Introduction
Prostate cancer is the second most frequently
diagnosed cancer in men worldwide, with 1.1 million new cases
estimated to have occurred in 2012 (1). Progression into the androgen-independent
stage of prostate cancer is considered to be primarily due to
resistance to apoptosis, and is accompanied by increased
proliferation and survival of cells within the primary or
metastatic tumors (2). At present,
there are no effective therapies available for curing or
controlling the androgen-independent stage of prostate cancer, due
to the high rate of apoptosis resistance. The primary therapeutic
modalities used to treat prostate cancer are surgery, radiation and
hormone therapy (3,4). These modalities have been demonstrated
to have certain curative effects on early-stage prostate cancer;
however, they have various limitations, including injury to the
surrounding normal tissues, drug resistance and tumor recurrence
(4–6).
Gene therapy is a promising method for the treatment
of human diseases. Among the numerous types of anticancer
treatment, gene therapy has gained attention in clinical trials, as
it has low side-effects compared with chemotherapy and radiotherapy
(7). Several methods have been
developed for the delivery of DNA into cells, including chemically
facilitated, vector-mediated, mechanical (8) and electric pulse (9) transfection. In the current clinical
protocols for gene therapy, virus-derived and non-virus-derived
vectors are primarily used to deliver DNA into cells (10). Although viral vectors have high
transfection efficiencies over a wide range of cell targets, major
limitations have occurred during clinical trials, such as the
induction of the immune response to viruses and insertional
mutagenesis (11). These unwanted
side-effects have increased support for the use of non-viral
methods of gene transfer. Non-viral vectors, such as liposomes, are
a promising alternative to viral vectors, as they are safe,
versatile, easy to prepare and simple to scale up. However,
non-viral vectors generally have low transfection efficiencies
(12,13).
The P53 protein is a tumor suppressor that has a
regulatory function associated with cell apoptosis. Furthermore,
the P53 gene is the most commonly mutated gene in a large
proportion of human cancer types. Therefore, the introduction of
wild-type P53 into tumors may be a novel strategy for treating
cancer, by inducing apoptotic death in cancer cells. As loss of P53
function is common in prostate cancer (14), restoring P53 activity is an attractive
target for prostate cancer gene therapy (15).
Several previous reports (16–18) have
described the combined use of sonoporation and microbubbles to
achieve delivery of naked plasmid DNA into cancer cells in tissue
culture-based systems. Sonoporation is an emerging and promising
physical method for cancer gene therapy that typically operates at
a frequency of 1–35 MHz, and has several advantages over other
nonphysical methods of nucleic acid delivery; for example,
sonoporation also has the ability to deliver drugs or small
molecules (19). However, the
transfer efficiency depends on ultrasound frequency, intensity
(20) and the number of microbubbles
(21). Transfer efficiency increases
with increasing ultrasound energy while the frequency decreases
(22). The presence of microbubbles
can also improve transfection efficiency (23). However, due to the high energy
involved in ultrasound treatment, cell lysis frequently occurs and
possibly masks other effects on the surviving cells (24).
Our previous study identified that low-frequency and
low-energy ultrasound combined with microbubbles improved
liposome-mediated transfection of pEGFP DNA into human prostate
cancer cells. Our previous study also demonstrated that
low-frequency and low-energy ultrasound combined with microbubbles
could induce cell membrane damage but resulted in minimum cell
death. Thus, we proposed that the rapid collapse of microbubbles
during sonoporation has major role in gene delivery into cells
(25).
The present study evaluated the improvement of
liposome-mediated transfection of wild-type P53 DNA into human
prostate cancer cells by using low-frequency and low-energy
ultrasound combined with SonoVue microbubbles. The aim of the study
was to develop a novel gene therapy technique that may be used to
treat androgen-independent prostate cancer.
Materials and methods
Cell culture
A human androgen-independent prostate cancer cell
line, PC-3 (null P53), was obtained from the Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were grown in Dulbecco's modified Eagle medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in humidified
air with 5% CO2. PC-3 cells were counted under an optical
microscope (Olympus CX23; Olympus, Tokyo, Japan) using a
hemocytometer (Qiujing XB-K-25; Shanghai Qiujing Biochemical
Reagent and Instrument Co., Ltd., Shanghai, China) and resuspended
at a density of 1×105 cells/ml. The cells were
transferred into polystyrene test tubes (1 ml cells/tube; Weierkang
Medical Supplies Co., Ltd., Taizhou, China) with diameters of 13
mm. Polystyrene test tubes and a Mylab90 ultrasound imaging system
(Esaote, Genoa, Italy) were used, which did not absorb sound waves.
If the sound wave was absorbed, it would make the sound energy
decay. Exposure to ultrasound resulted in no significant effect on
the acoustic permeation ratio. The bottom of the tube was planar,
which allowed it to more closely contact the ultrasound probe.
Ultrasound apparatus and
microbubbles
A low-frequency ultrasonic therapy machine (Shanghai
Institute of Ultrasound in Medicine, Shanghai, China) and a
microbubble echo-contrast agent (SonoVue; Bracco, Milan, Italy)
were used to perform gene transfection. Ultrasound was generated
using a 21-kHz ultrasound probe covered with (Shuangyi Medical
Equipment Co., Tianjin, China) transmission gel and the spatial
average temporal average intensity was 46 mW/cm2. The
duty cycle was controlled at 20% (2 sec on, 8 sec off) for 5 min.
