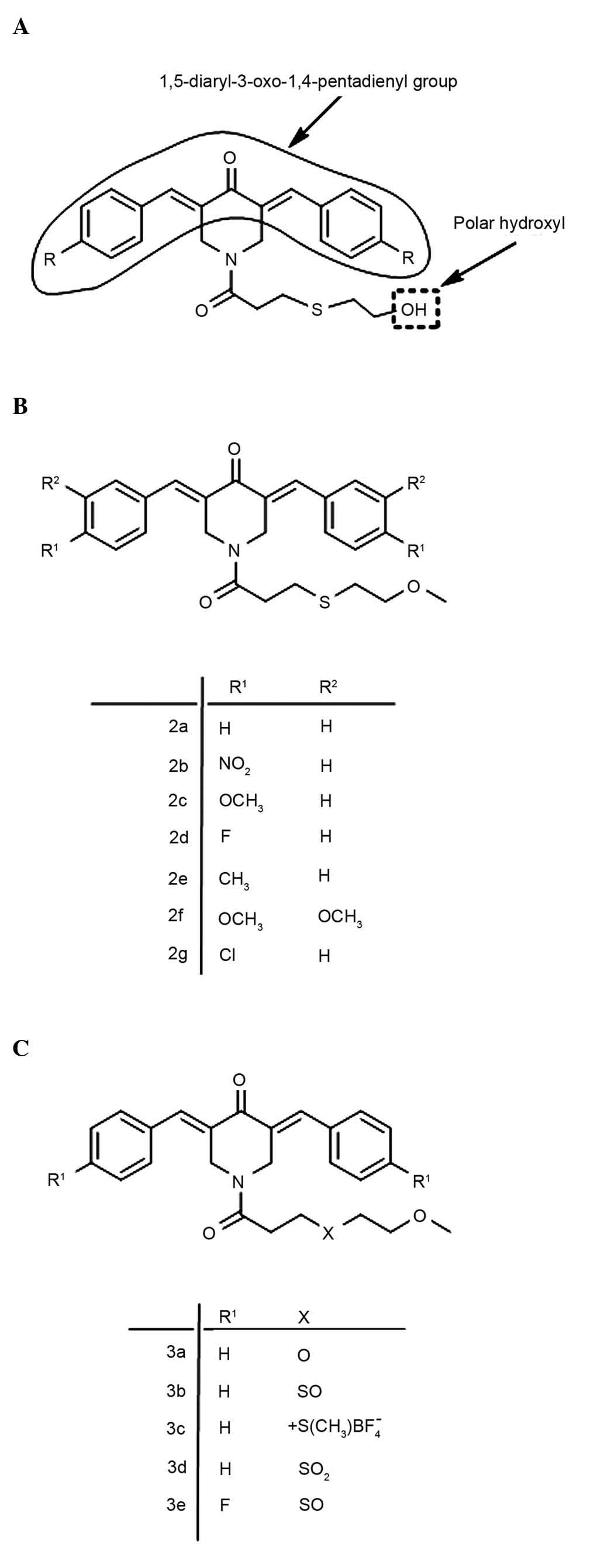
Introduction
Leukemia, lymphoma and myeloma are a diverse
category of malignant hematological neoplastic diseases that are
initiated primarily in the blood, bone marrow and lymphoid organs.
An estimated 52,380 newly diagnosed cases and 24,090 mortalities
were associated with leukemia in 2014 (1). Non-Hodgkin lymphoma (NHL), the most
common type of lymphoma, was diagnosed in 70,800 individuals, with
18,990 of them succumbing to the disease, in 2014 (1). Myeloma is the second most common type of
blood cancer, with 24,050 novel cases and 11,090 mortalities
reported in 2014 (1). In terms of
prevalence, 917,086 patients with the above blood malignancies were
living in 2014 in the USA, with NHL (530,919 patients) accounting
for over half of these cases (1).
According to the Leukemia and Lymphoma Society, approximately every
3 min, 1 person is diagnosed with one of the aforementioned blood
neoplasms in the USA (1,2). However, identifying a cure for all the
types of blood cancer is extremely challenging, as they are highly
heterologous in nature and possess great genetic variability and
diverse biochemical alterations (1,3). Recently,
several anti-lymphoma therapies have been developed that have
improved the survival of patients with blood cancer, including
novel chemotherapeutic agents and the use of combinatorial
therapeutic strategies that combine cytotoxins with immune
modulators such as monoclonal antibodies directed to specific
receptors (4–8).
