathologically effective in 73.7% of patients (30). The DSS rate of the previous study was
88.8%, which is similar to the present results (30). These results indicate that increasing
the total radiation dose may not affect the patient outcome, but it
yields an effective primary tumor response. The consistent patient
outcome may be due to the difference in surgical margins in the
resection between the present and previous studies. In the present
patients, the primary tumors were resected with safety margins ≥10
mm from the tattoos around the tumor, regardless of the clinical
response. Although not stated, in previous studies, the tumors may
be resected at a narrower safety margin in the case of a good
clinical response, which would result in a higher risk of
recurrence.
In order to maximize outcomes, the risk factors for
locoregional recurrence in patients receiving S-1 chemotherapy with
concurrent radiotherapy were also examined. It is notable that all
patients with locoregional recurrence demonstrated a poor
histological tumor response of grade I or IIa. Out of the patients
with stage T4 OSCC, Nomura et al found that the 3-year LRC
rate for grade 0-III responses was 73%, which was decreased
compared with the rate for grade IV pathological responses (93%),
although this difference was not statistically significant
(31). Furthermore, the residual
cancer cells in the patients with locoregional recurrence were more
frequently identified in the muscular layers, but not submucosal
layer. These results indicate that an increase in the surgical
margins at the bottom of the tumor in the patients with little or
no histopathological response is required.
At present, the standard treatment for oral cancer
remains surgery alone, with radiotherapy or concomitant
chemoradiotherapy subsequent to surgery recommended for high-risk
cases (32), as pre-operative
chemotherapy has failed to significantly improve the OS rate in
certain previous randomized controlled trials (33,34). In
addition, Yanamoto et al hypothesized that neoadjuvant
chemotherapy may increase the risk of local recurrence and lead to
poor outcomes in OSCC patients (35).
Overall, promising results were obtained in the present study,
although limited by its retrospective nature, and in other similar
studies (6–9,23,24,27).
However, the efficacy of pre-operative chemoradiotherapy remains
controversial, and a definitive conclusion cannot yet be reached.
Additional controlled studies with a large sample size and
randomized prospective design are required to resolve this clinical
question.
Acknowledgements
This study was supported by a Grant-in-Aid (grant
no. 26463014) from the Japanese Ministry of Education, Culture,
Sports, Science and Technology.
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
PD
|
progressive disease
|
|
SD
|
stable disease
|
|
OS
|
overall survival rate
|
|
DSS
|
disease-specific survival rate
|
|
LRC
|
locoregional control rate
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Funk GF, Karnell LH, Robinson RA, Zhen WK,
Trask DK and Hoffman HT: Presentation, treatment and outcome of
oral cavity cancer: A national cancer data base report. Head Neck.
24:165–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gowen GF and Desuto-Nagy G: The incidence
and sites of distant metastases in head and neck carcinoma. Surg
Gynecol Obstet. 116:603–607. 1963.PubMed/NCBI
|
|
4
|
Fletcher GH and Evers WT: Radiotherapeutic
management of surgical recurrences and postoperative residuals in
tumors of the head and neck. Radiology. 95:185–188. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen EE, Lingen MW and Vokes EE: The
expanding role of systemic therapy in head and neck cancer. J Clin
Oncol. 22:1743–1752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirita T, Ohgi K, Shimooka H, Yamanaka Y,
Tatebayashi S, Yamamoto K, Mishima K and Sugimura M: Preoperative
concurrent chemoradiotherapy plus radical surgery for advanced
squamous cell carcinoma of the oral cavity: An analysis of
long-term results. Oral Oncol. 35:597–606. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freier K, Engel M, Lindel K,
Flechtenmacher C, Mühling J, Hassfeld S and Hofele C: Neoadjuvant
concurrent radiochemotherapy followed by surgery in advanced oral
squamous cell carcinoma (OSCC): A retrospective analysis of 207
patients. Oral Oncol. 44:116–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohr C, Bohndorf W, Carstens J, Härle F,
Hausamen JE, Hirche H, Kimmig H, Kutzner J, Mühling J and Reuther
J: Preoperative radiochemotherapy and radical surgery in comparison
with radical surgery alone. A prospective, multicentric, randomized
DOSAK study of advanced squamous cell carcinoma of the oral cavity
and the oropharynx (a 3-year follow-up). Int J Oral Maxillofac
Surg. 23:140–148. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klug C, Berzaczy D, Voracek M and Millesi
W: Preoperative chemoradiotherapy in the management of oral cancer:
A review. J Craniomaxillofac Surg. 36:75–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirita T, Yamanaka Y, Imai Y, Yamakawa N,
Aoki K, Nakagawa Y, Yagyuu T and Hasegawa M: Preoperative
concurrent chemoradiotherapy for stages II–IV oral squamous cell
carcinoma: A retrospective analysis and the future possibility of
this treatment strategy. Int J Oral Maxillofac Surg. 41:421–428.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirasaka T, Shimamoto Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harada K, Kawaguchi S, Supriatno S, Onoue
T, Yoshida Ha and Sato M: Combined effects of the oral
fluoropyrimidine anticancer agent, S-1 and radiation on human oral
cancer cells. Oral Oncol. 40:713–719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada H and Omura K: Preoperative
concurrent chemotherapy with S-1 and radiotherapy for locally
advanced squamous cell carcinoma of the oral cavity: Phase I trial.
J Exp Clin Cancer Res. 29:332010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events v4.0 (CTCAE). http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed.