The diameter of the ultrasound probe was 13 mm, which was the same
as the diameter of the test tube. In all of the experiments, a
clamp was attached to a metal stud to keep the transducer facing
directly upward (25).
Upon use, SonoVue was reconstituted in 5 ml
phosphate-buffered saline (PBS) that was 2–5 µm in diameter.
Preparation of plasmid DNA
Wild-type P53 plasmid DNA (donated by the Center
Laboratory of Sixth People's Hospital Affiliated to Shanghai Jiao
Tong University) was prepared using an EZNA Plasmid Miniprep kit II
(Omega Bio-Tek, Inc., Norcross, GA, USA). Wild-type P53 plasmid DNA
was identified by two restriction sites; enzyme digestion
(SalI or XhoI; Fermentas; Thermo Fisher Scientific,
Inc.) was performed, followed by electrophoresis. Briefly, the
amplified P53 DNA fragment was purified from the corresponding band
in the agarose gel and incubated with SalI/XhoI at 37°C for 2 h.
Next, digestion products were purified from the corresponding band
in the agarose gel. The processed P53 fragment was then cloned into
EZNA using T4 DNA ligase (Thermo Fisher Scientific, Inc.). The
recombinant plasmid was transformed into DH5α (Beyotime Institute
of Biotechnology, Nanjing, China) and confirmed by PCR of the
bacterial solution and enzymatic digestion.
Cell lysis
PC-3 cells were divided into four groups, as
follows: The control group, the SonoVue alone group, the ultrasound
alone group and the ultrasound combined with SonoVue group. Prior
to ultrasound irradiation, 200 µl SonoVue was added to each SonoVue
alone and ultrasound combined with SonoVue group test tube.
Immediately after exposure to ultrasound, the cell numbers were
counted under an optical microscope (×200 magnification; 4
images/group; Olympus CX23; Olympus) using a hemocytometer (Qiujing
XB-K-25; Shanghai Qiujing Biochemical Reagent and Instrument Co.,
Ltd.). Cell lysis was measured using the following formula: Cell
lysis (%) = [1 - (number of viable cells per image following
therapy / total number of cells per image prior to therapy)] ×
100.
Gene transfer
Transfection was performed using a Lipofectamine
2000 kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, at a plasmid:liposome (Invitrogen;
Thermo Fisher Scientific, Inc.) ratio of 1:2. Prior to ultrasound
irradiation, these transfection reagents were added to the
suspension of PC-3 cells in each sample. PC-3 cells were
resuspended in polystyrene sample test tubes at a density of
1×105 cells/ml and divided into eight groups, as
follows: Group A (SonoVue + wild-type P53), group B (ultrasound +
wild-type P53), group C (SonoVue + ultrasound + wild-type P53),
group D (liposome + wild-type P53), group E (liposome +SonoVue +
wild-type P53), group F (liposome + wild-type P53+ultrasound),
group G (liposome + wild-type P53 + ultrasound + SonoVue) and the
control group (wild-type P53). In groups A, C, E and G, 200 µl
SonoVue was added to each tube. Following ultrasound exposure, the
cell suspensions were transferred onto 12-well plates.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Wild-type P53 mRNA expression was examined by
RT-qPCR. Total cellular RNA was extracted from frozen tumor tissues
using the RNAiso Plus kit (Takara Biotechnology, Co., Ltd., Dalian,
China), according to the manufacturer's protocol, and purified
using cold 75% ethanol precipitation. A DNase step was performed
using a PrimeScript™ RT reagent kit with gDNA Eraser (Takara
Biotechnology, Co., Ltd.). RNA concentration and quality were
measured using the NanoDrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). A volume of 5 µl of purified RNA was used
as the template for cDNA synthesis in the presence of 15 µl of 5X
RT buffer, 1 µl random primer (100 pmol/µl), 1 µl reverse
transcriptase and 8 µl RNAse-free water, all purchased from Takara
Biotechnology Co., Ltd. The reverse transcriptase was incubated at
25°C for 10 min, 60°C for 10 min and 70°C for 10 min. The
subsequent PCR mixture (50 µl) consisted of 1 µl cDNA, 25 µl of 2X
PCR buffer, 0.6 µl of each primer (25 pmol/µl) and 22.8 µl
RNAse-free water, all purchased from Takara Biotechnology Co., Ltd.
The primers used (Takara Biotechnology Co., Ltd.) were as follows:
P53 forward, 5′-GACAGCCAAGTCTGTGACTTG-3′ and reverse,
5′-CGCTATCTGAGCAGCGCTCATG-3′; and glyceraldehyde 3-phosphate
dehydrogenase, forward 5′-TGACAACAGCCTCAAGATCATC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTGG-3′. Amplification was performed for 1
cycle at 95°C for 3 min, followed by 40 cycles at 95°C for 10 sec
and 60°C for 10 sec. The cycle fluorescence intensity generated
using SYBR Green Master mix (Takara Biotechnology, Co., Ltd.) was
recorded for each sample across 5 repeats using an ABI PRISM® 7500
Sequence Detection System (Thermo Fisher Scientific, Inc.), and the
mean fluorescence intensity was calculated and statistically
analyzed. The relative expression of each gene was calculated and
normalized using the 2-∆∆Cq method (26).