A major focus for numerous studies involves the
synthesis and evaluation of novel anti-leukemic/lymphoma cytotoxins
(9–16). The present study focuses on the
development of conjugated unsaturated ketones or enones, which have
a preferential or exclusive affinity for thiols, in contrast to
amino or hydroxyl groups (17). Amino
and hydroxyl substituents are present in nucleic acids. Therefore,
the genotoxic problems associated with a number of current
anticancer drugs may be avoided with the use of enones (18). In particular, the
1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore has been mounted on
heterocyclic and cycloaliphatic scaffolds, and is considered to
align at a primary binding site (19). The extent of this interaction may also
be influenced by the nature of the groups placed on the piperidyl
nitrogen atom (19). For example, the
N-acyl group may form additional bonds with various atoms and
groups in cells, thus increasing the magnitude of the interaction
at the primary binding site (19). A
previous study revealed that the introduction of a
3-(2-hydroxyethylthio)propionyl group
(−COCH2CH2SCH2CH2OH) on
the piperidyl nitrogen atom led to a series of potent cytotoxins
(20), whose general structure is
represented in Fig. 1A. However,
these compounds contain a terminal polar hydroxyl group that may be
easily metabolized to acid analogs and impede cellular penetration
(21). In the present study, the
terminal polar hydroxyl group was masked by the corresponding
methoxy analogue (Fig. 1B). In
addition, the importance of the sulfur atom in the N-acyl side
chain, in association with cytotoxic potencies, was evaluated by
replacing it with related groups. The structures of the resulting
compounds are presented in Fig. 1C.
The aim of the present study was to evaluate the cytotoxicity of
novel 1-acyl-3,5-bis(benzylidene)-4-piperidones on a limited number
of leukemia cell lines. The results demonstrate that the two most
active piperidones investigated in the present study are able to
induce apoptosis in the cell lines tested.
Materials and methods
Compounds for cytotoxic
evaluations
The structures of the 12 compounds used in the
present study are presented in Fig. 1B
and C. The synthesis of these compounds was conducted at the
College of Pharmacy and Nutrition, University of Saskatchewan
(Saskatoon, Canada), details of which have recently been published
(22). In brief, a similar
methodology was used as described previously (20) in which aryl-aldehydes were condensed
with 4-piperidone and the resultant product was acylated with a
number of acyl chlorides to give the compounds in series 2 and 3.
The purified piperidones were characterized by proton nuclear
magnetic resonance (NMR), carbon-13 NMR and organic elemental
analysis of their carbon, hydrogen, nitrogen and sulfur content
(results not shown) (22).
Cell lines and culture conditions
The human T-lymphocyte leukemia Jurkat (TIB-152;
American Type Culture Collection, Manassas, VA, USA) (23), pre-B acute lymphoblastic leukemia
Nalm-6 (ACC-128; DSMZ, Braunschweig, Germany) (24) and T lymphoblast acute lymphoblastic
leukemia CCRF-CEM (American Type Culture Collection) (25) cell lines were grown in HyClone™
RPMI-1640 medium (GE Healthcare Life Sciences, Marlborough, MA,
USA) supplemented with 10% heat-inactivated HyClone™ fetal bovine
serum (FBS; GE Healthcare Life Sciences), 100 U/ml penicillin and
100 µg/ml streptomycin (15140–122; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Human non-malignant epithelial mammary MCF-10A
cells (CRL-10317; American Type Culture Collection) were grown in
Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 Ham media
(51445C; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10%
FBS, 10 µg/ml recombinant human insulin (Sigma-Aldrich), 20 ng/ml
epidermal growth factor (Peprotech Inc., Rocky Hill, NJ, USA), 0.5
µg/ml hydrocortisone (Sigma-Aldrich), 2.5 mM L-glutamine
(35050-061; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin. Nalm-6 and Jurkat cells were derived
from male donors, whereas CEM and MCF-10A cells were derived from
female donors (25). Cells that were
growing at 60–75% confluence in the exponential growth phase were
counted and seeded into 24- and 96-well plates (cat no. 167008;
Thermo Fisher Scientific, Inc.) at a density of 100,000 and 10,000
cells/well in 1,000 µl and 200 µl culture medium, respectively.
Cell incubation was performed at 37°C in a humidified atmosphere
containing 5% CO2. To procure high cell viability, the
cells were prepared as previously described (26).
