Introduction
Esophageal cancer affects >45 million people
worldwide, and the incidence is rapidly increasing (1). The 5-year survival rate for patients
with esophageal cancer has gradually improved to ~20% in the past
30 years (2), however, the disease
still has a poor prognosis. Since early-stage esophageal cancer has
few symptoms, the cancer is often found in the locally advanced or
metastatic state, and ~40% of patients have distant metastatic
lesions at the time of presentation (3). Moreover, despite adequate initial
treatment, patients remain at risk of developing recurrence.
Recurrence often occurs at the lymph nodes, bones,
lungs and liver (4). Treatment of
recurrent esophageal cancer depends on the initial treatment, the
histology of the primary tumor and the location of the recurrence.
The salvage therapy for recurrent esophageal cancer is aimed at
providing a survival benefit or improving the patient's quality of
life, and it rarely results in complete remission.
Combination chemotherapy is considered to be more
effective than single-agent chemotherapy, but has more toxic
effects for the patient (5). The
regimen for salvage therapy remains controversial. In the present
study, due to the condition of the patient, S-1 administration was
selected as salvage chemotherapy and greater than expected
effectiveness was observed.
Case report
A 74-year-old male presented to the Department of
Pathology, Sanikukai Hospital (Tokyo, Japan), in February 2006
after experiencing dysphagia and weight loss of 5 kg over the
preceding 6 months. The patient drank 360 ml of rice wine twice a
week, and had smoked 15 cigarettes per day for the past 55 years.
Gastrointestinal fibroscopy (GIF) and computed tomography (CT)
scans were performed. GIF revealed a 3-4-cm Borrmann type 2 lesion
covering ~one-third of the circumference of the esophagus, 35 cm
from the incisor teeth. The lesion was biopsied and diagnosed as
squamous cell carcinoma by examination of the pathological
specimen. CT scans show showed enlarged lymph nodes around the
lesser curvature of the stomach at the cardiac end. The patient was
clinically staged as T3N1M0, according to the 6th edition of the
American Joint Committee on Cancer tumor-node-metastasis staging
system (6). Surgery was not
recommended due to the age of the patient.
The patient was referred to the University of Tokyo
Hospital (Tokyo, Japan) for chemoradiotherapy on April 2006.
Positron emission tomography CT imaging revealed primary lesions in
the lower esophageal region and in the cardiac end of the stomach.
Further CT scans revealed a 60-mm dorsal dominant lesion with
circumferential wall thickening that was infiltrating the proper
muscular layer in the mid and lower thoracic esophagus. A 70×50-mm
enhanced lymph node was noted infiltrating the proper muscular
layer at the cardiac end of the stomach, and an enlarged lymph node
was present around the left renal vein. These findings were
compatible with the clinical stage, T3N1M1. The patient was
administered 2 cycles of chemotherapy (28 days/cycle) comprising a
combination of cisplatin (CDDP; 75 mg/m2) on day 1 and
5-fluorouracil (5-FU; 1,000 mg/m2) on days 1–4. The
patient was concurrently administered radiotherapy, with a clinical
target volume that included the whole esophageal tract,
para-esophageal lymph nodes, lesser curvature lymph nodes and
para-aortic lymph nodes. A 10 MV X-ray parallel-opposed pair
4-field oblique box and 50.4 Gy in 1.8-Gy daily fractions were
used. Acute adverse events, including grade 2 dermatitis, grade 4
anemia, grade 4 leukopenia and grade 3 thrombocytopenia, as
assessed by the Common Terminology Criteria for Adverse Events
(version 3.0) (7), were noted;
granulocyte colony-stimulating factor administration and a
transfusion of 4 units of red blood cell concentrate in mannitol
adenine phosphate and 10 units of platelet concentrate were
subsequently required. Following the concurrent chemoradiotherapy,
CT scans showed that the thickening of the esophageal wall was
reduced and that the lymph nodes at the cardiac end of the stomach
and para-aorta were markedly reduced in volume. GIF revealed no
clear abnormalities, with the exception of a scar-like lesion on
the right wall of the esophagus, 32–38 cm from the incisor teeth.
