Introduction
Since the international phase III trial
demonstrating the survival benefit of trastuzumab for patients with
human epidermal growth factor receptor 2 (HER2)-positive gastric
cancer (1), tissue HER2 assessment
has become a routine practice in patients with advanced gastric
cancer. In contrast to HER2-positive breast cancer, which generally
exhibits homogenous HER2 overexpression, gastric cancer frequently
exhibits intratumoral heterogeneity of HER2 overexpression
(2–5).
In addition, recent studies have demonstrated substantial
inter-laboratory, as well as inter-observer discordance in tissue
HER2 assessments (6,7). This may be responsible for generating
HER2 false-negative results, depriving the patient of the
opportunity for anti-HER2 treatment.
Serum HER2 is the HER2 extracellular domain that
sheds from the surface of cancer cells into the circulation, and
may be quantified by chemiluminescence immunoassay (CLIA). The
clinical significance of serum HER2 has been reported in breast
cancer (8–13); however, few studies have investigated
this in gastric cancer. The current report presents our experience
with an illustrative case of HER2-positive metastatic gastric
cancer that was initially diagnosed as HER2-negative and salvaged
by serum HER2.
Case report
In October 2012, a 56-year-old male presented to
Sapporo Medical University Hospital (Sapporo, Japan) with dysphagia
and weight loss. The patient's past history was unremarkable, and
physical examination revealed mild epigastric tenderness and a
palpable liver. The baseline laboratory tests demonstrated elevated
liver enzymes (aspartate transaminase, 148 IU/l, normal range,
11–39 IU/l; alanine transaminase, 89 IU/l, normal range, 5–40 IU/l;
alkaline phosphatase, 1,292 IU/l, normal range, 110–370 IU/l; and
lactate dehydrogenase, 2,976 IU/l, normal range, 119–229 IU/l) and
elevated carbohydrate antigen 19-9 (131.9 U/ml; normal range,
0.0–37.0 U/ml). Upper gastrointestinal endoscopy revealed a
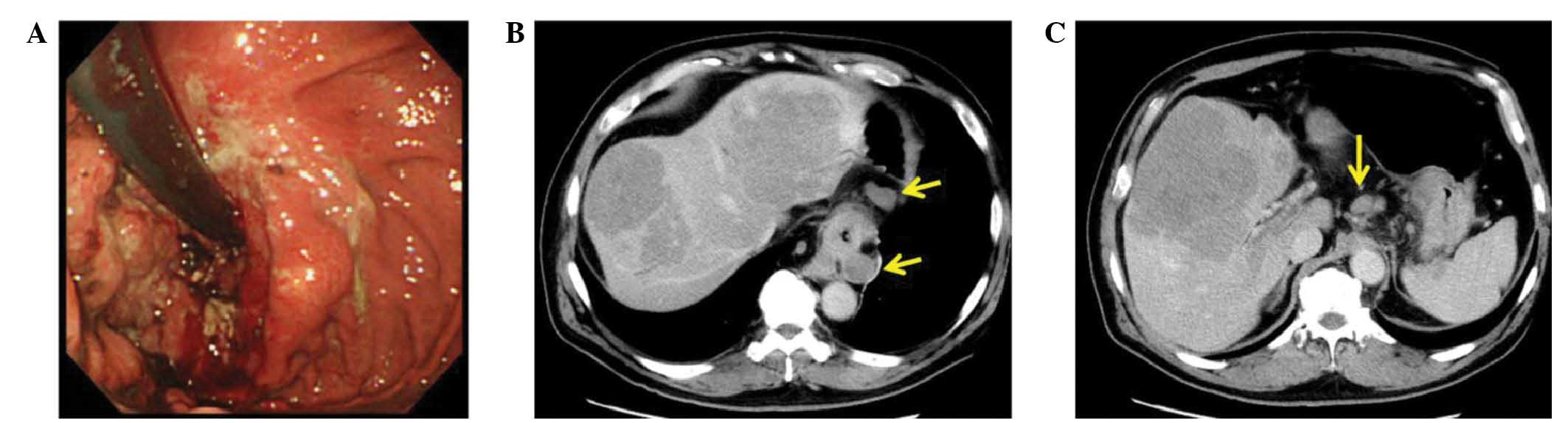
Borrmann type III cancer (14) from
the cardia to the lower esophagus (Fig.