Differential nuclear staining (DNS)
bioimaging assay
The cytotoxicity of the experimental compounds was
tested on the various cell lines via live-cell DNS bioimaging
assays (27). Cells in 96-well
microplates, seeded as described above, were treated with various
concentrations of experimental compounds (0.25–100 µM) for 24 and
48 h. A total of three controls were analyzed in each experimental
plate, which included a compound solvent control [dimethyl
sulfoxide (DMSO; D12345; Thermo Fisher Scientific, Inc.)]; cells
treated with hydrogen peroxide (H2O2; 349887;
Sigma-Aldrich) as a positive control for toxicity; and untreated
cells to monitor the background of dead cells. To differentially
label the cells, a mixture of two DNA-intercalating fluorescent
dyes, consisting of 1 µg/ml Hoechst (H3570; Thermo Fisher
Scientific, Inc.) and propidium iodide (PI; 0219545880; MP
Biomedicals, Santa Ana, CA, USA), was added to each well 1 h prior
to image recording (27). Hoechst
stain is a blue fluorescent dye that may easily cross cell
membranes of healthy and dead cells, and stains nuclear DNA, thus
identifying the total number of cells (27). PI (red) only stains cells with
compromised plasma membrane, thus indicating the number of dead
cells (28). The fluorescence signals
emitted by the stained cell nuclei were captured in two separate
channels, in fulfillment with each individual fluorophore emission
requirements (27). In order to
capture acceptable numbers of regions of interest (equivalent to
number of nuclei/cells), 2×2 image montages between four adjacent
image fields were captured per well, using a 10x objective lens and
a BD Pathway 855 Bioimaging System (BD Biosciences, San Jose, CA,
USA). Image collection and data analysis that measured the
percentage of dead cells in each individual experimental well was
achieved using BD AttoVision version 1.6.2 software (BD
Biosciences), and every test condition was assessed in
quintuplicate. The 50% cytotoxic concentration (CC50)
was calculated using a linear interpolation equation with a
gradient of concentrations, as previously described (29).
Apoptosis and necrosis assays
Disruption of phosphatidylserine (PS) membrane
asymmetry, which is an early event of apoptotic cell death, was
detected via flow cytometry upon treatment of cells with
1-(2-methoxyethylthio-propionyl)-3,5-bis(benzylidene)-4 piperidone
(2a) and
3,5-bis(4-fluorobenzylidene)-1-[3-(2-methoxyethylsulfinyl)-propionyl]-4
piperidone (3e). Following incubation with the above compounds,
cells were stained with fluorescein isothiocyanate (FITC)-labeled
annexin V and PI (IM2375; Beckman Coulter, Inc., Miami, FL, USA),
and monitored using flow cytometry (Cytomics FC 500 Flow Cytometer;
Beckman Coulter, Inc.). Flow cytometry readily detects PS exposure
on the external leaflet of the plasma membrane due to the high
affinity of annexin for binding to PS on the cellular membrane
(15). Jurkat cells seeded in 24-well
plates were subsequently exposed to piperidones at their
CC50 concentration (1.4 µM) for 24 h, and the treated
cells were then harvested from each well in a pre-chilled ice-water
cytometric tube (60818-437; VWR International, Houston, TX, USA),
washed and processed as previously described (13). In each experimental plate, the
following controls were included: Untreated cells; cells treated
with compound solvent (0.2% v/v DMSO); and cells exposed to 1 mM
H2O2 as a positive control for cytotoxicity.
The total percentage of apoptotic cells was determined by the
addition of the annexin V-FITC+ early and late phases of
apoptosis. Cells that stained only with PI, but not with annexin
V-FITC, were considered to be the necrotic population. A total of
~5,000 individual cells/sample were collected and analyzed with CXP
software version 2.2 (Beckman Coulter, Inc.).
Detection of mitochondrial membrane
potential (ΔΨm) disruption
Jurkat cells, seeded in a 24-well plates as
described earlier, were exposed to CC50 concentrations
of 2a and 3e for 6 h, and labeled with 2 µM
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) fluorophore, following the manufacturer's protocol
(MitoProbe™; Thermo Fisher Scientific, Inc.). Upon labeling with
JC-1, cells with polarized mitochondria emitted a red fluorescence
signal, while cells with depolarized mitochondria emitted a green
fluorescence signal (11,13). A flow cytometry protocol was used to
monitor the percentage distribution profile of cells emitting red
or green signals (11,13). As a control for the dissipation of
ΔΨm, 1 mM H2O2 was utilized. In addition, a
solvent control (1% v/v DMSO) and untreated control cells were
analyzed. Data collection and analysis was performed using CXP
software.