Accordingly, this treatment was noted to have resulted in a
complete response (CR). Follow-up examinations included a physical
examination, the assessment of laboratory data, GIF and CT
scans.
The patient showed no signs of recurrence after the
initial treatment, however, in December 2013, the patient
experienced dysphagia and a CT scan (Aquilion LB; Toshiba Medical
Systems Corporation, Tokyo, Japan) showed a swollen lymph node
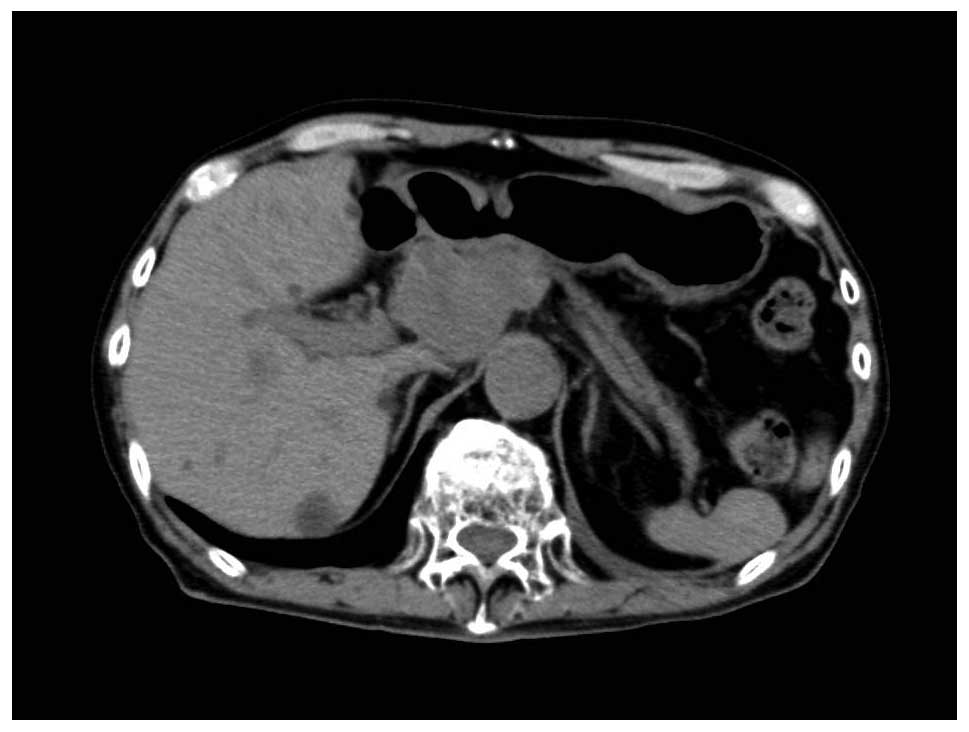
around the common hepatic artery. In March 2014, the lymph node
around the common hepatic artery had increased to 38 mm in diameter
and the sub-pyloric lymph node had increased to 22 mm in diameter
(Fig. 1); this was diagnosed as
metastatic recurrence. The patient decided to receive salvage
chemotherapy consisting of S-1 monotherapy following consideration
of the diminished renal function, which may have been caused by
initial treatment of CDDP administration. The patient's serum
creatinine level was 1.49 mg/dl and the blood urea nitrogen level
was 29.7 mg/dl. S-1 was administered twice a day during a 2-week
period, followed by a 2-week rest period (80 mg/day for 14
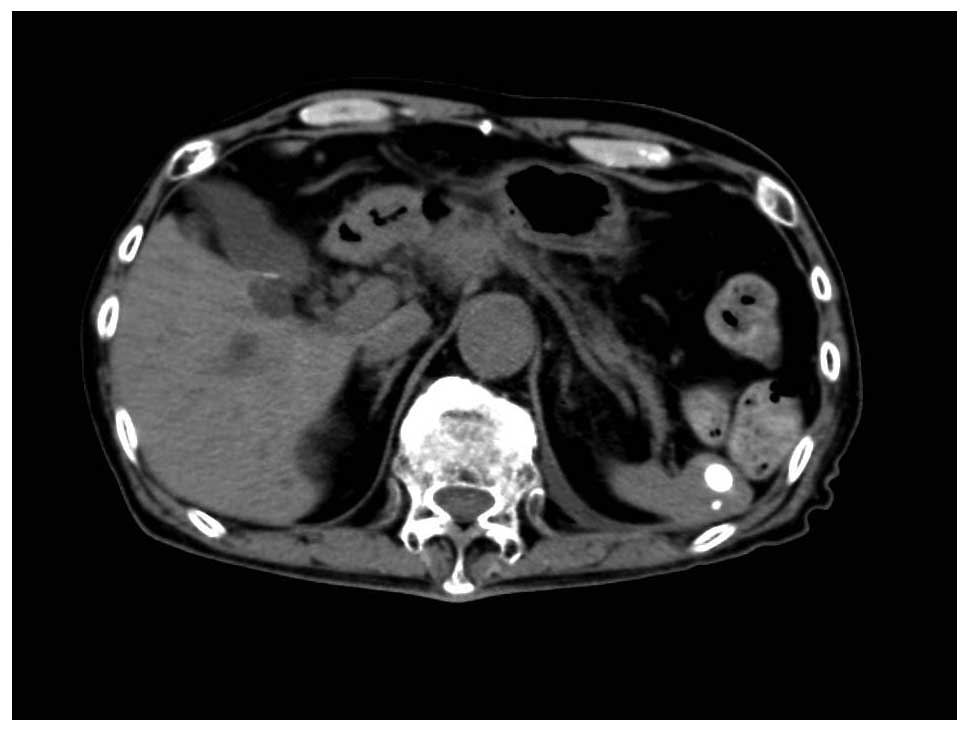
days/cycle). After 2 cycles of S-1 monotherapy, CT scans (Aquilion
LB; Toshiba Medical Systems Corporation) showed that the existing
lymph node lesions in the abdominal area were markedly reduced in
volume. Follow-up examinations included a physical examination,