1A), and computed tomography (CT) imaging revealed multiple
liver and nodal metastases (Fig. 1B).
The pathological diagnosis of three biopsy specimens was moderately
to poorly differentiated tubular adenocarcinoma.
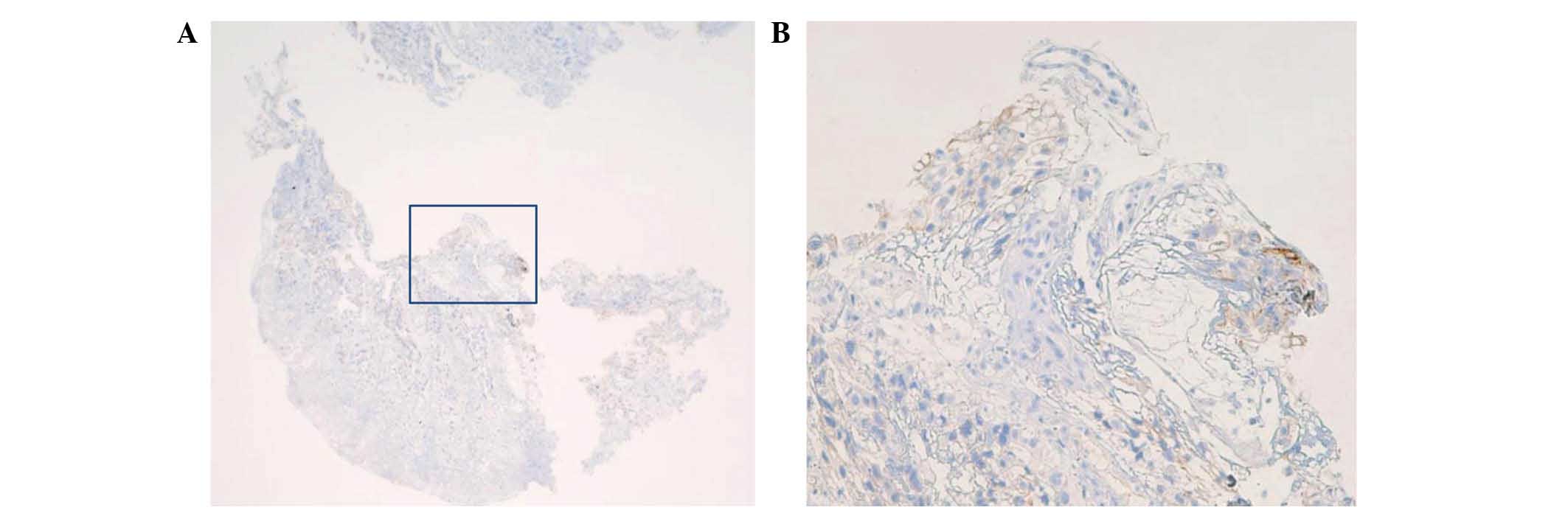
Immunohistochemistry (IHC) for HER2 was performed on
formalin-fixed, 4-µm thick, paraffin-embedded tissue sections
(SurgiPath Paraplast; Leica Biosystems, Wetzlar, Germany) using a
monoclonal rabbit PATHWAY anti-HER2 antibody (4B5; Bench Mark GX;
Roche Diagnostics K.K., Tokyo, Japan). The tissue yielded a score
of 1+ according to a scoring criteria specifically developed for
gastric cancer (2). Staining was
scored as follows: 0, no reactivity or no membrane staining; +1,
tumor cell cluster with a faint/barely perceptible membranous
reactivity; +2, tumor cell cluster with a weak to moderate
complete, basolateral, or lateral membranous reactivity; +3, tumor
cell cluster with a strong complete, basolateral, or lateral
membranous reactivity. Tissues with a score of +3 or +2 in addition
to fluorescence in situ hybridization (FISH) positivity were
considered as HER2 positive. Thus, the patient was diagnosed as
HER2-negative (Fig. 2). Based on this
diagnosis, S-1 plus cisplatin combination chemotherapy (S-1, 40
mg/m2, twice daily, days 1–21; cisplatin, 60
mg/m2, day 8, every 5 weeks) was commenced.