Live-cell detection of caspase-3
activation
Jurkat cells were incubated for 6 h with
CC50 concentrations of 2a and 3e. Subsequently,
caspase-3 activation was measured using the fluorogenic NucView™488
Caspase-3/7 substrate for live cells, following the manufacturer's
protocol (Biotium, Inc., Hayward, CA, USA). This caspase-3/7
substrate penetrates live cells with undamaged plasma membranes,
and permits the identification of living cells with active
caspase-3 (9). Cells exhibiting a
green fluorescence signal, indicative of caspase-3 activation, were
examined via flow cytometry (Cytomics FC 500 Flow Cytometer). The
same positive and negative controls used in the preceding
experiments were included and analyzed in the present series of
experiments. CXP software was used for data acquisition and
analysis.
Statistical analysis
Each data point was derived from ≤3 replicates. Data
are presented as the mean ± standard deviation to determine
experimental variability. Two-tailed paired Student's
t-tests were performed to determine the statistical
significance of differences among experimental samples and
controls. P<0.01 was considered to indicate a statistically
significant difference for comparisons of two treatments.
Results and Discussion
Cytotoxicity examined by DNS
assay
A well-established criterion that is used to define
cell death is the irreversible disruption of the plasma membrane,
which may be easily visualized with PI fluorescent dye that
specifically enters dead or dying cells (11). In the present study, PI was employed
to quantify the cytotoxicity of all the experimental compounds
tested on various cell lines using the live-cell DNS assay
(27). As indicated in Table I, a wide disparity in the cytotoxic
potency of the compounds was observed in series 2 and 3 at the two
time points tested. At 24 h, the 2a and 3e CC50 values
for the three malignant cell lines ranged between 0.9 and 1.8 µM,
and between 1.4 and 3.4 µM, respectively. In addition, 48 h
subsequent to treatment, only 2a and 3e exhibited consistent
sub-micromolar CC50 values for all the malignant cells
tested, with values ranging between 0.5 and 0.9 µM, and between 0.3
and 0.6 µM, respectively. The most potent compound in series 2 was
the unsubstituted analogue 2a. Therefore, in series 2, substituents
in the aryl rings may decrease the cytotoxic potencies. Analysis of
the toxicity of compounds in series 3a-d revealed that replacement
of the sulfur atom of 2a with other groups led to an increase in
CC50 values, and resulted in less toxic compounds.
Subsequent to incubation of the various cell lines with piperidones
for 48 h, the unsubstituted compound in series 2 and the 2
analogues that contained strongly-withdrawing substituents (2b and
2g), exhibited CC50 values in the sub-micromolar range.
In series 3, the most potent piperidone was 3e, with a
CC50 value that ranged between 0.3 and 0.6 µM subsequent
to 48 h incubation. A comparison of the various sensitivities of
the cell lines to the compounds in series 2 and 3 was performed.
The combined CC50 values of series 2 and 3 compounds
subsequent to 24 h incubation on Nalm-6, CEM and Jurkat cells
ranged between 1.8–126.4, 0.9–99.4 and 0.7–65.6 µM, respectively.
Subsequent to 48 h incubation, the CC50 values ranged
between 0.9–11.7, 0.6–6.3 and 0.3–5.6 µM, respectively. These
assays revealed that the most potent and promising compounds were
2a and 3e. These piperidones were additionally tested on the human
non-cancerous MCF-10A cell line. As shown in Table I, 2a possessed decreased cytotoxic
activity (<15%) at the highest concentration used (8 µM),
following 24 and 48 h incubation with the non-cancerous cells.
Notably, the CC50 values were not determined for cells
with decreased cytotoxicity, as 2a was not soluble at high
concentrations. The cytotoxicity of 3e was 10.6 (24 h) and 6.5 (48
h)-fold higher in the T-lymphocyte leukemia cells than in the
non-malignant cells (Table I).
Varying degrees of 3e selective cytotoxicity were also observed on
Nalm-6 and CEM cells (Table I).
