Introduction
Osteosarcoma is the most commonly observed primary
malignant cancer of the bone in children, and possesses high
incidence and mortality rates (1).
The tumor predominantly arises from the metaphyses of the long
bones with active bone growth and repairation, such as the knee
joint, lower femur and upper tibia. As osteosarcoma is considered
to be a radioresistant tumor, chemotherapy is the primary approach
for the treatment of osteosarcoma (2). However, the currently utilized
chemotherapy regimens demonstrate low efficacy for the treatment of
this tumor (3). Current
chemotherapeutic drugs, including ifosfamide, cisplatin and
high-dose methotrexate, have a number of side-effects and their use
may result in acquired drug resistance in osteosarcoma cells
(4). Furthermore, the prognosis of
osteosarcoma is poor and >30% of patients succumb to pulmonary
metastases within 5 years of diagnosis (5). Therefore, there is an urgent requirement
for the development of novel effective therapeutic drugs for the
treatment of osteosarcoma.
Yes-associated protein (YAP), a transcriptional
co-activator, is a key regulator of the Hippo signaling pathway
(6). When YAP is recruited to the
nucleus, transcription of cell proliferation-promoting and
anti-apoptotic genes is continuously activated (7,8). High
expression of YAP has been observed in a number of types of tumor,
including osteosarcoma, hepatocellular, colorectal, ovarian, breast
and lung cancer cases, as well as gastric carcinoma, and has been
reported to be correlated with a poor prognosis (9–12). These
findings suggest that YAP may contribute to a malignant cellular
phenotype and therefore may be an important target for anticancer
drugs (13).
Dobutamine is a synthetic catecholamine developed by
Eli Lilly and Company in the 1970s (14). It has been widely used as an inotropic
drug for hemodynamic support in the treatment of congestive heart
failure, as well as cardiogenic and septic shock (15). A previous study demonstrated that
dobutamine is able to attenuate YAP-dependent transcription by
inhibiting its nuclear translocation (16).
In the present study, the effect of dobutamine on
the proliferation, apoptosis and invasiveness of the MG-63 human
osteosarcoma cell line was investigated. The results of the present
study demonstrated the potential effectiveness of dobutamine for
the treatment of osteosarcoma.
Materials and methods
The MG-63 human osteosarcoma cell line was purchased
from the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Dulbecco's Modified Eagle's Medium (DMEM) and
fetal bovine serum (FBS) were obtained from GE Healthcare Life
Sciences (Logan, UT, USA). Propidium iodide (PI) and dobutamine
were obtained from Sigma-Aldrich (St. Louis, MO, USA). The Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
obtained from Beckman Coulter, Inc. (Brea, CA, USA).
Cell culture
MG-63 cells were grown in medium at 37°C in an
atmosphere with 5% CO2. Culture medium supplemented with
10% FBS, 100 U/ml penicillin (Gibco; Thermo Fisher Scientific Inc.,
Waltham, MA, USA), 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific Inc.) and DMEM was used for MG-63 culture.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The influence of dobutamine treatment on cell
viability was determined using an MTT assay. Cells were seeded into
a 96-well plate (Corning, New York, NY, USA) overnight at 37°C and
incubated with various concentrations of dobutamine (1, 5, 10, 25
and 50 µM) for 12, 24, 48 and 72 h. Following the indicated
treatments, the cells were incubated with MTT (0.25 mg/ml;
Sigma-Aldrich) in phosphate-buffered saline (PBS; Gibco; Thermo
Fisher Scientific Inc.) for 4 h at 37°C, followed by removal of the
medium and addition of 1 ml 100% dimethyl sulfoxide (Beyotime
Institute of Biotechnology, Shanghai, China) to solubilize the
MTT-formazan product. The absorbance at 490 nm was determined using
an automatic multi-well spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The inhibitory rate of cell growth was
calculated as [1-treatment group/control group)]x100. The growth
curve was drawn using time as the abscissa and inhibition rate as
the ordinate. Each dobutamine dose was used in triplicate, and the
MTT assay was repeated at least twice.
