Introduction
Tumor angiogenesis is the process of novel blood
vessel formation from existing vessels, which is critical for tumor
growth, progression and metastasis (1). Several studies have revealed that tumor
angiogenesis has prognostic value; there is an association between
angiogenesis and increased risk of local regional recurrence,
distant metastasis and decreased survival in patients with head and
neck cancer (2–4). Traditionally, vascular endothelial cell
growth factor (VEGF) staining of tumor tissues has been used to
evaluate angiogenesis (5,6). It has been reported that VEGF is a
prognostic factor for the survival of patients with esophageal
squamous cell carcinoma, and positive expression of VEGF is
associated with a poorer prognosis (5,7,8).
Current advances in functional imaging techniques
have resulted in perfusion computed tomography (CTP), which is an
attractive technique to assess tumor vascularity and indirectly
reflects tumor angiogenesis (9–11). The
development of novel vessels leads to physiological changes, and
tumor angiogenesis is associated with increased perfusion, blood
volume and permeability, which alters contrast enhancement during
CTP. CTP quantifies these physiological changes and identifies the
extent of tumor angiogenesis in the head and neck (12). In theory, the degree of neovascularity
is different between benign and aggressive lesions. Consequently,
CTP aids in the early detection of malignancy, assessment of
responses to therapy and differentiation between residual or
recurrent tumors and fibrous post-therapeutic alterations (11,13–15). As a
clinical tool, CTP has been a promising technique in evaluating the
angiogenesis of head and neck lesions (HNLs) in vivo and in
differentiating malignant from benign lesions (5,13,16,17).
VEGF is an angiogenic molecular marker (7,8), and CTP
provides a quantitative measure of tumor perfusion, which reflects
tumor angiogenesis (9–11). Therefore, CTP findings may be a
predictive indicator of intratumoral VEGF. There have been a few
studies evaluating the use of CTP in the differential diagnosis
between benign and malignant HNLs (13,16,17).
However, to the best of our knowledge, there has been only one
study concerning the correlation of CTP with VEGF in a group of
HNLs, which demonstrated a positive correlation between MTT and
VEGF (17). The aim of the present
study was to prospectively study the correlation of CTP parameters
with VEGF expression, and determine the value of CTP combined with
VEGF in the differential diagnosis between malignant and benign
HNLs.
Materials and methods
Patients
The current prospective study was performed with
approval from the Institutional Review Board affiliated to the Eye
Ear Nose & Throat Hospital, Fudan University (Shanghai, China),
and informed written consent was obtained from each patient prior
to enrolment. In total, 41 patients (23 men and 18 women; age
range, 25–74 years; mean age ± standard deviation, 45.22±13.82
years) with HNLs were included in this study, who underwent
surgical resection for HNLs from October 2008 and March 2010.
Primary tumor sites consisted of the parotid gland (n=20), temporal
bone [n=11, including external auditory canal (n=7), mastoid
process (n=3) and petrous portion (n=1)], parapharyngeal space
(n=7), infratemporal fossa (n=1), jugular foramen (n=1) and
pterygopalatine fossa (n=1). All the histopathological diagnoses
were confirmed by surgical specimens.
CTP imaging
CT scans were performed with a 16-row multi-slice CT
scanner (Somatom 16, Sensation; Siemens Healthcare, Erlangen,
Germany). An unenhanced CT of the neck was performed prior to CTP.
On transverse CT images, the slice revealing the maximal transverse
tumor diameter was selected to measure perfusion. Two adjacent
12-mm sections were selected at the level of the tumor. A total of
50 ml nonionic iodinated contrast agent (300 mg/ml; Ultravist 300;
Bayer Health Care, Whippany, NJ, USA) was injected at 4 ml/sec
using a Optivantage power injector (Cincinnati, OH, USA). At 4 sec
into the injection, a continuous acquisition was initiated using
the following parameters: 80 kV, 100 mA, 2×12 mm sections, with a
temporal resolution of 1 picture/sec for a 40 sec duration.
Following the completion of the perfusion acquisition, dual-phase
imaging (arterial and venous acquisitions) was performed. Fixed
delays of 25–30 sec for the arterial phase and 90 sec for the
venous phase were included. To increase radiation protection, the
volume acquisition was reduced to the area of the lesion in the
arterial phase. The venous phase scanning (routine scanning) was
between the skull base and thoracic inlet. All the lesions were
subdivided into benign (Group A and B) and malignant lesions (Group
C) upon pathological examination (Table
I). Subsequently, two head and neck radiologists (Department of
Radiology, Eye Ear Nose & Throat Hospital, Shanghai, China; 25
years of experience in radiology) blindly reviewed the dual-phase
images and divided the benign lesions into two groups (Group A,
benign hypovascular lesions; Group B, benign hypervascular lesions)
(Table I). Imaging criteria for
benign hypervascular lesions consisted of arterial contrast
enhancement and washout on delayed images. For benign hypovascular
lesions imaging features were defined as delayed enhancement of the
venous phase, which had no obvious enhancement on the arterial
phase (18).
 | Table I.Histopathological diagnosis, TDC types
and CTP parameters in 41 patients with head and neck lesions. |
Table I.