The patient was enrolled onto our clinical trial
investigating the association between serum HER2 and tissue HER2
status in gastric cancer (15). The
serum HER2 level of this patient measured by CLIA (ADVIA
Chemilumi-Centaur-HER2/neu assay™; Siemens Healthcare Diagnostics,
Tokyo, Japan) was 53.3 ng/ml, which was higher than the upper limit
of normal for breast cancer (15.2 ng/ml). In addition to the
clinical features consistent with HER2-positive gastric cancer,
such as the junctional location, differentiated histology and liver
metastasis, the elevated serum HER2 level prompted the reevaluation
of the tissue HER2 status.
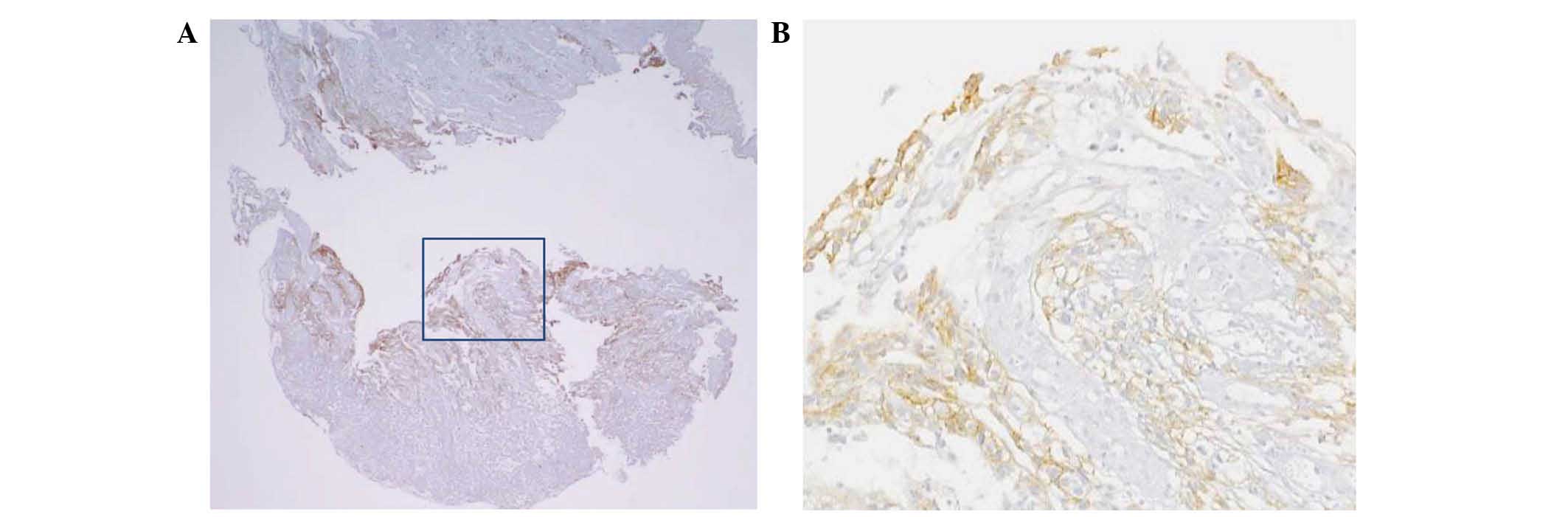
The second IHC analysis, performed on
formalin-fixed, 4-µm thick, paraffin-embedded tissue sections using