assessment of tumor markers and CT scans. The patient achieved a CR
and has exhibited no signs of recurrence for 9 months (Fig. 2). However, in June 2015 a CT scan
revealed multiple lymphadenopathy of the abdominal lymph nodes. The
patient was subsequently administered chemotherapy with tegafur
(300 mg/kg, daily) for 2 months, but succumbed due to progressive
disease in December 2015.
Discussion
There is no consensus as to the best regimen for the
chemotherapeutic treatment of advanced, recurrent or metastatic
esophageal cancer. The active single agents for squamous cell
carcinoma are CDDP, 5-FU, bleomycin, paclitaxel, mitomycin,
mitoguazone, vinorelbine and methotrexate. Combination chemotherapy
with CDDP is widely used for esophageal cancer treatment. The
response rate of targeted CDDP/5-FU treatment for advanced and
recurrent cases of squamous cell carcinoma has been recorded at 36%
(8). Whereas two-drug regimens are
preferred due to a lower toxicity, three-drug regimens are adopted
for more medically fit patients with a good performance status.
Combination treatment with CDDP and fluoropyrimidine is regarded as
first-line therapy for esophageal and esophagogastric junction
cancers (9). For advanced
esophagogastric cancer, combination chemotherapy with epirubicin
plus CDDP/5-FU has shown a good response rate of 40.7%, a 1-year
survival rate of 37.7% and a median survival time of 9.9 months
(10).