Western blot analysis
The protein expression of wild-type P53 was examined
by western blotting 24 h after the PC-3 cells were transfected with
wild-type P53, as previously described (27). Briefly, the cell line was solubilized
in cold radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). The proteins were separated using 12%
sodium dodecyl sulfate polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene difluoride membrane (Thermo
Fisher Scientific Inc.). The membrane was incubated with PBS
containing 5% milk at room temperature for 2 h. Next, the membrane
was incubated with Tris-buffered saline with 0.1% Tween 20
containing 5% milk and mouse anti-human P53 (catalog no. ab1101;
dilution, 1:1,000; Abcam, Cambridge, UK) or β-actin (catalog no.
ab8226; dilution, 1:500; Abcam) monoclonal antibodies overnight at
4°C. Following the incubation, the membrane was incubated again
with horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (catalog no. ab6789; dilution, 1:2,000; Abcam) at room
temperature for 1 h. An enhanced chemiluminescence kit (Beyotime
Institute of Biotechnology) was used to perform chemiluminescence
detection. The relative protein expression was analyzed by
Image-Pro® Plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA), represented as the density ratio vs.
β-actin.
Cell proliferation
Immediately after gene transfection, each group of
cells was seeded at 3×103 cells/well in 96-well plates.
After 24 h, 100 µl Cell Counting Kit-8 (Dojindo Laboratories,
Kumamoto, Japan) was added and the plates were incubated for 3 h.
The optical density for each well was measured using a microculture
plate reader (FL600; Bio-Tek, Winooski, VT, USA) at a wavelength of
450 nm (28).
Cell apoptosis
Following ultrasound treatment, each group of cells
was incubated for an additional 24 h in 6-well plates. The cells
were evaluated for apoptosis using a fluorescein isothiocyanate
(FITC)-labeled Annexin V and propidium iodide (PI) double staining
kit (Becton Dickinson, Franklin Lakes, NJ, USA), as previously
described (29). After the 24-h
incubation, cells were harvested, washed twice with PBS and
resuspended with 0.5 ml PBS at a cell density of 1×106
cells/ml. Annexin V (5 µl) and PI (10 µl) were added to the wells
in the dark. After 10 min incubation, the cells were analyzed by
flow cytometry (BD FACSCalibur; Becton Dickinson) to determine the
levels of apoptosis. Annexin V-FITC(+)/PI(−) cells were considered
early apoptotic cells, while cells that were Annexin
V-FITC(+)/PI(+) were considered late apoptotic cells. Therefore,
the total apoptotic cell count equaled the sum of the
Annexin-V-FITC(+)/PI(−) and the Annexin-V-FITC(+)/PI(+) cells
(30).
Statistical analysis
Data are expressed as mean ± standard deviation.
SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA) was used
to perform analysis of variance and t-tests to determine
differences among the groups. A least significant difference
post-hoc test was also performed. P<0.05 was considered to
indicate a statistically significant difference. Experiments were
repeated three times.
Results
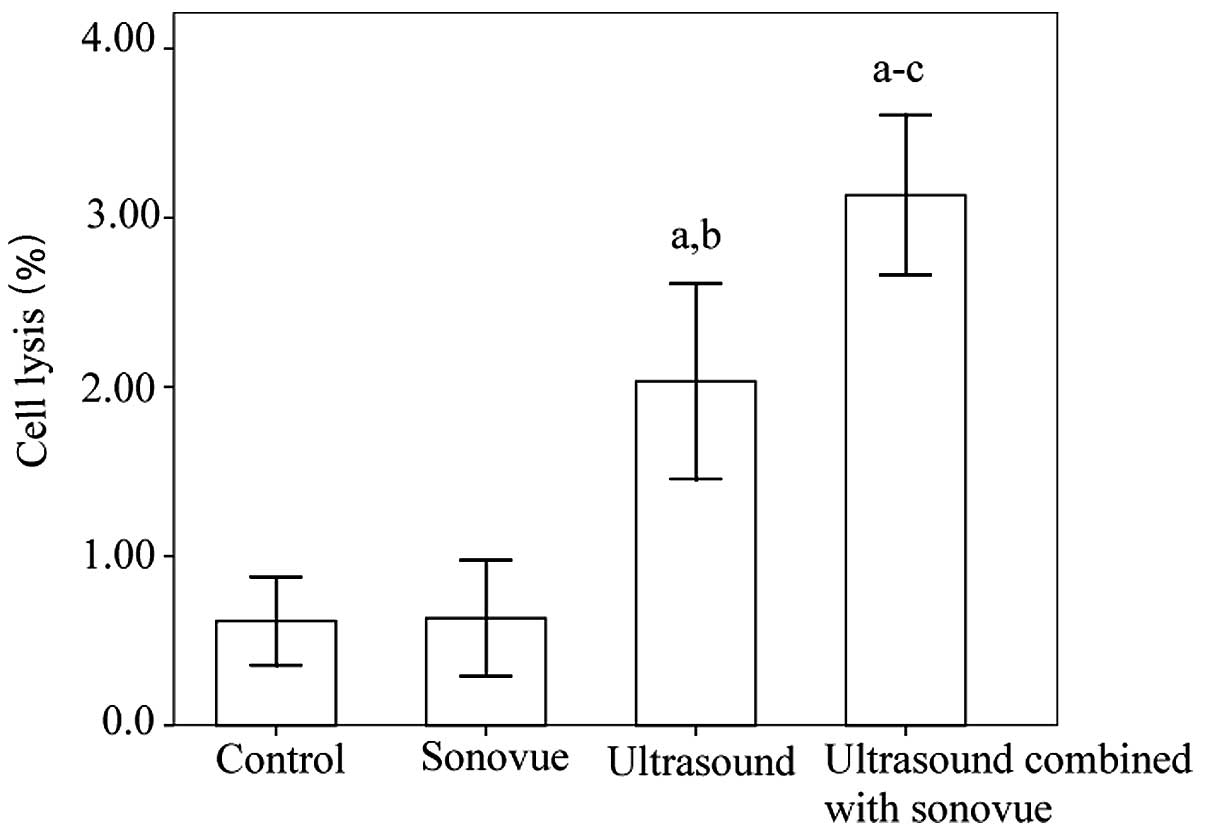
Detection of cell lysis
Cell lysis analysis is presented in Fig. 1. To assess cell lysis, the cells were
examined immediately after ultrasound exposure. The ultrasound
group and the ultrasound combined with SonoVue group exhibited
significantly greater cell lysis compared the control group and the
SonoVue alone group (P<0.001). Furthermore, significantly higher
levels of cell lysis were observed in the ultrasound combined with
SonoVue group compared with the ultrasound group. There was no
significant difference between the control group and the SonoVue
alone group (P=0.945).