Flow cytometric analysis
The rate of apoptosis and percentage of cells in G1,
S and G2/M phases was measured by flow cytometry. Following
treatment of the experimental groups in MTT for 24 h, cells were
harvested, trypsinized, washed twice with PBS and resuspended in
binding buffer (Beyotime Institute of Biotechnology). The cells
were subsequently stained with Annexin V-FITC and PI according to
the manufacturer's protocol and analyzed by flow cytometry. The
cell suspension was incubated with 50 µg/ml PI solution and 50 U/ml
RNase (Beyotime Institute of Biotechnology) for 30 min in order to
observe the cell cycle stage. Flow cytometric analysis was
performed on a BD FACSCaliber™ using CellQuest software, version
5.1 (BD Biosciences, Franklin Lakes, NJ, USA).
Cell invasion analysis
The effect of treatment with dobutamine on the
invasion of MG-63 cells was investigated using Transwell chambers
with polycarbonate filters (pore size of 8 µm; Beyotime Institute
of Biotechnology). MG-63 cells were seeded into the upper chamber
at a density of 1×105 cells/ml and incubated in 0.6 ml
DMEM medium containing 10% FBS and various concentrations (1, 5,
10, 25 and 50 µM) of dobutamine. The lower chamber was filled with
0.6 ml DMEM medium containing 20% FBS. Following 24 h of incubation
at 37°C, cells on the upper filter that had not migrated through
were removed by wiping, and the remaining cells were fixed in 4%
paraformaldehyde (Beyotime Institute of Biotechnology) for a total
of 1 h. Cells that had migrated through the filter were stained
using hematoxylin (Beyotime Institute of Biotechnology) and
visualized and counted under a microscope (Olympus IX53; Olympus,
Tokyo, Japan).
Western blot analysis
The present study examined the levels of protein
expression of caspase-3 and caspase-9 in MG-63 cells prior to and
following treatment with dobutamine. The cells were treated with
the different concentrations of dobutamine (1, 5, 10, 25 and 50
µM). The cells were then harvested in 5 ml of medium, pelleted by
centrifugation (1,000 × g for 5 min at 4°C), washed twice using
ice-cold PBS and lysed in ice-cold HEPES buffer (50 mmol/l; pH7.5),
10 mmol/l NaCl, 5 mmol/l MgCl2, 1 mmol/l
ethylenediaminetetraacetic acid (all Beyotime Institute of
Biotechnology), 110% glycerol (v/v), 1% Triton X-100 (v/v 1X
complete), a cocktail of SigmaFast protease inhibitors (1X
complete; Sigma-Aldrich), followed by treatment with 1 mg/l
dobutamine on ice for 30 min. The cell lysates were clarified by
centrifugation (15,000 × g for 10 min at 4°C), and the supernatants
were analyzed immediately or stored at −80°C until required.
Equivalent quantities of protein (50 µg) from total cell lysates
were resolved by sodium dodecyl sulfate polyacrylamide gel
electrophoresis using precast 12% BIS-TRIS gradient gels and
transferred onto polyvinylidene difluoride membranes. Membranes
were blocked overnight at 4°C using blocking buffer [5% skimmed
dried milk (v/v), 150 mmol/l NACl, 10 mmol/l Tris (pH 8.0) and
0.05% Tween 20 (v/v); Beyotime Institute of Biotechnology].
Proteins were detected by incubation in blocking buffer overnight
at 4°C with the following primary antibodies: Mouse anti-human
monoclonal caspase-3 antibody (1:10,00 dilution; sc-65496), mouse
anti-human monoclonal caspase-9 antibody (1:10,00 dilution;
sc-56073) and mouse anti-human monoclonal GADPH antibody (1:10,00
dilution; sc-47778) (all Santa Cruz Biotechnology, Inc, Dallas, TX,
USA). Unbound antibody was removed by washing with Tris-buffered
saline (pH7.2) containing 0.5% Tween 20 (TBS-T; Beyotime Institute
of Biotechnology). The membrane was subsequently incubated at room
temperature with horseradish peroxidase-conjugated secondary
antibody. Subsequent to washing with TBS-T three times, bands were
visualized by enhanced chemiluminescence system (Pierce
Biotechnology, Rockford, IL, USA), and the protein intensities were
quantified using AlphaEaseFC 4.1.0 software (Alpha Innotech, San
Leandro, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS
version 15.0 (SPSS Inc., Chicago, IL, USA). Comparisons between two
samples (experimental and control group) were employed by Student's
t-test. P<0.05 was considered to represent a statistically
significant difference.