Histopathological diagnosis, TDC types
and CTP parameters in 41 patients with head and neck lesions.
|
|
| TDC types, n | CTP parameters |
|---|
|
|
|
|
|
|---|
| Histopathologic
diagnosis | Total, n | I | II | III | MIP, Hu | BV, ml/100 ml | BF, ml/100
ml/min | MTT, sec | CP, ml/100
ml/min |
|---|
| Benign lesions
(GA+B) | 29 | 14 | 4 | 11 | 73.8±34.1 | 6.7±7.2 | 73.7±51.1 | 9.4±4.1 | 26.6±23.1 |
| Benign
hypovascular lesions (GA) | 16 | 12 | 0 | 4 | 55.3±30.1 | 2.8±2.2 | 39.1±19.9 | 9.9±4.2 | 17.6±11.3 |
|
Pleomorphic
adenoma | 9 | 6 | 0 | 3 | 59.0±12.6 | 3.1±2.5 | 42.5±23.7 | 8.6±3.3 | 20.6±8.9 |
|
Lymphoepithelial
cyst | 2 | 2 | 0 | 0 |
38.8 | 2.1 | 32.7 | 9.1 | 3.9 |
|
Branchial cleft
cyst | 2 | 2 | 0 | 0 |
25.6 | 2.7 | 38.6 | 11.9 | 24.7 |
|
Schwannoma | 1 | 1 | 0 | 0 |
28.5 | 1.0 | 38.0 | 8.0 | 3.7 |
|
Venous
hemangioma | 1 | 1 | 0 | 0 |
48.5 | 0.6 | 19.5 | 13.6 | 4.0 |
|
Osteochondroma | 1 | 0 | 0 | 1 | 147.8 | 5.6 | 43.6 | 17.8 | 31.2 |
| Benign
hypervascular lesions (GB) | 13 | 2 | 4 | 7 | 96.5±23.6 | 11.5±8.4 | 116.2±45.1 | 8.7±4.1 | 37.7±28.9 |
|
Kimura's
disease | 3 | 0 | 0 | 3 | 79.3±14 | 9.4±5.2 | 101.5±45.8 | 8±3.9 | 35.3±0.9 |
|
Warthin's
tumor | 2 | 0 | 2 | 0 | 108.7 | 23.1 | 174.9 | 5.3 | 20.6 |
|
Papillary
epithelioma | 2 | 0 | 2 | 0 | 97.8 | 7.9 | 97.1 | 7.4 | 25.5 |
|
Intradermal
nevus | 1 | 0 | 0 | 1 | 75.9 | 22.1 | 164.8 | 17.7 | 119.7 |
|
Chondroblastoma | 1 | 0 | 0 | 1 | 142.1 | 6.8 | 96.2 | 6.3 | 39.9 |
|
Glomus tumor | 1 | 1 | 0 | 0 | 78.4 | 4.5 | 73.7 | 7.4 | 45.7 |
|
Granulosa cell
tumor | 1 | 0 | 0 | 1 | 77.9 | 3.8 | 44.4 | 13.3 | 22.8 |
|
Basal cell
adenoma | 1 | 0 | 0 | 1 | 134.2 | 14.2 | 156.6 | 12.9 | 0.4 |
|
Giant cell
granuloma | 1 | 1 | 0 | 0 | 95.4 | 8.0 | 127.2 | 6.7 | 64.4 |
|
Malignant lesions
(GC) | 12 | 0 | 6 | 6 | 79.1±25.1 | 12.9±12.5 | 112.4±76.6 | 8.4±2.8 | 33.2±22.6 |
|
Rhabdomyosarcoma | 2 | 0 | 2 | 0 | 77.9 | 18.7 | 185.2 | 6.6 | 49.7 |
|
Adenoid cystic
carcinoma | 2 | 0 | 0 | 2 | 76.5 | 4.8 | 48.9 | 10.0 | 21.9 |
|
Malignant
schwannoma | 2 | 0 | 1 | 1 | 65.3 | 10.7 | 112.1 | 6.7 | 40.5 |
|
Mucoepidermoid
carcinoma | 1 | 0 | 1 | 0 | 145.7 | 45.9 | 241.2 | 10.1 | 21.8 |
|
Squamous cell
carcinoma | 2 | 0 | 2 | 0 | 88.1 | 13.7 | 120.6 | 12.1 | 41.5 |
|
WDC | 1 | 0 | 0 | 1 | 43.4 | 1.4 | 37.2 | 4.3 | 15.4 |
|
Epithelioid
sarcoma | 1 | 0 | 0 | 1 | 79.5 | 7.1 | 57.5 | 9.3 | 17.8 |
|
MEMT | 1 | 0 | 0 | 1 | 65.6 | 5.0 | 79.3 | 6.6 | 36.7 |
CTP imaging postprocessing
Quantitative evaluation of the CTP data was
performed in consensus by the two radiologists, using commercially
available body perfusion software (syngo® Volume
Perfusion CT Body; Siemens Healthcare). The Patlak approach was
used for analysis (19). After the CT
data set was corrected for motion with an integrated motion
correction algorithm (20), a
circular region of interest (ROI) was placed in the input artery,
either the ipsilateral external or internal carotid artery, since
there was substantial anatomic variation, which rendered it
challenging to use the same artery in different patients.