an alternative polyclonal rabbit antibody from the HercepTest™ kit
(Dako A/S, Glostrup, Denmark), demonstrated intensive membranous
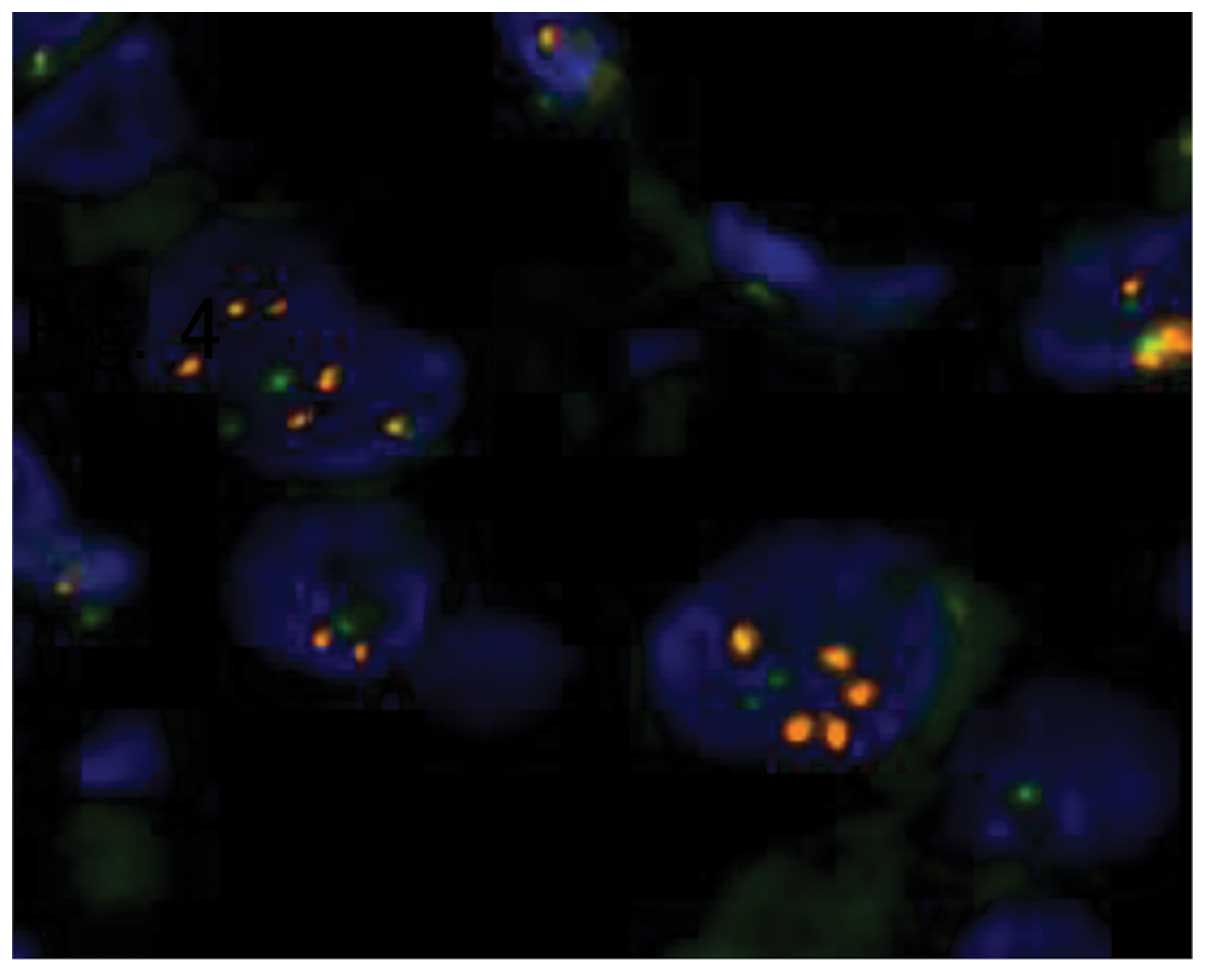
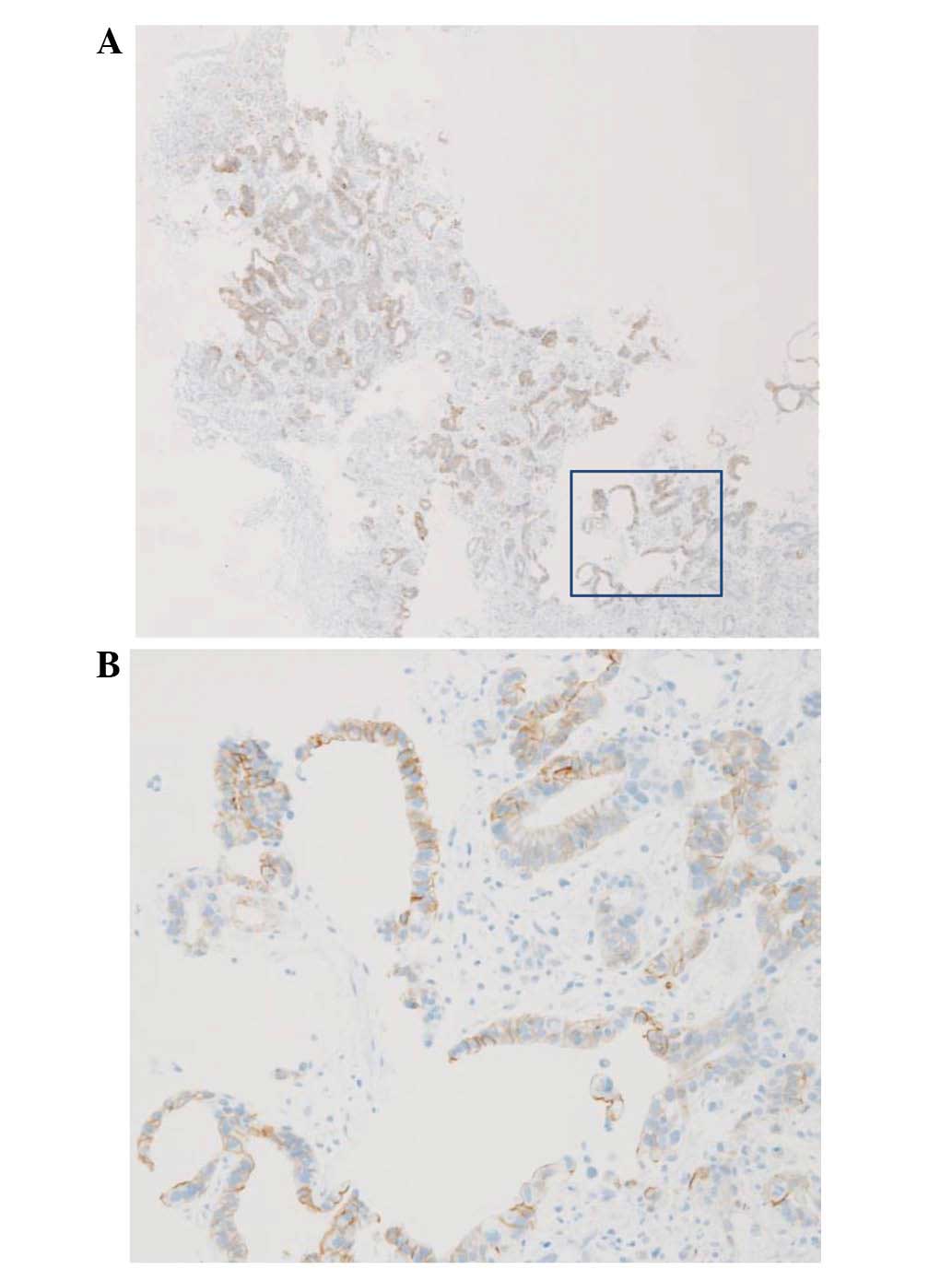
staining, judged to be HER2 score 3+ (Fig. 3). In addition, the HER2/chromosome 17
centromere ratio assessed by FISH was 2.48, also interpreted as
HER2-positive (Fig. 4). Four biopsy
samples were additionally taken at a follow-up endoscopy, and IHC
for these re-biopsy specimens also confirmed a HER2 score of 3+
(Fig. 5). Based on the final
diagnosis, the treatment protocol was changed from S-1 plus
cisplatin to capecitabine, cisplatin and trastuzumab combination
chemotherapy (XP+H; capecitabine, 2,000 mg/m2, twice
daily, days 1–14; cisplatin, 80 mg/m2, day 1, every 3
weeks; trastuzumab, 8 mg/kg in the first cycle followed by 6 mg/kg,
day 1) from the third course of chemotherapy. This regimen was
well-tolerated, with grade 1 fatigue and grade 1 anorexia observed
(16). The patient's symptoms were
greatly relieved by the treatment, and CT imaging demonstrated
regression of liver as well as nodal metastases. A partial response
was maintained for four months; however, the disease progressed
following 5 cycles of XP+H. Despite second and third-line treatment
[paclitaxel (80 mg/m2 on days 1, 8 and 15, every 4
weeks) and nab-paclitaxel (260mg/m2, every 3 weeks)
respectively], the patient succumbed to the disease 10 months after
the initial presentation.