Results and Discussion
Dobutamine inhibits the proliferation
of MG-63 osteosarcoma cells
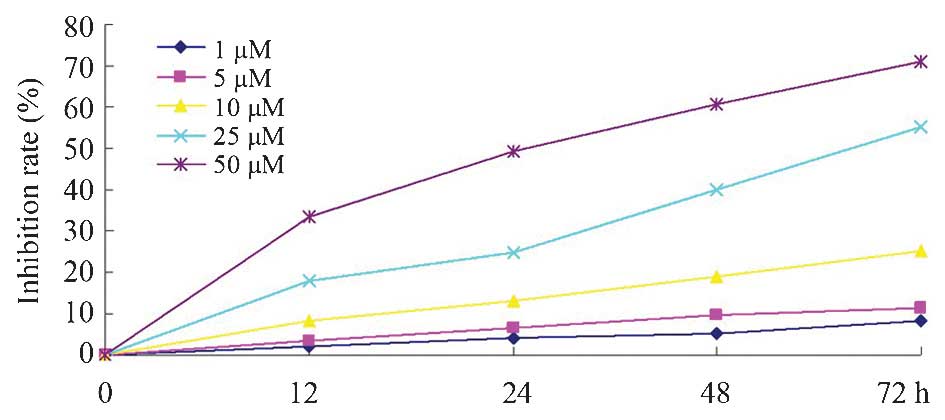
The results of the present study revealed that
dobutamine significantly inhibited cell proliferation in a time-
and concentration-dependent manner compared with the control group.
As demonstrated by the proliferation inhibition graph (Fig. 1), treatment with 10, 25 and 50 µM
dobutamine had a significant inhibitory effect on the survival of
MG-63 cells (P=0.032).
Dobutamine augments cell apoptosis and
arrests the cell cycle
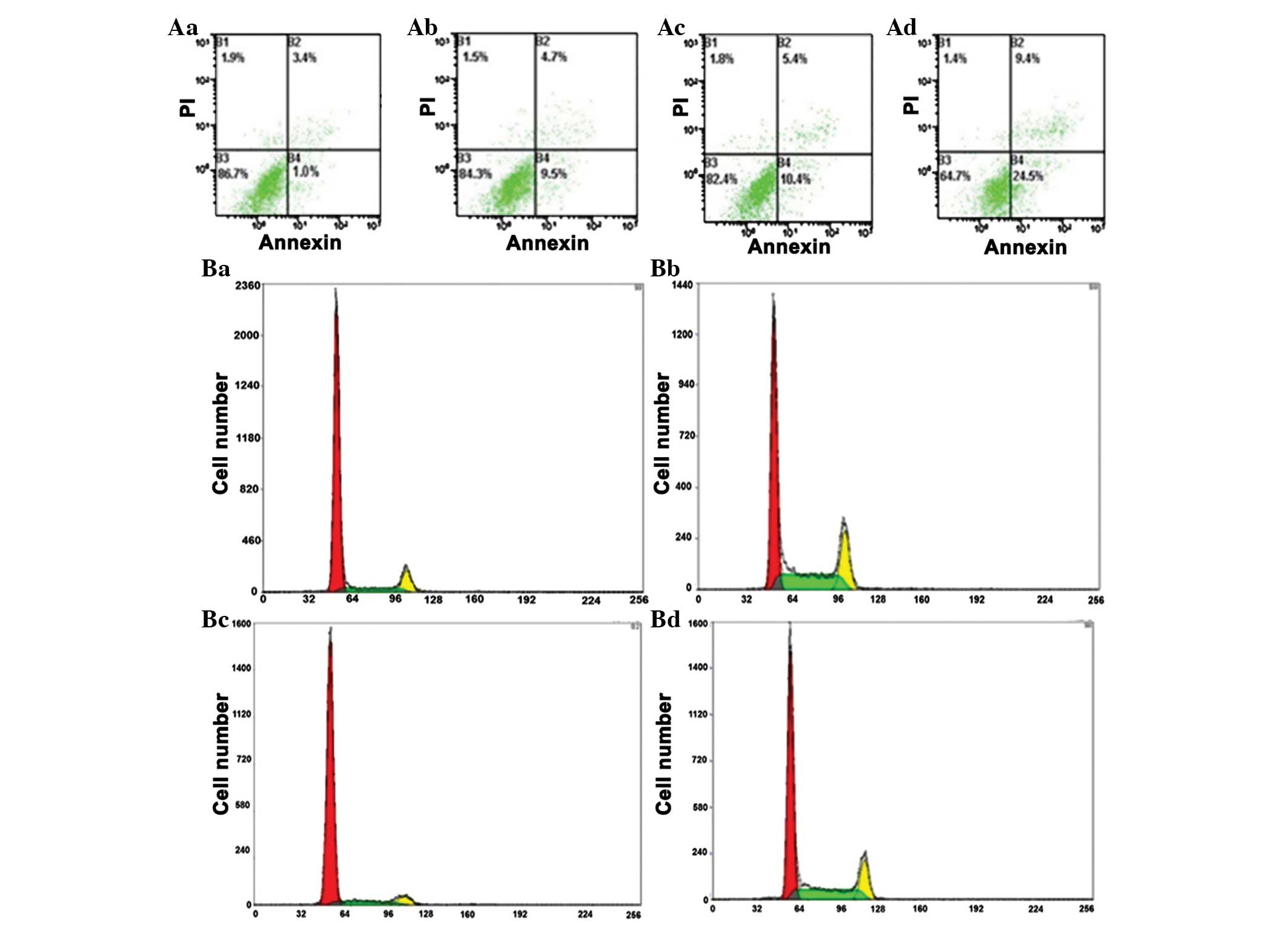
Annexin V/PI staining was used to measure
dobutamine-induced apoptosis. Compared with the control group,
dobutamine induced a significant increase in apoptotic death,
following pretreatment of MG-63 cells with 5, 10, 25 and 50 µM for