Subsequently, color coded maps of maximum intensity projection
(MIP), blood volume (BV), blood flow (BF), mean transit time (MTT)
and capillary permeability (CP) were generated. A ROI was drawn
within the greatest dimension plane of the tumor (range, 19–1032
mm2). If homogeneous enhancement was identified, the ROI
was drawn to cover ≥50% of the tumor surface. If heterogeneous
enhancement was identified, the regions with the highest
enhancement were selected. Care was taken to avoid regions of
adjacent normal vasculature and those that had no enhancement,
including calcification and cystic, necrotic and hemorrhagic
components (21). Subsequently, the
time density curves (TDCs) and the five parametric values of CTP
(MIP, BV, BF, MTT and CP) were generated for selected ROIs. For CTP
imaging post-processing, the commercially available body perfusion
software (Syngo Volume Perfusion CT Body, VA 40; Siemens
Healthcare) was used.
VEGF immunohistochemistry and
assessment
All patients underwent surgical resection as soon as
possible following the CTP scan. Two pathologists and two
radiologists jointly performed the processing of all surgical
specimens and matching with CT images. Subsequent to a 24 h
fixation in formalin (Sinopharm Chemical Reagent Co., Ltd,
Shanghai, China), the surgical specimens were sliced in the
transverse plane at the level of the maximal tumor diameter (range,
0.9–5.7 cm). The macroscopic appearance of the transversely sliced
surgical specimen was compared with the appearance of the
corresponding tumor plane on transverse CT images to ensure maximal
consistency of the regions subjected to pathological examination
with the ROIs selected on the CT images. Subsequently a 3–5 mm
thick slice was cut from the specimen in this plane, and all tissue
slices were embedded in paraffin. From the paraffin, 4-µm thick
sections were cut and incubated with phosphate-buffered saline
(Sinopharm Chemical Reagent Co., Ltd), 3%
H2O2 solution (Sinopharm Chemical Reagent
Co., Ltd) and citrate buffer (Sinopharm Chemical Reagent Co., Ltd).
The Histostain™ Plus Kit (Fuzhou Maixin Biological Technology
Development Co., Ltd., Fuzhou, China), rabbit anti-VEGF polyclonal
antibody (dilution, 1:100; catalog no. ab46154; Abcam, Cambridge,
MA, USA) and DAB Horseradish Peroxidase Color Development Kit
(Fuzhou Maixin Biological Technology Development Co., Ltd.) were
used for the detection of VEGF. VEGF expression was visualized
using a microscope (Eclipse Ci; Nikon Corporation, Tokyo, Japan).
In addition to histopathological analysis, routine tissue samples
for hematoxylin and eosin staining (Shanghai Hongqiao Le Xiang
Medical Technology Co., Ltd, Shanghai, China) were also obtained.
All the immunohistochemical results were evaluated by the two
pathologists, and consensus was reached when there was a
discrepancy.
Assessment of VEGF
According to the criteria established by Volm et
al (22) for evaluating VEGF
expression, a score was designated corresponding to the sum of the
following: (a) Percentage of positive cells (0, 0% immunopositive
cells; 1, <25% positive cells; 2, 26–50% positive cells; and 3,
>50% positive cells); and (b) Staining intensity (0, negative;
1, weak; 2, moderate; and 3, high). The sum of a + b had a maximum
score of 6. Scores between 0 and 2 were regarded as negative,
scores of 3 and 4 as weakly positive and scores between 5 and 6 as
strongly positive. At low magnification (x40), 10 fields with the
highest density of VEGF positive expression in the tumor cells were
initially determined. The 10 fields were composed of 6 counting
regions in the central portion and 4 in the peripheral portion;
necrotic areas were avoided. VEGF staining extent and intensity
were assessed in each of 10 fields at high magnification (x400) for
each field. The total scores for 10 fields were averaged and
recorded as the final score of VEGF expression for each tumor
specimen.
Statistical analysis
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analysis. P<0.05 was considered to
indicate a statistically significant difference. The Mann-Whitney
test was used in the comparison of CT perfusion parameters and VEGF
expression between any two groups. Pearson's correlation
coefficients were calculated to assess the correlation between
perfusion measurements (MIP, BV, BF, MTT, CP) and VEGF.
Results
Characteristics of dynamic enhancement
TDC
The obtained TDCs were classified into three types
on the basis of the time to peak (TTP) and the washout ratio (WR)
(23). These TDC parameters were
calculated using the following equations: TTP = time required to
reach the enhancement density peak; WR (%) =[(density at
peak)-(density at 44 sec after the start of contrast medium
injection)] × 100 / [(density at peak)-(density at the start of
contrast medium injection)] (21).
The three types of TDC were classified as follows: Type I TDCs, TTP
>30 sec and no washout; type II, TTP ≤30 sec and WR >30%;
type III, TTP <30 sec and WR <30%. Table I summarizes the various types of TDC
in the three groups.