However, 2a exhibited <15% cytotoxicity towards the
non-cancerous cell line at 24 and 48 h at the highest soluble
concentration tested (8 µM). Furthermore, 2a also demonstrated
selective toxicity towards leukemia cells (Table I). These findings demonstrate that the
piperidone analogues 2a and 3e possessed selective cytotoxicity
towards the three leukemia cell lines tested at sub-micromolar
concentrations.
 | Table I.CC50a values of experimental compounds
upon exposure to human leukemia/lymphoma cells for 24 or 48 h. |
Table I.
CC50a values of experimental compounds
upon exposure to human leukemia/lymphoma cells for 24 or 48 h.
| A, 24 h
treatment |
|---|
|
|---|
| Cell line | 2a | 2b | 2c | 2d | 2e | 2f | 2g | 3a | 3b | 3c | 3d | 3e |
|---|
| Nalm-6 | 1.80 | 23.80 | 79.07 |
5.20 | –b | 46.60 | –b | 29.90 | 24.30 | 126.40 | 27.00 |
3.40 |
| CEM | 0.90 |
2.70 | 45.20 | 25.10 | 99.40 | 52.50 | 5.80 |
5.20 |
3.10 |
21.50 |
3.10 |
2.90 |
| Jurkat | 1.40 |
4.60 | 12.10 |
7.30 |
4.60 | 34.00 | 0.70 |
5.30 | 65.60 |
23.10 |
3.00 |
1.40 |
| MCF-10A | –c | – | – | – | – | – | – | – | – | – | – | 14.80 |
|
| B, 48 h
treatment |
|
| Cell line | 2a | 2b | 2c | 2d | 2e | 2f | 2g | 3a | 3b | 3c | 3d | 3e |
|
| Nalm-6 | 0.90 | 1.50 | 6.20 | 0.90 | 3.60 | 10.70 | 0.50 | 10.60 | 7.00 | 10.50 | 11.70 | 0.50 |
| CEM | 0.60 | 0.60 | 6.30 | 2.00 | 3.20 |
5.00 | 1.30 |
1.40 | 1.50 |
6.30 |
1.30 | 0.30 |
| Jurkat | 0.50 | 0.30 | 5.60 | 1.50 | 1.70 |
8.00 | 0.80 |
1.50 | 2.00 |
3.40 |
0.40 | 0.60 |
| MCF-10A | –c | – | – | – | – | – | – | – | – | – | – | 3.90 |
Cell death analysis by PS
externalization
Apoptosis and necrosis are the two major modes of
cell death, and PS externalization is a biochemical event that
distinguishes between the two (30).
PS is an aminophospholipid that is predominantly confined in the
inner leaflet of the plasma membrane of resting cells (31). The presence of PS at the surface of
cells is indicative of the loss of phospholipid membrane asymmetry,
and is also a distinctive event of apoptosis (32). Assays that detect PS externalization
are centered on the high-affinity binding of annexin V to PS
(11). FITC-labeled annexin V, a 36
kDa Ca2+-dependent PS-binding protein, may readily
identify the event (33). The
compounds with the lowest CC50 across all cell lines
were 2a and 3e (Table I). Therefore,
the 2a and 3e piperidones were used to elucidate the mechanism of
cell death induction on T-lymphocyte leukemia cells. The detection
of PS exposure on the external leaflet of the cellular membrane was
examined by flow cytometry using a live-cells mode protocol. Jurkat
cells were individually exposed for 48 h to the CC50
values of 2a and 3e determined at 24 h (1.4 µM; Table I). As shown in Fig. 2, PS externalization was clearly
detected subsequent to 48 h of exposure to 2a and 3e (29,34). Cells
treated with 2a or 3e exhibited significantly increased (>60%)
numbers of annexin V-FITC+ cells, compared with the
solvent controls (P<0.0001; Fig.
2). Notably, 2a and 3e induced increased PS externalization,
similarly to the positive control used in the present study (1 mM
H2O2; Fig. 2).
These findings suggest that compounds 2a and 3e are likely to
induce cell death via apoptosis rather than necrosis.