24 h (P=0.028; Table I). The
percentage of MG-63 cells in G2/M phases was significantly
increased at dobutamine concentrations of 25 and 50 µM (P=0.007 and
P=0.003, respectively), and the percentage of cells in S-phase was
significantly decreased (P=0.039) compared with the control group
(Fig. 2).
 | Table I.Cell cycle phase distribution and
apoptosis of MG-63 cells. |
Table I.
Cell cycle phase distribution and
apoptosis of MG-63 cells.
|
| Cell cycle phase |
|
|---|
|
|
|
|
|---|
| Group | G0/G1 | S | G2/M | Apoptosis, % |
|---|
| Control | 54.12±6.53 | 25.60±5.33 | 20.28±1.79 | 2.2±0.4 |
| 1 µM dobutamine | 55.19±3.16 | 25.37±3.66 | 19.44±5.01 | 2.5±1.1 |
| 5 µM dobutamine | 53.96±4.58 | 23.18±6.34 | 22.86±4.27 | 2.9±1.7 |
| 10 µM dobutamine | 49.82±2.99 | 26.78±5.92 | 23.40±6.52 | 7.0±2.5a |
| 25 µM dobutamine | 50.62±5.27 | 21.50±4.59 |
27.88±4.55b | 11.6±4.7c |
| 50 µM dobutamine | 51.21±6.52 | 18.39±2.79 |
30.40±7.26d | 13.2±1.8e |
Dobutamine reduces the migration and
invasion of MG-63 cells
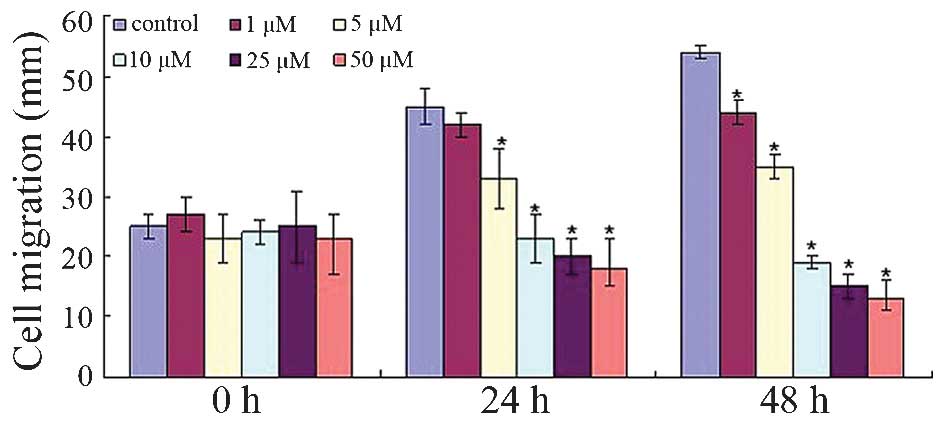
To investigate whether dobutamine treatment affects
osteosarcoma cell movement, the migratory rate of the MG-63 cells
was observed. Fig. 3 demonstrates
that dobutamine significantly decreased cell migration from the
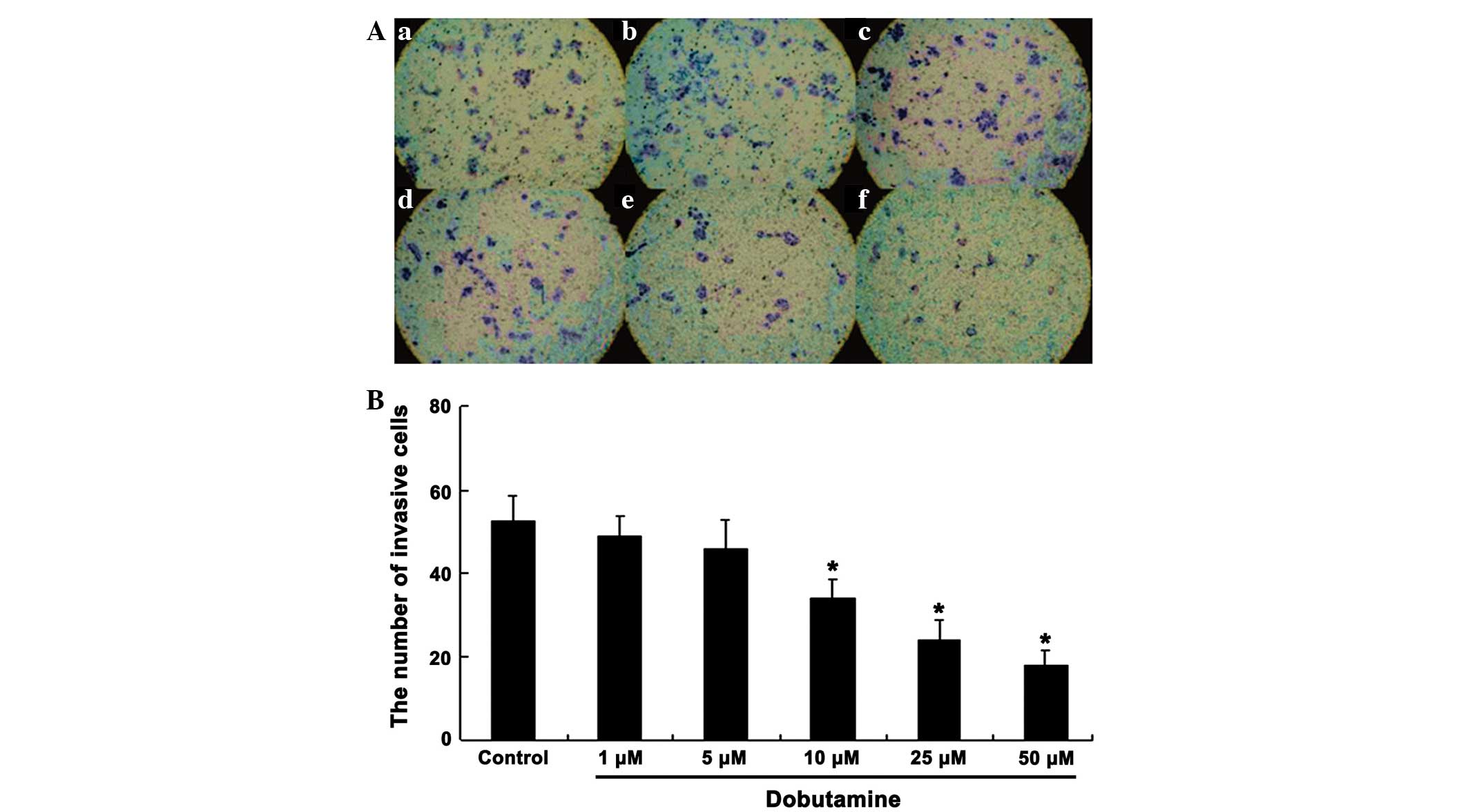
edge of the wound (P=0.041). Similarly, the cell invasion/Transwell
assay showed that a large number of cells passed through the filter
in the control group, whereas the cells passing through the filter
were markedly reduced following dobutamine treatment. Furthermore,
treatment with dobutamine reduced the number of invasive cells in a
concentration-dependent manner (Fig.