TDC of type I (Fig. 1)
was more frequently identified in Group A compared with Groups B
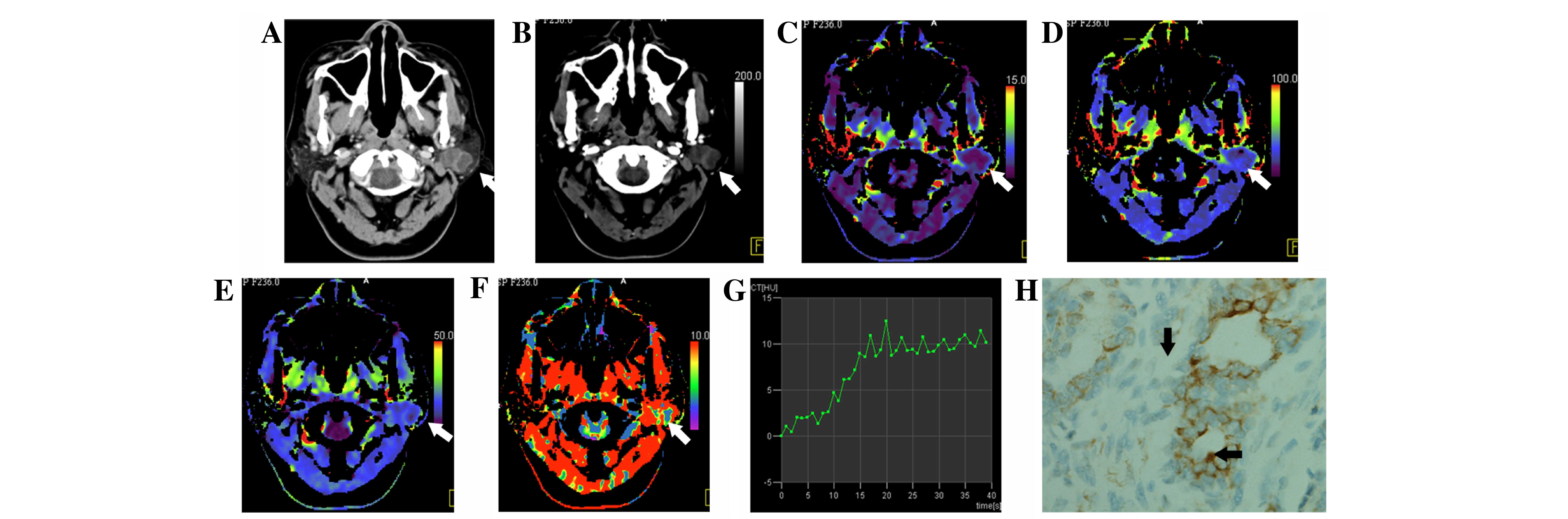
and C (P=0.003 and P<0.001) respectively). Type II (Fig. 2) was only identified in Groups B and
C. There was no significant difference in TDC of type III among the
three groups. TDC of type I was more frequently identified in
benign tumors (Groups A and B) compared with malignant tumors
(Group C) (P=0.003). Malignant tumors primarily had TDC of type II
and III.
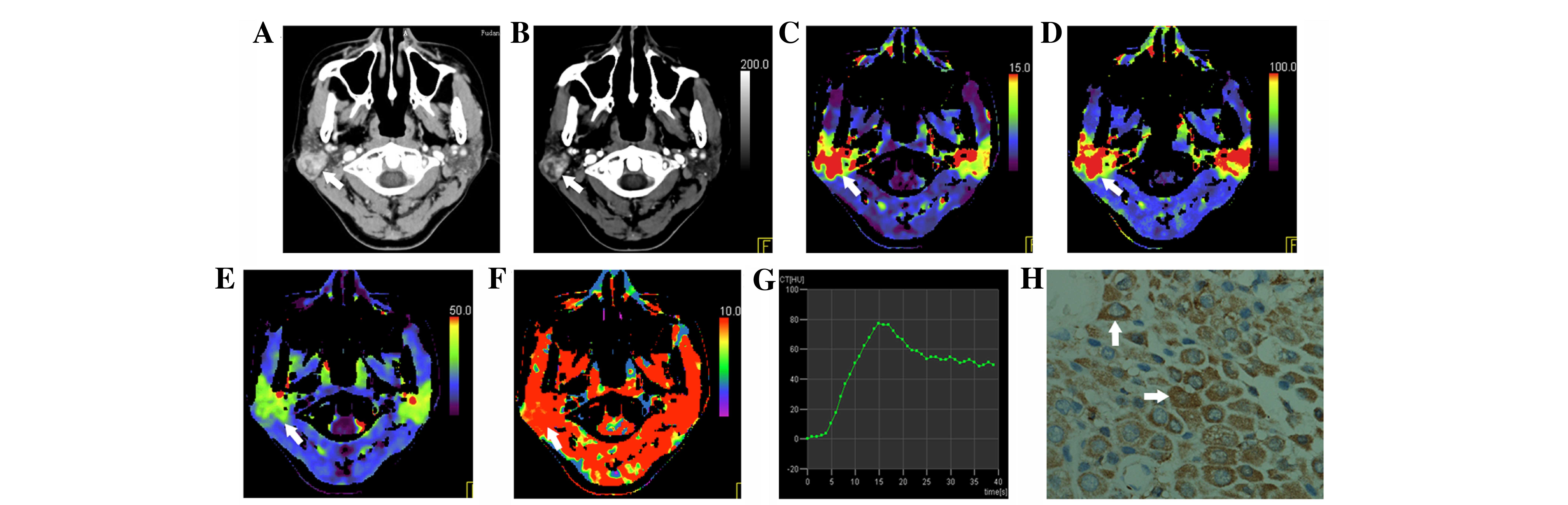
 | Figure 1.A 50-year-old female of Group A with
pleomorphic adenoma in the left parotid. (A) Axial
contrast-enhanced CT shows an irregular mass with heterogeneous
enhancement (arrow). (B-F) CT perfusion functional maps show that
the lesion has relatively (B) increased MIP, and decreased (C) BV,
(D) BF, (E) CP and (F) MTT compared with the contralateral normal
parotid gland. (G) Time density curve of type I; a steep ascending
phase with no descending phase. (H) Vascular endothelial growth
factor expression is weakly positive; brown staining is observed in
the tumour cytoplasm and cytomembrane (arrow; magnification, ×400).