S-1 is an oral fluoropyrimidine that consists of
tegafur, which is a 5-FU prodrug, combined with gimeracil and
oteracil potassium. Gimeracil (5-chloro-2, 4-dihydroxypyridine)
acts as a potent inhibitor of 5-FU degradation, and oteracil
potassium reduces the gastrointestinal toxicity caused by 5-FU
(11). S-1 monotherapy is considered
as the second- or third-line chemotherapy for unresectable and
recurrent esophageal squamous cell carcinoma (12). While S-1 monotherapy is safe and well
tolerated in elderly patients, it is also associated with modest
response rates and infrequent responses (13). However, several reported cases from
Japan have shown a marked response to S-1/CDDP chemotherapy in
patients with recurrent esophageal cancer (14,15).
In a previous study, we reported a CR in a patient
who presented with advanced esophageal cancer and abdominal bulky
lymph node metastasis, and was treated with concurrent
chemoradiotherapy using docetaxel, CDDP and 5-fluorouracil
(16). Although S-1 administration
alone is not considered to exhibit a significant effect on
recurrent lesions, the treatment in the present study showed
unexpected effectiveness. Overall, the reported therapy may be
considered as an option for the treatment of patients with a poor
performance status and locally advanced, recurrent or metastatic
esophageal cancer.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
CDDP
|
cisplatin
|
|
CR
|
complete response
|
|
CT
|
computed tomography
|
|
GIF
|
gastrointestinal fiberscopy
|
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enzinger PC and Mayer RJ: Esophageal
Cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quint LE, Hepburn LM, Francis IR, Whyte RI
and Orringer MB: Incidence and distribution of distant metastases
from newly diagnosed esophageal carcinoma. Cancer. 76:1120–1125.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ilson DH: Esophageal cancer chemotherapy:
Recent advances. Gastrointest Cancer Res. 2:85–92. 2008.PubMed/NCBI
|
|
6
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: Esophagus. American Joint
Committee on Cancer (AJCC) Cancer Staging Manual (6th). Springer.
(New York, NY). 90–95. 2002.
|
|
7
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iizuka T, Kakegawa T, Ide H, Ando N,
Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M, et
al: Phase II evaluation of cisplatin and 5-fluorouracil in advanced
squamous cell carcinoma of the esophagus: A Japanese esophageal
oncology group trial. Jpn J Clin Oncol. 22:172–176. 1992.PubMed/NCBI
|
|
9
|
The National Comprehensive Cancer Network
(NCCN) Guidelines Version 1.2014: Esophageal and Esophagogastric
Junction Cancers. http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdfAccessed.
March 01–2015
|
|
10
|
Cunningham D, Okines AF and Ashley S:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 362:858–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akutsu Y, Kono T, Uesato M, Hoshino I,
Narushima K, Hanaoka T, Tochigi T, Semba Y, Qin W and Matsubara H:
S-1 monotherapy as second- or third-line chemotherapy for
unresectable and recurrent esophageal squamous cell carcinoma.
Oncology. 84:305–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiraki M, Yunotani S, Noguchi R, Shinozaki
Y, Tani H, Sakai M, Ishimitsu T and Tabuchi M: Recurrence of
esophageal cancer treated by combination TS-1/CDDP therapy. Gan To
Kagaku Ryoho. 32:219–221. 2005.(In Japanese). PubMed/NCBI
|
|
15
|
Kanamori N, Fujii M, Takahashi T,
Wakabayashi K, Kochi M, Sou K and Takayama T: A patient with
esophageal cancer recurrence responding to S-1 combined with
cisplatin (CDDP). Gan To Kagaku Ryoho. 34:1459–1461. 2007.(In
Japanese). PubMed/NCBI
|
|
16
|
Kubota K, Mafune K, Yamada K, Yamashita H,
Kuroda J, Aikou S and Kaminishi M: Complete regression of advanced
esophageal cancer with abdominal bulky lymph node metastasis
treated by concurrent chemoradiotherapy using docetaxel, cisplatin
and 5-fluorouracil. Esophagus. 6:183–187. 2009. View Article : Google Scholar
|