4). The number of invasive cells in the dobutamine groups was
significantly reduced compared with that in the control group
(P=0.039 for 10 µM, P=0.015 for 25 µM and P=0.011 for 50 µM)
(Fig. 4).
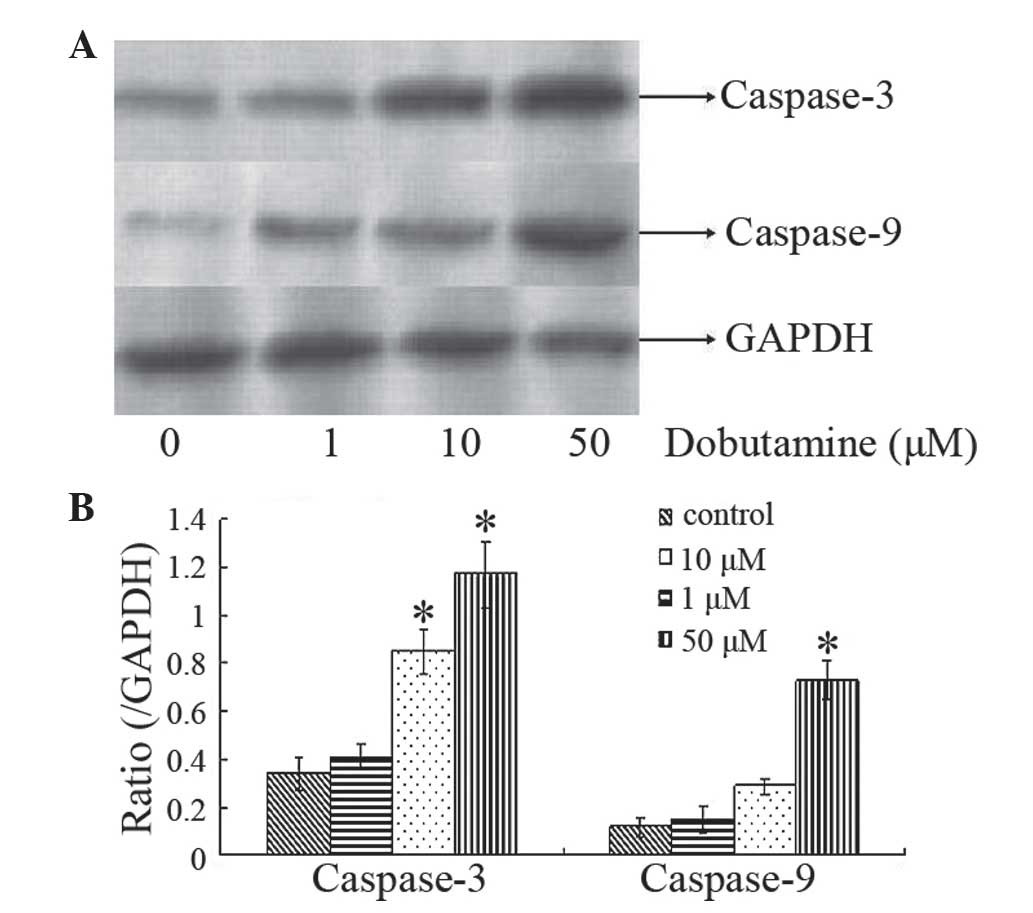
Dobutamine induces expression of
caspase-3 and caspase-9
Caspases are a family of endoproteases that provide
crucial links in cell regulatory networks that control inflammation
and cell death (17). Caspase-3 and 9
are crucial mediators in apoptosis signaling pathways (18). Western blot analysis was used to
investigate the expression of caspase-3 and caspase-9 in MG-63
cells following dobutamine treatment. Protein expression analysis
indicated that caspase-3 levels were increased following treatment
with dobutamine at the concentrations of 10 and 50 µM for 72 h
(P=0.011 and P=0.013, respectively), and caspase-9 levels were
increased following treatment with dobutamine at a concentration of
50 µM for 72 h (P=0.031) (Fig. 5).