CT, computed tomography; MIP, maximum intensity projection; BV,
blood volume; BF, blood flow; MTT, mean transit time; CP, capillary
permeability. |
CTP parameters
The values of the CTP parameters for each tumor are
presented in Table I, and the
differences between the CTP parameters among the three groups are
summarized in Table II. MIP, BV and
BF were all statistically increased in Groups B and C compared with
Group A (Table II). CP was increased
in Group B compared with Group A (P=0.002). Group B had increased
MIP values compared with Group C (P=0.044).
 | Table II.Comparison of computed tomography
perfusion parameters and VEGF among the three groups of head and
neck patients. |
Table II.
Comparison of computed tomography
perfusion parameters and VEGF among the three groups of head and
neck patients.
| Group | MIP, Hu | BV, ml/100 ml | BF, ml/100
ml/min | MTT, sec | CP, ml/100
ml/min | VEGF
expression |
|---|
| A |
|
|
Range | 21.6–147.8 | 0.5–9.2 | 15.8–83.6 | 3.0–17.8 | 1.2–33.8 | 0–5.5.0 |
| M | 55.3 | 2.3 | 35.8 | 9.8 | 16.0 | 3.4 |
| B |
|
|
Range | 64.1–142.1 | 3.8–33.5 | 44.4–188.6 | 3.0–17.7 | 0.4–119.7 | 1.0–4.8 |
| M | 91.7 | 8.0 | 105.2 | 7.6 | 34.5 | 3.7 |
| C |
|
|
Range | 43.4–145.7 | 1.4–45.9 | 37.2–249.4 | 4.3–13.5 | 15.2–81.5 | 3.0–5.6 |
| M | 76.4 | 9.1 | 87.2 | 9.2 | 23.9 | 5.0 |
| A vs. B |
|
| Z
score | −3.667 | −3.946 | −3.992 | −1.323 | −3.064 | −0.256 |
|
P-value |
<0.001a |
<0.001a |
<0.001a | 0.186 |
0.002a |
0.798 |
| A vs. C |
|
| Z
score | −2.693 | −3.389 | −3.296 | −0.882 | −1.811 | −2.262 |
|
P-value |
0.007a |
0.001a |
0.001a |
0.378 |
0.070 | 0.024b |
| B vs. C |
|
| Z
score | −2.013 | −0.272 | −0.761 | −0.082 | −0.870 | −2.510 |
|
P-value |
0.044b |
0.786 |
0.446 |
0.935 |
0.384 |
0.012b |
| A+B vs. C |
|
| Z
score | −0.602 | −1.948 | −1.633 | −0.501 | −0.659 | −2.713 |
|
P-value | 0.547 |
0.051 |
0.102 |
0.616 |
0.510 |
0.007a |
VEGF expression and its association
with CTP parameters
The results of VEGF expression in the three groups
are presented in Table II. The
Mann-Whitney test revealed a significant difference of VEGF
expression in Group C vs. A (P=0.024) and Group C vs. B (P=0.012).
VEGF expression of malignant tumors (Group C) was significantly
higher compared with benign tumors (Groups A + B; P=0.007). The
correlation between perfusion parameters and VEGF is presented in
Table III. VEGF expression was not
correlated with any CTP parameters (P>0.05).
 | Table III.Pearson's correlation for computed
tomography perfusion parameters and VEGF. |
Table III.