March 08–2016
|
|
15
|
Sobin LH and Wittekind C: TNM
classification of malignant tumours (6th). Wiley-Liss, Inc. New
York: 2002.
|
|
16
|
Yuasa K, Kawazu T, Nagata T, Kanda S,
Ohishi M and Shirasuna K: Computed tomography and ultrasonography
of metastatic cervical lymph nodes in oral squamous cell carcinoma.
Dentomaxillofac Radiol. 29:238–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuasa K, Kawazu T, Kunitake N, Uehara S,
Omagari J, Yoshiura K, Nakayama E and Kanda S: Sonography for the
detection of cervical lymph node metastases among patients with
tongue cancer: Criteria for early detection and assessment of
follow-up examination intervals. Am J Neuroradiol. 21:1127–1132.
2000.PubMed/NCBI
|
|
18
|
Gale N, Pilch BZ, Sindransky D, El Naggar
A, Westra W, Califano J, Johnson N and MacDonald DG: Epithelial
precursor lesions. In: World Health Organization Classification of
Tumors. Pathology and Genetics of Head and Neck Tumors. Barnes L,
Eveson JW, Reichart P and Sidransky D: IARC Press. (Lyon). 177–179.
2005.
|
|
19
|
Yamamoto E, Miyakawa A and Kohama G: Mode
of invasion and lymph node metastasis in squamous cell carcinoma of
the oral cavity. Head Neck Surg. 6:938–947. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauera EA, Therasseb P, Bogaertsc J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S and
Mooney M: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimosato Y, Oboshi S and Baba K:
Histological evaluation of effects of radiotherapy and chemotherapy
for carcinomas. Jpn J Clin Oncol. 1:19–35. 1971.
|
|
22
|
Slotman GJ, Doolittle CH and Glicksman AS:
Preoperative combined chemotherapy and radiation therapy plus
radical surgery in advanced head and neck cancer. Five-year results
with impressive complete response rates and high survival. Cancer.
69:2736–2743. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giralt JL, Gonzalez J, del Campo JM,
Maldonado J, Sanz X, Pamias J, Eraso A, Bescos S and Raspall G:
Preoperative induction chemotherapy followed by concurrent
chemoradiotherapy in advanced carcinoma of the oral cavity and
oropharynx. Cancer. 89:939–945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mücke T, Konen M, Wagenpfeil S, Kesting
MR, Wolff KD and Hölzle F: Low-dose preoperative chemoradiation
therapy compared with surgery alone with or without postoperative
radiotherapy in patients with head and neck carcinoma. Ann Surg
Oncol. 18:2739–2747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iguchi H, Kusuki M, Nakamura A, Nishiura
H, Kanazawa A, Takayama M, Sunami K and Yamane H: Concurrent
chemoradiotherapy with pirarubicin and 5-fluorouracil for
resectable oral and maxillary carcinoma. Acta Otolaryngol Suppl.
554:55–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Numico G, Benasso M, Vannozzi MO, Merlano
M, Rosso R, Viale M and Esposito M: Hydration regimen and
hematological toxicity of a cisplatin-based chemotherapy regimen.
Clinical observations and pharmacokinetic analysis. Anticancer Res.
18:1313–1318. 1998.PubMed/NCBI
|
|
27
|
Harada H, Omura K, Tomioka H, Nakayama H,
Hiraki A, Shinohara M, Yoshihama Y and Shintani S: Multicenter
phase II trial of preoperative chemoradiotherapy with S-1 for
locally advanced oral squamous cell carcinoma. Cancer Chemother
Pharmacol. 71:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukuda M, Ishitoya J, Mikami Y, Matsuda
H, Horiuchi C, Taguchi T, Satake K, Kawano T, Takahashi M and
Nishimura G: Analysis of feasibility and toxicity of concurrent
chemoradiotherapy with S-1 for locally advanced squamous cell
carcinoma of the head and neck in elderly and/or cases with
comorbidity. Cancer Chemother Pharmacol. 64:945–952. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukuda M, Kida A, Fujii M, Kono N,
Yoshihara T, Hasegawa Y and Sugita M: Chemotherapy Study Group of
Head and Neck Cancer: Randomized scheduling feasibility study of
S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br
J Cancer. 93:884–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyawaki A, Hijioka H, Ikeda R, Ishida T,
Nozoe E and Nakamura N: Analysis of the outcome of concurrent
neoadjuvant chemoradiotherapy with S-1 compared to super-selective
intra-arterial infusion for oral squamous cell carcinoma. Oncol
Lett. 3:995–1001. 2012.PubMed/NCBI
|
|
31
|
Nomura T, Murakami R, Toya R, Teshima K,
Nakahara A, Hirai T, Hiraki A, Nakayama H, Yoshitake Y and Ota K:
Phase II study of preoperative concurrent chemoradiation therapy
with S-1 in patients with T4 oral squamous cell carcinoma. Int J
Radiat Oncol Biol Phys. 76:1347–1352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bernier J, Domenge C, Ozashin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, et al: Postoperative irradiation with or
without concomitant chemotherapy for locally advanced head and neck
cancer. N Engl J Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. Meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Licitra L, Grandi C, Guzzo M, Mariani L,
Lo Vullo S, Valvo F, Quattrone P, Valagussa P, Bonadonna G,
Molinari R and Cantù G: Primary chemotherapy in resectable oral
cavity squamous cell cancer: A randomized controlled trial. J Clin
Oncol. 21:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yanamoto S, Yamada S, Takahashi H,
Yoshitomi I, Kawasaki G, Ikeda H, Minamizato T, Shiraishi T, Fujita
S and Ikeda T: Clinicopathological risk factors for local
recurrence in oral squamous cell carcinoma. Int J oral Maxillofac
Surg. 41:1195–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|