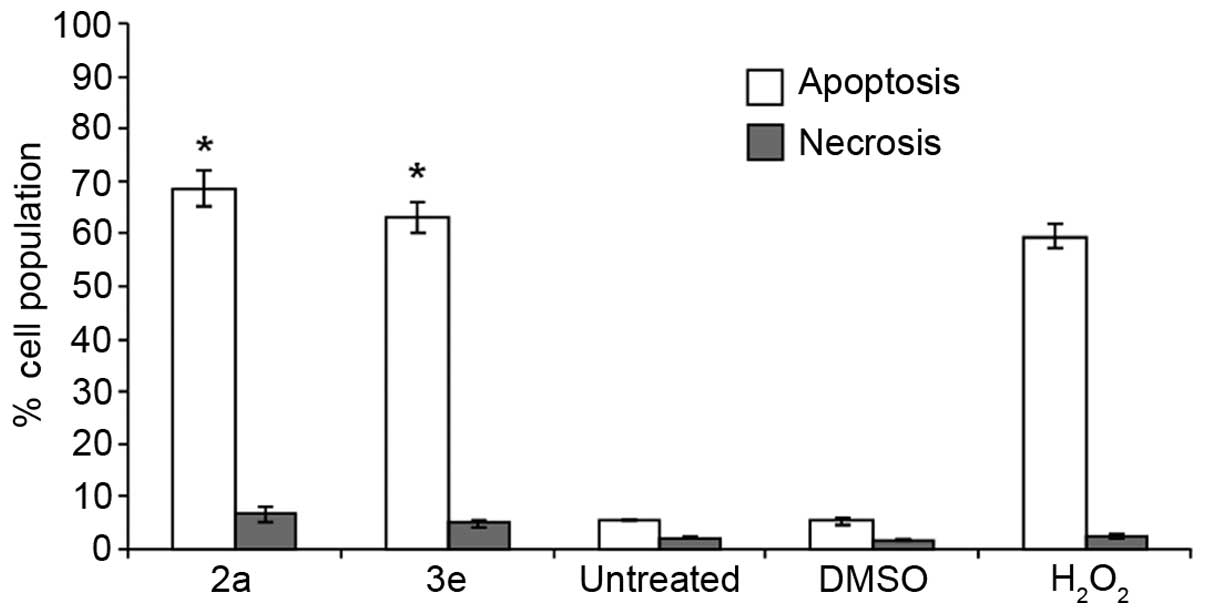 | Figure 2.Exposure of Jurkat cells to compounds
2a and 3e results in phosphatidylserine externalization. The cell
death mechanism (apoptosis or necrosis) was monitored via flow
cytometry upon co-staining the cells with annexin V-FITC and PI.
Cells were exposed for 48 h to 2a and 3e at their 50% cytotoxic
concentration (1.4 µM, as determined at 24 h). The total percentage
of apoptotic (annexin V-FITC+) Jurkat cells is expressed
as the sum of percentages of cells at the early and late stages of
apoptosis (open bars). Cells that were stained only with PI due to
their compromised plasma membrane integrity, but which did not
exhibit FITC signal, are considered to be necrotic cells (grey
bars). Each bar represents the mean ± standard deviation of 3
independent replicates. The following controls were included:
Untreated cells, as a negative control; cells treated with 0.2% v/v
dimethyl sulfoxide, as a control for solvent effects; and cells
exposed to 1 mM H2O2, as a positive control.
Statistically significant differences between cells treated with
compounds and solvent-treated control cells are denoted by
asterisks (*P<0.0001), and were analyzed using the two-tailed
Student's t-test. FITC, fluorescein isothiocyanate; PI,
propidium iodide; DMSO, dimethyl sulfoxide;
H2O2, hydrogen peroxide. |
ΔΨm status of treated cells
Apoptosis may be initiated via the intrinsic or the
extrinsic pathway (34–36). The intrinsic pathway is generally
characterized by a perturbation of the mitochondrial function,
whereas the extrinsic pathway is activated by membrane death
receptor signaling (35). In order to
gain additional insight into the succession of events induced by 2a
and 3e, ΔΨm integrity was examined at an early incubation time
point (6 h). The mitochondrion is the principal energy-producing
organelle in animals, and is considered to be important in cell
survival and death (36). The
stimulus that elicits the dissipation of the ΔΨm is an initial
distinctive biochemical feature of the intrinsic apoptosis pathway
(37). The loss of ΔΨm provokes the
release of downstream pro-apoptotic proteins into the cytosol,
consequently activating several apoptotic effectors such as
caspase-3 (37). Additionally,
impaired mitochondrial function may negatively impact cellular
energy levels leading to ATP depletion and cell death (36). At present, there are several approved
anticancer agents that induce apoptotic cell death by disrupting
the ΔΨm, including etoposide, doxorubicin and
1-β-D-arabinofuranosylcytosine (38).