Pearson's correlation for computed
tomography perfusion parameters and VEGF.
|
| MIP, Hu | BV, ml/100 ml | BF, ml/100
ml/min | MTT, sec | CP, ml/100
ml/min |
|---|
|
|
|
|
|
|
|
|---|
| VEGF
expression | r | P | r | P | r | P | r | P | r | P |
|---|
| ++ |
0.292 | 0.312 | 0.283 | 0.327 | 0.408 | 0.148 | −0.466 | 0.100 |
0.100 | 0.734 |
| + |
0.106 | 0.640 | 0.048 | 0.830 | 0.019 | 0.933 |
0.108 | 0.600 |
0.170 | 0.448 |
| – | −0.498 | 0.393 | −0.002 | 0.998 | 0.004 | 0.996 |
0.349 | 0.600 | −0.129 | 0.837 |
Discussion
CTP is performed to evaluate blood flow patterns of
lesions, and the various appearances of the TDCs of HNLs primarily
represent the differences in blood perfusion (17). In the present study, TDC of type I was
more frequently identified in Group A (benign hypovascular lesions)
compared with Group B (benign hypervascular lesions) (P=0.003),
which indicated that TDC of type I may be used to distinguish
between benign hypovascular and hypervascular lesions. In addition,
TDC of type I was more frequently identified in benign tumors
(Groups A and B) compared with malignant tumors (Group C)
(P=0.003). This may be due to the decreased blood supply and intact
vascular wall observed benign tumors, particularly in benign
hypovascular lesions (17).
Consequently, TDC of type I may aid in the differentiation between
benign and malignant HNLs. Additionally in the present study,
malignant tumors primarily were TDC type II and III, and type II
was only identified in Groups B and C. In malignant tumors in the
present study, due to the abundant novel vessels, loosely
interconnected endothelium and immature basement membrane (17), the contrast agent dispersed quickly
following injection. Therefore, these were classified as TDC of
type II, and may be used to differentiate Group A from Groups B and
C. In addition, certain tumors in Group B, including Warthin's
tumors and papillary epitheliomas, also had TDC of type II, which
may be attributed pathologically to their extensive capillary
networks and numerous leaky blood vessels (24). Therefore, the various types of TDC may
reflect the alterations in blood microcirculation and may aid in
distinguishing between benign and malignant HNLs, and between
benign hypovascular and hypervascular lesions.
There are three main differences in CTP parameters
in the differentiation of malignant and benign HNLs (13,16,17).
Rumboldt et al (16)
demonstrated that benign head and neck lesions had a lower BF and
longer MTT compared with malignant lesions. Conversely, Bisdas
et al (13) reported that the
BV and BF of benign parotid tumors were significantly higher than
those of malignant tumors. In the present study, MIP, BV and BF
were all statistically higher in Groups B and C compared with Group
A (P<0.01). As MIP may reflect the overall level and average
degree of perfusion, its increase in Groups B and C may reveal more
abundant angiogenesis than Group A. A significantly high BV in
Groups B and C may reflect an increased vascular bed in the process
of angiogenesis (the growth of novel vessels from pre-existing
ones), while a high BF may reflect the formation of a number of
arteriovenous shunts in tumor angiogenesis. Therefore, MIP, BV and
BF reflect the extent of tumor angiogenesis in HNLs and may be of
great value in the differentiation of Group A from Groups B and C.
However, there were no significant differences in BV and BF between
Group B and Group C (P>0.05). This may be due to the numerous
microvessels and high cellularity-stromal grade observed in benign
hypervascular lesions, which results in a high WR (13), followed by MIP, BV and BF increases.
Additionally in the present study, the necrotic regions of
malignant tumors had an apparent affect on CTP parameters, which
could reduce MIP, BV and BF. Furthermore, CP was higher in Group B
than in Group A (P=0.002), and this may aid in differentiating
between benign hypovascular and hypervascular lesions. The
difference may be due to the leaky blood vessels of certain tumors
in Group B, including Warthin's tumors and papillary epitheliomas
(24), which has led to the capillary
permeability increase.
The importance of angiogenesis in tumor growth is
well-established (14). Although
there are numerous types of vascular growth factors, VEGF is
crucial in tumor angiogenesis due to its specialized role in
promoting tumor karyokinesis (25).
Yang et al (17) demonstrated
that high VEGF expression was significantly different between
malignant and benign HNLs. In the present study, there were no
significant differences in VEGF expression between Group A and
Group B. However, the VEGF expression in Group C was significantly
higher than that Groups A and B (P=0.024 and P=0.012,
respectively), which indicated that VEGF expression may be useful
to differentiate between malignant and benign HNLs.
CTP assesses tumor vascularity quantitatively in
vivo with vascular parameters, including BV, BF, MTT and CP,
and it has been used as a tool for the assessment of antivascular
chemotherapy (15,26). Therefore, the vascular parameters of
CTP may be an appropriate surrogate for angiogenesis and may have
certain correlations with histological measures of angiogenesis,
including VEGF.