Discussion
Past studies have demonstrated that 7–34% of gastric
cancer cases overexpress HER2 (17–19), and
the rate was reported to be 22% in a recent large-scale
international prospective trial (1).
Since the introduction of trastuzumab, standardization and
optimization of HER2 testing has been highlighted in the management
of advanced gastric cancer. However, HER2 assessment by IHC and
FISH present a substantial risk of false-negative results for
gastric cancer, particularly when biopsy samples were used for the
diagnosis (i.e. unresectable cases) (2–7). Serum
HER2, a simple and less invasive method, assesses a different
aspect of HER2 status from IHC and FISH. Although the clinical
utility of serum HER2 in gastric cancer remains uncertain, a number
of investigators have reported that serum HER2 level correlates
with tissue HER2 status in gastric cancer (15,20–22). In
the present case, the high serum HER2 level was the primary reason
for the IHC reevaluation, and tissue HER2 positivity was eventually
proven. Discrepancy of the IHC results may be due to use of a
different primary antibody, as the same biopsy samples were used
for the initial and second IHC.
There are numerous risk factors for diagnostic error
of HER2 status in gastric cancer, including insufficient number of
biopsy specimens, inadequate formalin fixation protocol,
inexperienced laboratory staff and intratumoral HER2 heterogeneity
(2–7).
As trastuzumab has demonstrated a significant positive effect on
treatment for unresectable HER2-positive gastric cancer, efforts
must be made to minimize HER2 false-negative cases. Recent reviews
concluded that serum HER2 is not useful for breast cancer
management (11,23). However, serum HER2 may be useful to
identify HER2 false-negative gastric cancer as HER2 overexpression
in gastric cancer is frequently heterogeneous.
In conclusion, in the present case, serum HER2 was
useful to identify a HER2-positive gastric cancer that was
initially diagnosed as HER2-negative by IHC. This case suggests
that serum HER2 may be useful to salvage tissue HER2 false-negative
patients who are able to benefit from anti-HER2 treatment. Serum
HER2 is expected to compensate for the aforementioned drawbacks of
IHC in gastric cancer management. Large scale prospective studies
are required.
References
|
1
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Luo H, Li Y, Li J, Cai Z, Su X,
Dai D, Du W, Chen T and Chen M: Intratumoral heterogeneity
determines discordant results of diagnostic tests for human
epidermal growth factor receptor (HER) 2 in gastric cancer
specimens. Cell Biochem Biophys. 62:221–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HE, Park KU, Yoo SB, Nam SK, do Park
J, Kim HH and Lee HS: Clinical significance of intratumoral HER2
heterogeneity in gastric cancer. Eur J Cancer. 49:1448–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rüschoff J, Hanna W, Bilous M, Hofmann M,
Osamura RY, Penault-Llorca F, van de Vijver M and Viale G: HER2
testing in gastric cancer: A practical approach. Mod Pathol.