These findings indicated that dobutamine may induce cancer cell
apoptosis and cell death.
Recent reports have demonstrated that YAP is highly
expressed in human osteosarcoma MG-63 cells (19). The results of the present study
indicate that the inhibitory effect of dobutamine may be associated
with the inhibition of YAP translocation. Silencing of the YAP gene
by RNA interference led to a similar effect to that caused by
dobutamine (20). In addition, the
present study found that dobutamine arrests the cell cycle at the
G2/M transition stage and augments cell apoptosis. Previous studies
have demonstrated that YAP activates cell apoptosis in response to
DNA damage via interaction with p73 in several cancer cell lines
(21). The findings of the present
study may result in a novel application for dobutamine in the
treatment of cancer.
In conclusion, the results of the present study
demonstrated that dobutamine was able to significantly suppress
osteosarcoma cell growth by inhibiting cell proliferation, inducing
cell apoptosis and redistributing cell cycle phases. These findings
indicate that dobutamine may become a novel therapeutic agent for
the treatment of osteosarcoma. However, additional in vivo
studies are required in order to confirm the effectiveness and
safety of dobutamine in the treatment of osteosarcoma.
Acknowledgements
This study was supported by grants from the National
Science and Technology Support Program (no. 2012BAI10B02) and the
National Science Foundation of China (no. 81571641), and an
internal grant from China-Japan Friendship Hospital, Beijing, China
(no. 2014-3-MS-18).
References
|
1
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamplot JD, Denduluri S, Qin J, Li R, Liu
X, Zhang H, Chen X, Wang N, Pratt A, Shui W, et al: The current and
future therapies for human osteosarcoma. Curr Cancer Ther Rev.
9:55–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loh AH, Navid F, Wang C, Bahrami A, Wu J,
Neel MD and Rao BN: Management of local recurrence of pediatric
osteosarcoma following limb-sparing surgery. Ann Surg Oncol.
21:1948–1955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating Yorkie, the Drosophila
homolog of YAP. Cell. 122:421–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roden R and Wu TC: How will HPV vaccines
affect cervical cancer? Nat Rev Cancer. 6:753–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castle PE, Dockter J, Giachetti C, Garcia
FA, McCormick MK, Mitchell AL, Holladay EB and Kolk DP: A
cross-sectional study of a prototype carcinogenic human
papillomavirus E6/E7 messenger RNA assay for detection of cervical
precancer and cancer. Clin Cancer Res. 13:2599–2605. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molden T, Kraus I, Karlsen F, Skomedal H
and Hagmar B: Human papillomavirus E6/E7 mRNA expression in women
younger than 30 years of age. Gynecol Oncol. 100:95–100. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tuttle RR and Mills J: Dobutamine:
Development of a new catecholamine to selectively increase cardiac
contractility. Circ Res. 36:185–196. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roesslein M, Froehlich C, Jans F, Piegeler
T, Goebel U and Loop T: Dobutamine mediates cytoprotection by
induction of heat shock protein 70 in vitro. Life Sci.
98:88–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bao Y, Nakagawa K, Yang Z, Ikeda M,
Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H and Hata
Y: A cell-based assay to screen stimulators of the Hippo pathway
reveals the inhibitory effect of dobutamine on the YAP-dependent
gene transcription. J Biochem. 150:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita E, Egashira J, Urase K, Kuida K and
Momoi T: Caspase-9 processing by caspase-3 via a feedback
amplification loop in vivo. Cell Death Differ. 8:335–344.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YH, Li B, Shen L, Shen Y and Chen
XD: The role and clinical significance of YES-associated protein 1
in human osteosarcoma. Int J Immunopathol Pharmacol. 26:157–167.
2013.PubMed/NCBI
|
|
20
|
Zhou Z, Zhu JS and Xu ZP: RNA interference
mediated YAP gene silencing inhibits invasion and metastasis of
human gastric cancer cell line SGC-7901. Hepatogastroenterology.
58:2156–2161. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lapi E, Di Agostino S, Donzelli S, Gal H,
Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X and
Blandino G: PML, YAP, and p73 are components of a proapoptotic
autoregulatory feedback loop. Mol Cell. 32:803–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|