Different results to the present study, have been
demonstrated in prior studies that focused on the correlation
between CTP parameters and VEGF expression. Zhang et al
(27) revealed that there was a
positive correlation between BV, BF, PS and VEGF, and a negative
correlation between MTT and VEGF in a malignant rabbit VX2 liver
tumor model. In addition, Yang et al (17) reported a significant correlation
between VEGF expression and MTT in HNLs. In the present study, no
correlation was observed between VEGF expression and any CTP
measurements in all three groups, in accordance with the findings
of previous studies where no association was demonstrated between
the expression levels of VEGF and any perfusion CT parameter in
colorectal cancer (28), gastric
adenocarcinoma (29), and renal cell
carcinoma (30). Although VEGF is a
key stimulatory factor that promotes tumor angiogenesis (31), it is not the only factor that
influences tumor angiogenesis. Other factors, including
platelet-derived endothelial cell growth factor, cyclooxygenase-2
and interleukin-8, also participate in tumor angiogenesis, which
may lead to alterations in CTP parameters (31). Furthermore, VEGF expression is
upregulated according to alterations in the microenvironment,
including hypoxia and a decrease in pH (31), without an obvious impact on the
results of tumor CTP. Consequently, it is possible that there is no
correlation between VEGF expression and CTP measurements in HNLs;
however, this requires validation.
The present study had certain limitations. First,
due to the small sample size and overlap in the CTP data among the
three groups, a larger scale trial is required to validate the
differential diagnostic value of CTP in HNLs. Second, the perfusion
scanning time (40 sec) was not long enough in the present study,
and prolonged scans may provide more information in the
differential diagnosis of HNLs. Third, the CTP studies were
performed on a 16-row multi-slice CT scanner, and more precise data
would be acquired with thinner slices and a larger scanning area on
a more advanced multidetector CT.
In conclusion, the present study suggests that CTP
may differentiate between malignant and benign HNLs and between
benign hypovascular and hypervascular HNLs. In addition, VEGF may
differentiate between benign and malignant HNLs. Overall, CTP
combined with VEGF may be beneficial for the differentiation, and
reflect the angiogenesis, of HNLs.
Acknowledgements
The present study was supported by the Crossover
Study Fund of Basic & Clinical Medicine of Shanghai Medical
School of Fudan University (Shanghai, China; grant no. Z-259).
References
|
1
|
Lee TY, Purdie TG and Stewart E: CT
imaging of angiogenesis. Q J Nucl Med. 47:171–187. 2003.PubMed/NCBI
|
|
2
|
Li C, Fan J, Song X, Zhang B, Chen Y, Li
C, Mi K, Ma H, Song Y, Tao X and Li G: Expression of angiopoietin-2
and vascular endothelial growth factor receptor-3 correlates with
lymphangiogenesis and angiogenesis and affects survival of oral
squamous cell carcinoma. PLoS One. 8:e753882013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai W, Li Y, Zhou Q, Xu Z, Sun C, Tan X
and Lu L: Cetuximab inhibits oral squamous cell carcinoma invasion
and metastasis via degradation of epidermal growth factor receptor.
J Oral Pathol Med. 43:250–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Oliveira MV, Pereira Gomes EP, Pereira
CS, de Souza LR, Barros LO, Mendes DC, Guimarães AL and De Paula
AM: Prognostic value of microvessel density and p53 expression on
the locoregional metastasis and survival of the patients with head
and neck squamous cell carcinoma. Appl Immunohistochem Mol Morphol.
21:444–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen TW, Yang ZG, Chen HJ, Li Y, Tang SS,
Yao J, Dong ZH and He D: Quantitative assessment of first-pass s
using a low-dose method at multidetector CT in oesophageal squamous
cell carcinoma: Correlation with VEGF expression. Clin Radiol.
67:746–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling S, Deng D, Mo Y, Zhang X, Guan X and
Wei Q: Correlations between CT perfusion parameters and vascular
endothelial growth factor expression and microvessel density in
implanted VX2 lung tumors. Cell Biochem Biophys. 70:629–633. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JY, Jang KT, Shim YM, Kim K, Ahn G,
Lee KH, Choi Y, Choe YS and Kim BT: Prognostic significance of
vascular endothelial growth factor expression and microvessel
density in esophageal squamous cell carcinoma: Comparison with
positron emission tomography. Ann Surg Oncol. 13:1054–1062. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleespies A, Guba M, Jauch KW and Bruns
CJ: Vascular endothelial growth factor in esophageal cancer. J Surg
Oncol. 87:95–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meijerink MR, van Waesberghe JH, van der
Weide L, van den Tol P, Meijer S and van Kuijk C:
Total-liver-volume perfusion CT using 3-D image fusion to improve
detection and characterization of liver metastases. Eur Radiol.
18:2345–2354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon
OJ, Jeong YJ and Kim S: Solitary pulmonary nodules: Dynamic
enhanced multi-detector row CT study and comparison with vascular
endothelial growth factor and microvessel density. Radiology.
233:191–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trojanowska A, Trojanowski P, Drop A,
Jargiełło T and Klatka J: Head and neck cancer: Value of perfusion
CT in depicting primary tumor spread. Med Sci Monit.
18:CR112–CR118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miles KA: Tumour angiogenesis and its
relation to contrast enhancement on computed tomography: A review.