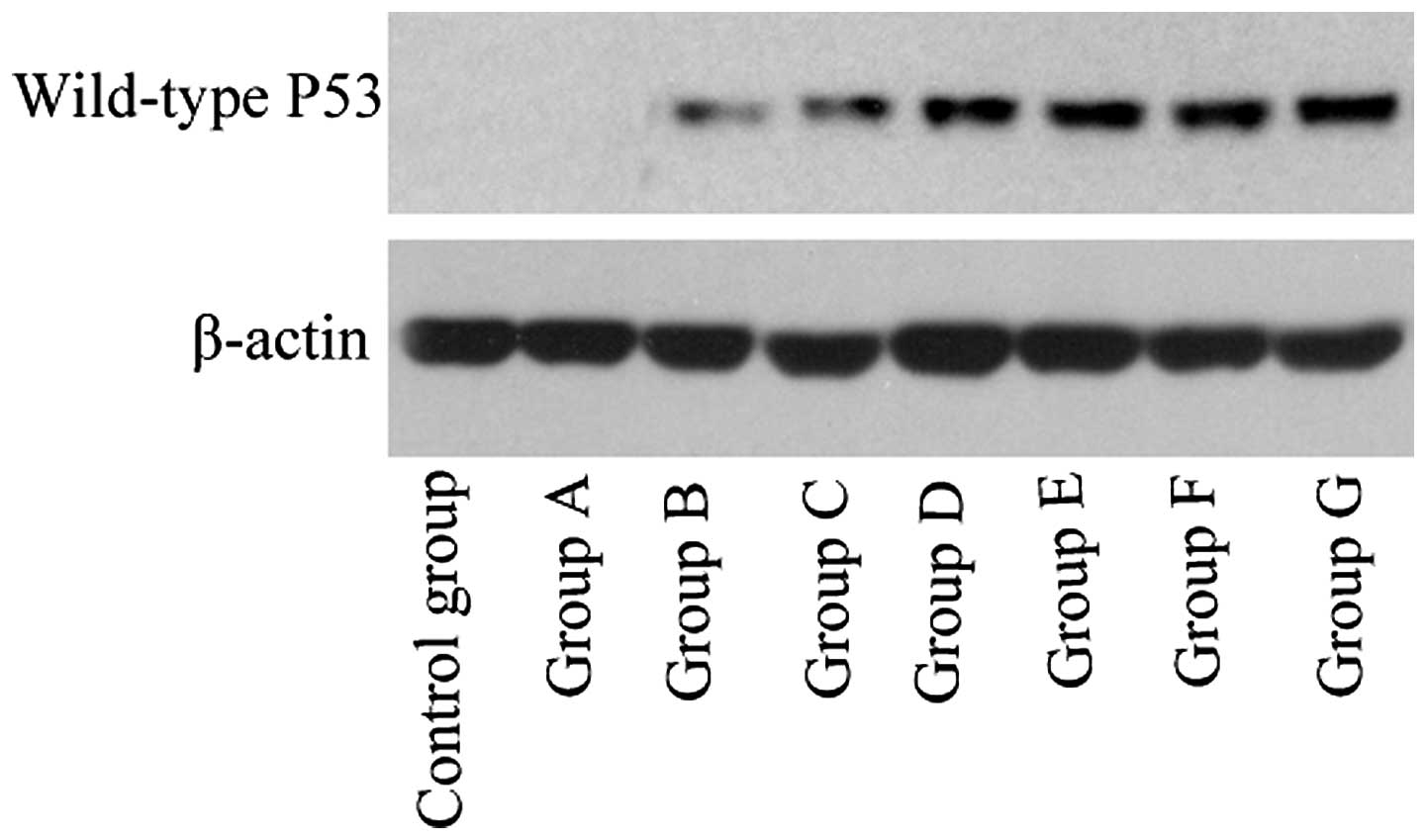
Detection of gene transfection
efficiency
The results for gene transfection efficiency, as
determined by RT-qPCR and western blotting, are presented in
Table I and Fig. 2.
 | Table I.Wild-type P53 transfection efficiency
of PC-3 cells in groups A-G and the control group following gene
transfection. |
Table I.