25:637–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang D, Lu N, Fan Q, Sheng W, Bu H, Jin
X, Li G, Liu Y, Li X, Sun W, et al: HER2 status in gastric and
gastroesophageal junction cancer assessed by local and central
laboratories: Chinese results of the HER-EAGLE study. PLoS One.
8:e802902013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kushima R, Kuwata T, Yao T, Kuriki H,
Hashizume K, Masuda S, Tsuda H and Ochiai A: Interpretation of HER2
tests in gastric cancer: Confirmation of interobserver differences
and validation of a QA/QC educational program. Virchows Arch.
464:539–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carney WP, Neumann R, Lipton A, Leitzel K,
Ali S and Price CP: Monitoring the circulating levels of the
HER2/neu oncoprotein in breast cancer. Clin Breast Cancer.
5:105–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fornier MN, Seidman AD, Schwartz MK, Ghani
F, Thiel R, Norton L and Hudis C: Serum HER2 extracellular domain
in metastatic breast cancer patients treated with weekly
trastuzumab and paclitaxel: Association with HER2 status by
immunohistochemistry and fluorescence in situ hybridization and
with response rate. Ann Oncol. 16:234–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finn RS, Gagnon R, Di Leo A, Press MF,
Arbushites M and Koehler M: Prognostic and predictive value of HER2
extracellular domain in metastatic breast cancer treated with
lapatinib and paclitaxel in a randomized phase III study. J Clin
Oncol. 27:5552–5558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lennon S, Barton C, Banken L, Gianni L,
Marty M, Baselga J and Leyland-Jones B: Utility of serum HER2
extracellular domain assessment in clinical decision making: Pooled
analysis of four trials of trastuzumab in metastatic breast cancer.
J Clin Oncol. 27:1685–1693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leary AF, Hanna WM, van de Vijver MJ,
Penault-Llorca F, Rüschoff J, Osamura RY, Bilous M and Dowsett M:
Value and limitations of measuring HER-2 extracellular domain in
the serum of breast cancer patients. J Clin Oncol. 27:1694–1705.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kontani K, Kuroda N, Hashimoto S, Murazawa
C, Norimura S, Tanaka H, Ohtani M, Fujiwara-Honjo N, Kushida Y,
Date M, et al: Clinical usefulness of human epidermal growth factor
receptor-2 extracellular domain as a biomarker for monitoring
cancer status and predicting the therapeutic efficacy in breast
cancer. Cancer Biol Ther. 14:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borrmann R: Geschwülste des Magens und
Duodenums, Makroskopische Formen. Henke u. Lubarsch. Handbuch d.
sp. Path. usw., 1/IV. 864–871. 1926.
|
|
15
|
Saito M, Yamashita K, Arimura Y, Kaneto H,
Okuda H, Nojima M, Hagiwara T, Suzuki K, Adachi T, Goto A, et al:
Serum HER2 as an adjunct to assess HER2 status for advanced gastric
cancer: A prospective multicenter trial (SHERLOCK). Acta Oncol.
55:309–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). Version 4.0.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed.
January 05–2014
|
|
17
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
Topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai SQ, An X, Wang F, Shao Q, Chen YC,
Kong YN, Chen C, Li C, Luo HY, Liang Y, et al: Serum HER 2
extracellular domain level is correlated with tissue HER 2 status
in metastatic gastric or gastro-oesophageal junction
adenocarcinoma. PloS One. 8:e634582013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narita T, Seshimo A, Suzuki M, Murata J
and Kameoka S: Status of tissue expression and serum levels of HER2
in gastric cancer patients in japan. Hepatogastroenterology.
60:1083–1088. 2013.PubMed/NCBI
|
|
22
|
Oyama K, Fushida S, Tsukada T, Kinoshita
J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I,
et al: Evaluation of serum HER2-ECD levels in patients with gastric
cancer. J Gastroenterol. 50:41–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leyland-Jones B and Smith BR: Serum HER2
testing in patients with HER2-positive breast cancer: The death
knell tolls. Lancet Oncol. 12:286–295. 2011. View Article : Google Scholar : PubMed/NCBI
|