Eur J Radiol. 30:198–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bisdas S, Baghi M, Wagenblast J, Knecht R,
Thng CH, Koh TS and Vogl TJ: Differentiation of benign and
malignant parotid tumours using deconvolution-based perfusion CT
imaging: Feasibility of the method and initial results. Eur J
Radiol. 64:258–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
George ML, Dzik-Jurasz AS, Padhani AR,
Brown G, Tait DM, Eccles SA and Swift RI: Non-invasive methods of
assessing angiogenesis and their value in predicting response to
treatment in colorectal cancer. Br J Surg. 88:1628–1636. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahani DV, Kalva SP, Hamberg LM, Hahn PF,
Willett CG, Saini S, Mueller PR and Lee TY: Assessing tumour
perfusion and treatment response in rectal cancer with multisection
CT: Initial observations. Radiology. 234:785–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rumboldt Z, Al-Okaili R and Deveikis JP:
Perfusion CT for head and neck tumours: Pilot study. AJNR Am J
Neuroradiol. 26:1178–1185. 2005.PubMed/NCBI
|
|
17
|
Yang ZY, Meng QF, Xu QL, Li SR, Yan CG,
Xie HB, Yang XF, Peng Q and Lai YR: Correlation between CT
perfusion and vascular endothelial growth factor in neoplasm of
head and neck. Zhonghua Fang She Xue Za Zhi. 41:900–906. 2007.(In
Chinese).
|
|
18
|
Schmid-Tannwald C, Thomas S, Ivancevic MK,
Dahi F, Rist C, Sethi I and Oto A: Diffusion-weighted MRI of
metastatic liver lesions: Is there a difference between
hypervascular and hypovascular metastases? Acta Radiol. 55:515–523.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spira D, Neumeister H, Spira SM, Hetzel J,
Spengler W and von Weyhern CH: Assessment of tumor vascularity in
lung cancer using volume perfusion CT (VPCT) with histopathologic
comparison: A further step toward an individualized tumor
characterization. J Comput Assist Tomogr. 37:15–21. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reiner CS, Roessle M, Thiesler T, Eberli
D, Klotz E and Frauenfelder T: Computed tomography perfusion
imaging of renal cell carcinoma: Systematic comparison with
histopathological angiogenic and prognostic markers. Invest Radiol.
48:183–191. 2013.PubMed/NCBI
|
|
21
|
d'Assignies G, Couvelard A, Bahrami S,
Vullierme MP, Hammel P, Hentic O, Sauvanet A, Bedossa P,
Ruszniewski P and Vilgrain V: Pancreatic endocrine tumours: Tumour
blood flow assessed with perfusion CT reflects angiogenesis and
correlates with prognostic factors. Radiology. 250:407–416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volm M, Koomägi R and Mattern J:
Prognostic value of vascular endothelial growth factor and its
receptor Flt-1 in squamous cell lung cancer. Int J Cancer.
74:64–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Y, Lei GW, Wang SW, Zheng SW, Ge Y
and Wei FC: Diagnostic value of CT perfusion imaging for parotid
lesions. Dentomaxillofac Radiol. 43:201302372014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woo SH, Choi DS, Kim JP, Park JJ, Joo YH,
Chung PS, Kim BY, Ko YH, Jeong HS and Kim HJ: Two-phase computed
tomography study of warthin tumor of parotid gland: Differentiation
from other parotid gland tumors and its pathologic explanation. J
Comput Assist Tomogr. 37:518–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Byrne KJ, Koukourakis MI, Giatromanolaki
A, Cox G, Turley H, Steward WP, Gatter K and Harris AL: Vascular
endothelial growth factor, platelet-derived endothelial cell growth
factor and angiogenesis in non-small-cell lung cancer. Br J Cancer.
82:1427–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ng QS, Goh V, Milner J, Stratford MR,
Folkes LK, Tozer GM, Saunders MI and Hoskin PJ: Effect of nitric
oxide synthesis on tumour blood volume and vascular activity: A
phase I study. Lancet Oncol. 8:111–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Wang R, Lou H, Zou Y and Zhang M:
Functional computed tomographic quantification of angiogenesis in
rabbit VX2 soft-tissue tumour before and after interventional
therapy. J Comput Assist Tomogr. 32:697–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goh V, Halligan S, Daley F, Wellsted DM,
Guenther T and Bartram CI: Colorectal tumour vascularity:
Quantitative assessment with multidetector CT-do tumour perfusion
measurements reflect angiogenesis? Radiology. 249:510–517. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao J, Yang ZG, Chen HJ, Chen TW and Huang
J: Gastric adenocarcinoma: Can perfusion CT help to noninvasively
evaluate tumour angiogenesis? Abdom Imaging. 36:15–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Zhang J, Dai J, Feng X, Lu H and
Zhou C: Angiogenesis of renal cell carcinoma: Perfusion CT
findings. Abdom Imaging. 35:622–628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reinmuth N, Parikh AA, Ahmad SA, Liu W,
Stoeltzing O, Fan F, Takeda A, Akagi M and Ellis LM: Biology of
angiogenesis in tumours of the gastrointestinal tract. Microsc Res
Tech. 60:199–207. 2003. View Article : Google Scholar : PubMed/NCBI
|