Wild-type P53 transfection efficiency
of PC-3 cells in groups A-G and the control group following gene
transfection.
|
| Relative wild-type
P53 expression |
|---|
|
|
|
|---|
| Group | mRNA | Protein |
|---|
| Control | Null | Null |
| A | Null | Null |
| B | 0.16±0.01 | 0.24±0.04 |
| C |
0.75±0.10a |
0.42±0.05a |
| D |
1.49±0.14a,b |
0.60±0.05a,b |
| E |
1.74±0.17a,b |
0.66±0.03a,b |
| F |
2.80±0.19a–d |
0.77±0.03a–d |
| G |
4.00±0.51a–e |
0.87±0.02a–e |
In group G (liposome + wild-type P53 + ultrasound +
SonoVue), RT-qPCR and western blotting revealed obvious P53 mRNA
and protein expression, respectively, in the PC-3 cells transfected
with wild-type P53, while all other cell groups exhibited
significantly lower expression levels (P<0.001).
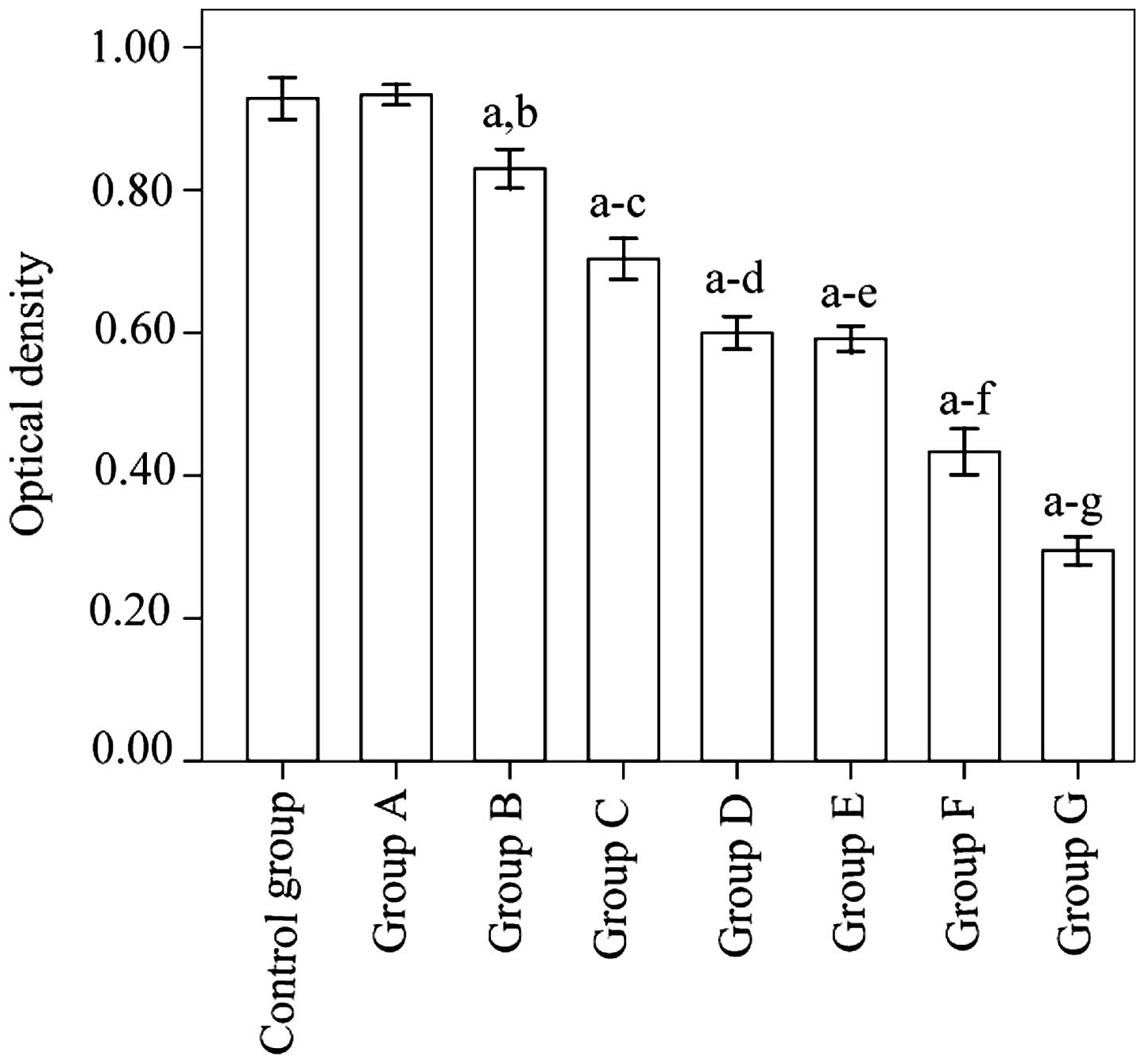
Measurement of cell proliferation
Cell proliferation levels in group G was
significantly suppressed relative to the control group
(P<0.001). Furthermore, the suppression observed in group G was
significantly greater than in any of the other groups (P<0.001)
(Fig. 3).
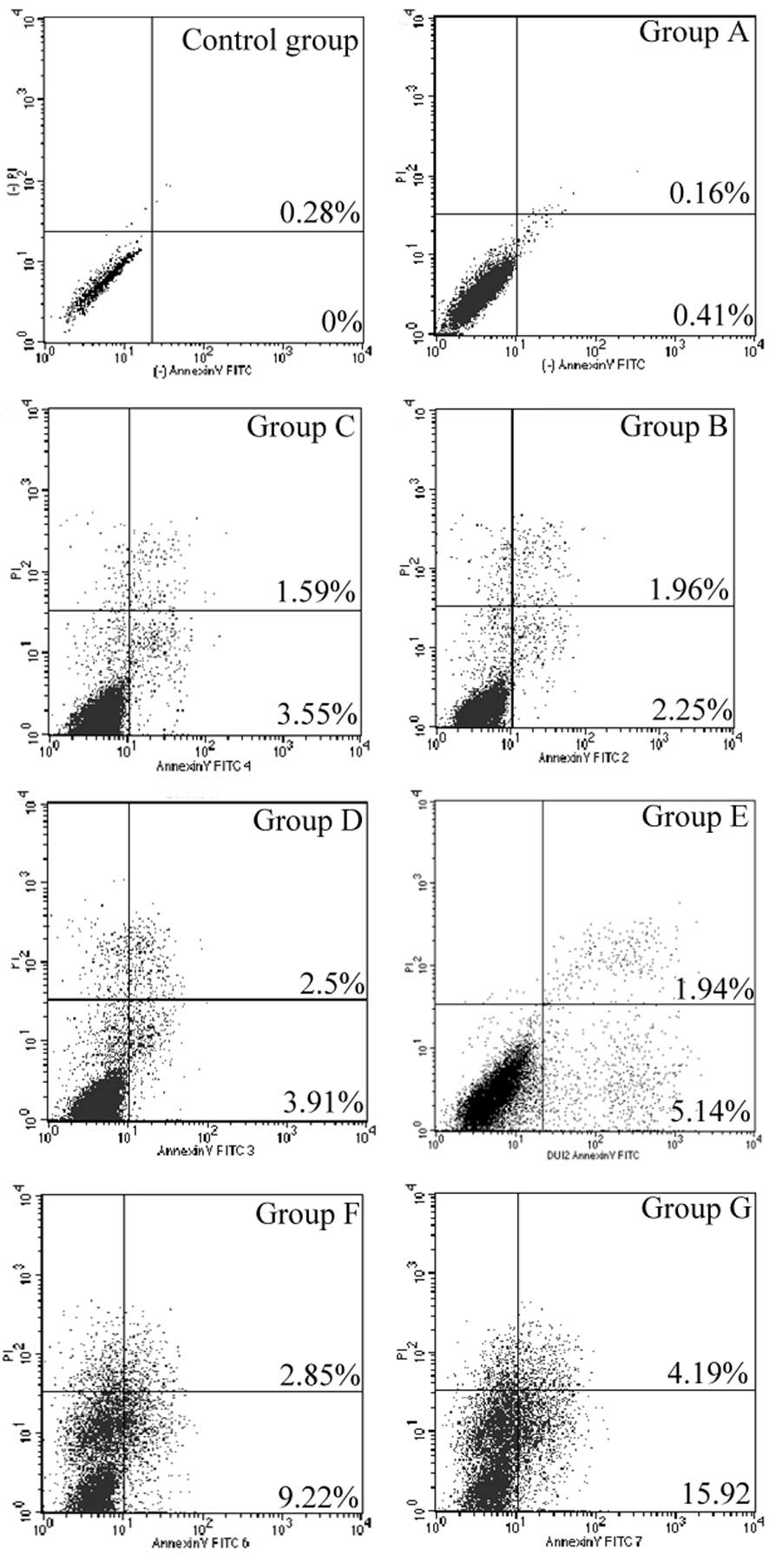
Detection of cell apoptosis
Cell apoptosis levels in group G were significantly
increased relative to the control group (P<0.001). In addition,
the proportion of cell apoptosis in group G was significantly
greater than in all the other groups (P<0.001) (Fig. 4; Table
II).
 | Table II.Apoptosis of PC-3 cells in groups A-G
and the control group following gene transfection. |
Table II.
Apoptosis of PC-3 cells in groups A-G
and the control group following gene transfection.
| Group | Apoptosis,% |
|---|
| Control | 0.37±0.05 |
| A | 0.48±0.05 |
| B |
4.10±0.26a,b |
| C |
5.78±0.35a–c |
| D |
6.81±0.25a–d |
| E |
6.91±0.24a–d |
| F |
12.69±0.72a–f |
| G |
21.23±1.58a–g |
Discussion
The PC-3 cell line was selected for use in the
current study, as it is a human androgen-independent prostate
cancer cell line that does not express P53 (wild-type or mutant).
Therefore, if wild-type P53 is detected in this cell line it must
be due to successful transfection (28).
In the present study, ultrasound significantly
combined with microbubbles improved wild-type P53 transfection
efficiency of liposomes, and the efficiency was greater in group G
compared with in groups A, B, C, D, E and F, and the control group.
Thus, gene transfection was improved by increasing cell
permeabilization using microbubbles and ultrasound to deliver
molecules into the cytoplasm. Collapsing microbubbles and
cavitation bubbles created by this collapse generate impulsive
pressures, including liquid jets and shockwaves, that may damage
cell membranes and cause transient membrane permeability, allowing
exogenous molecules to enter into cells. The impulsive pressures
can affect neighboring cells, as the shockwave propagation distance
from the center of a cavitation bubble is considerably larger than
the maximum radius of the cavitation bubble (31). Our previous study demonstrated that
low-frequency and low-energy ultrasound combined with microbubbles
could induce cell membrane damage. In addition, our previous study
used transmission electron microscopy to detect cell membrane
discontinuity and observed gaps in the cell membranes following
ultrasound exposure (25). The
results indicated that low-frequency and low-energy ultrasound may
induce cell membrane damage to improve gene delivery.
In the present study, transfection with wild-type
P53 suppressed PC-3 cell proliferation and increased apoptosis.
Loss of normal P53 function and/or defects in the P53 signaling
pathway, caused by missense mutations or deletions, occur in
>50% of types of human cancer, including prostate cancer. The
P53 protein is a tumor suppressor gene product that can induce
apoptosis, therefore, these molecular alterations are associated
with resistance to cell death (32).
Loss of P53 function as a result of mutation or deletion in the P53
gene occurs in 24% of primary prostate tumors (33). Considering the aforementioned
functions of P53 expression, the possibility of reactivating the
P53 pathway has been extensively investigated in several types of
cancer (34).
In previous studies, ultrasound frequencies of 1–3
MHz have been used (11,35). However, the present study used
low-frequency (21 kHz) and low-energy ultrasound, which is
associate with easy penetration of the organism, less tissue
absorption and reduced tissue injury. Low-frequency ultrasound
predominantly induces mechanical and cavitation effects, and the
temperature increase through the thermal effect is virtually
negligible (36). The results of the
present study demonstrated that low-frequency and low-energy
ultrasound combined with microbubbles minimally induced the lysis
of PC-3 cells. Cell lysis <5% was considered as minimal damage.
Considering these advantages, low-frequency and low-energy
ultrasound shows promise for future use in cancer therapy.
In conclusion, the present study demonstrated that
sonoporation, in the presence of microbubbles, was a promising
technique for improving liposome transfer of wild-type P53 genes
into prostate cancer cells and provided an experimental model for
clinical gene therapy. Additionally, liposome-mediated transfection
combined with low-frequency and low-energy ultrasound to induce the
destruction of microbubbles was revealed as a feasible and
efficient method for wild-type P53 delivery into prostate cancer
cells. Although the exact mechanisms underlying efficient gene
transfection remain to be elucidated, the rapid collapse of
microbubbles during sonoporation is considered to have a major role
in gene delivery into cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81271597 and
81401421).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu AH, Hu ZM, Qu JB, Liu SM, Syed AK, Yuan
HQ and Lou HX: Cyclic bisbibenzyls induce growth arrest and
apoptosis of human prostate cancer PC3 cells. Acta Pharmacol Sin.
31:609–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mangar SA, Huddart RA, Parker CC,
Dearnaley DP, Khoo VS and Horwich A: Technological advances in
radiotherapy for the treatment of localised prostate cancer. Eur J
Cancer. 41:908–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka G, Hirata Y, Goldenberg SL,
Bruchovsky N and Aihara K: Mathematical modelling of prostate
cancer growth and its application to hormone therapy. Philos Trans
A Math Phys Eng Sci. 368:5029–5044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lecornet E, Ahmed HU, Moore C and Emberton
M: Focal therapy for prostate cancer: A potential strategy to
address the problem of overtreatment. Arch Esp Urol. 63:845–852.
2010.PubMed/NCBI
|
|
6
|
Baumert H: Salvage treatments for
prostatic radiation failure. Cancer Radiother. 14:442–445. 2010.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verma IM and Somia N: Gene
therapy-promises, problems and prospects. Nature. 389:239–242.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wyber JA, Andrews J and D'Emanuele A: The
use of sonication for the efficient delivery of plasmid DNA into
cells. Pharm Res. 14:750–756. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whelan J: Electroporation and ultrasound
for gene and drug delivery. Drug Delivery Today. 11:585–586. 2002.
View Article : Google Scholar
|
|
10
|
Danialou G, Comtois AS, Dudley RW,
Nalbantoglu J, Gilbert R, Karpati G, Jones DH and Petrof BJ:
Ultrasound increase plasmid-mediated gene transfer to dystrophic
muscles without collateral damage. Mol Ther. 5:687–693. 2002.
View Article : Google Scholar
|
|
11
|
Negishi Y, Omata D, Iijima H, Takabayashi
Y, Suzuki K, Endo Y, Suzuki R, Maruyama K, Nomizu M and Aramaki Y:
Enhanced laminin-derived peptide AG73-mediated liposomal gene
transfer by bubble liposomes and ultrasound. Mol Pharm. 7:217–226.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Audouy SA, de Leij LF, Hoekstra D and
Molema G: In vivo characteristics of cationic liposomes as delivery
vectors for gene therapy. Pharm Res. 19:1599–1605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirko A, Tang F and Hughes JA: Cationic
lipid vectors for plasmid DNA delivery. Curr Med Chem.
10:1185–1193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin VC, Huang CY, Lee YC, Yu CC, Chang TY,
Lu TL, Huang SP and Bao BY: Genetic variations in TP53 binding
sites are predictors of clinical outcomes in prostate cancer
patients. Arch Toxicol. 88:901–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han L, Zhao J, Liu J, Duan XL, Li LH, Wei
XF, Wei Y and Liang XJ: A universal gene carrier platform for
treatment of human prostatic carcinoma by p53 transfection.
Biomaterials. 35:3110–3120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan C, Qian J, Li F and Li H:
Ultrasound-targeted microbubble destruction enhances
polyethylenimine-mediated gene transfection in vitro in human
retinal pigment epithelial cells and in vivo in rat retina. Mol Med
Rep. 12:2835–2841. 2015.PubMed/NCBI
|
|
17
|
Sugano M, Negishi Y, Endo-Takahashi Y,
Hamano N, Usui M, Suzuki R, Maruyama K, Aramaki Y and Yamamoto M:
Gene delivery to periodontal tissue using Bubble liposomes and
ultrasound. J Periodontal Res. 49:398–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Xie M, Wang X, Lv Q and Ding S:
Efficient gene delivery to myocardium with ultrasound targeted
microbubble destruction and polyethylenimine. J Huazhong Univ Sci
Technolog Med Sci. 28:613–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zolochevska O, Xia X, Williams BJ, Ramsay
A, Li S and Figueiredo ML: Sonoporation delivery of interleukin-27
gene therapy efficiently reduces prostate tumor cell growth in
vivo. Hum Gene Ther. 22:1537–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tachibana K and Tachibana S: Transdermal
delivery of insulin by ultrasonic vibration. J Pharm Pharmacol.
43:270–271. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newman CM and Bettinger T: Gene therapy
progress and prospects: Ultrasound for gene transfer. Gene Ther.
14:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan
W and Gao Y: Investigation into the impact of diagnostic ultrasound
with microbubbles on the capillary permeability of rat hepatomas.
Ultrasound Med Biol. 39:628–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Sonoporation: Gene transfer
using ultrasound. World J Methodol. 3:39–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marentis TC, Kusler B, Yaralioglu GG, Liu
S, Haeggström EO and Khuri-Yakub BT: Microfluidic sonicator for
real-time disruption of eukaryotic cells and bacterial spores for
DNA analysis. Ultrasound Med Biol. 31:1265–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai WK, Wu ZH, Shen E, Zhang JZ and Hu B:
The improvement of liposome-mediated transfection of pEGFP DNA into
human prostate cancer cells by combining low-frequency and
low-energy ultrasound with microbubbles. Oncol Rep. 27:475–480.
2012.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott SL, Earle JD and Gumerlock PH:
Functional p53 increases prostate cancer cell survival after
exposure to fractionated doses of ionizing radiation. Cancer Res.
63:7190–7196. 2003.PubMed/NCBI
|
|
28
|
Sicklick JK, Li YX, Jayaraman A, Kannangai
R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS
and Diehl AM: Dysregulation of the Hedgehog pathway in human
hepatocarcinogenesis. Carcinogenesis. 27:748–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XB, Liu QH, Wang P, Zhang K, Tang W
and Wang BL: Enhancement of apoptosis by sonodynamic therapy with
protoporphyrin IX in isolate sarcoma 180 cells. Cancer Biother
Radiopharm. 23:238–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang HY, Tsai KC, Cheng WH, Shieh MJ, Lou
PJ, Lin WL and Chen WS: The effects of power on-off durations of
pulsed ultrasound on the destruction of cancer cells. Int J
Hyperthermia. 23:371–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kodama T, Tomita Y, Koshiyama K and
Blomley MJ: Transfection effect of microbubbles on cells in
superposed ultrasound waves and behavior of cavitation bubble.
Ultrasound Med Biol. 32:905–914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ecke TH, Schlechte HH, Hübsch A, Lenk SV,
Schiemenz K, Rudolph BD and Miller K: TP53 mutation in prostate
needle biopsies-comparison with patients follow-up. Anticancer Res.
27:4143–4148. 2007.PubMed/NCBI
|
|
33
|
Gupta K, Thakur VS, Bhaskaran N, Nawab A,
Babcook MA, Jackson MW and Gupta S: Green tea polyphenols induce
p53-dependent and p53-independent apoptosis in prostate cancer
cells through two distinct mechanisms. PLoS One. 7:e525722012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Li Y, Hu J, Wang B, Zhao L, Ji K,
Guo B, Yin D, Du Y, Kopecko DJ, et al: Plasmid-based E6-specific
siRNA and co-expression of wild-type p53 suppresses the growth of
cervical cancer in vitro and in vivo. Cancer Lett. 335:242–250.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang JF, Wu CJ, Zhang CM, Qiu QY and Zheng
M: Ultrasound-mediated microbubble destruction facilitates gene
transfection in rat C6 glioma cells. Mol Biol Rep. 36:1263–1267.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manome Y, Nakayama N, Nakayama K and
Furuhata H: Insonation facilitates plasmid DNA transfection into
the central nervous system and microbubbles enhance the effect.
Ultrasound Med Biol. 31:693–702. 2005. View Article : Google Scholar : PubMed/NCBI
|