To determine the initial signal utilized by 2a and 3e to activate
cytotoxicity in T-lymphocyte leukemia Jurkat cells, the JC-1
reagent was utilized to detect changes in the ΔΨm (39). As shown in Fig. 3, the majority of Jurkat cells
(>75%) exhibited a green fluorescence signal following 6 h of
exposure to 2a and 3e, which is indicative of the dissipation of
the ΔΨm. The strong ΔΨm perturbation mediated by 2a and 3e
indicates that these compounds induced the intrinsic apoptotic
pathway as a mechanism that elicits cell death (Fig. 3).
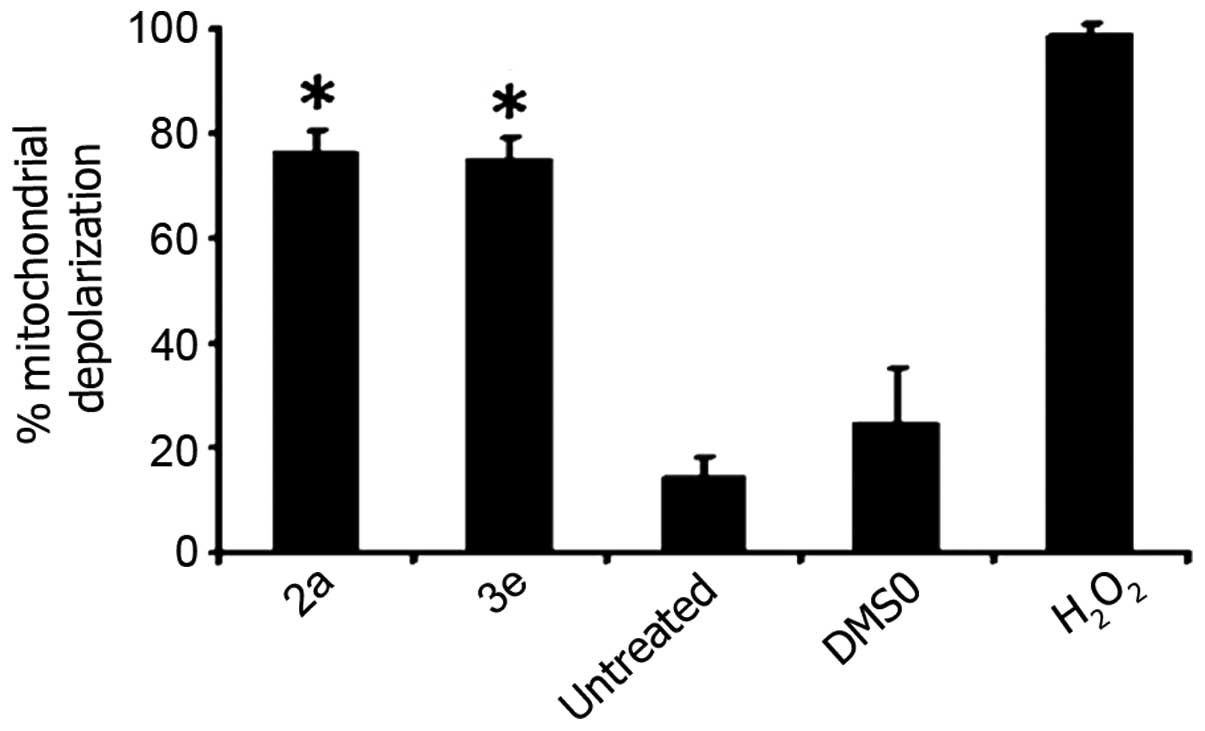 | Figure 3.Cytotoxicity mediated by 2a and 3e is
dependent of ΔΨm disruption in Jurkat cells. Cells were exposed for
6 h to 2a and 3e at their 50% cytotoxic concentration (1.4 µM, as
determined at 24 h). Variations in the ΔΨm were detected by
staining the cells with the aggregate-forming lipophilic cationic
fluorophore
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (2 µM), and monitored via flow cytometry. Percentages of
cells emitting green fluorescence signal (indicative of
mitochondrial depolarization) are depicted on the y axis. Each bar
represents the mean ± standard deviation of 4 independent
replicates. For the assays, three controls were included: Untreated
cells; cells treated with 0.1% v/v dimethyl sulfoxide; and cells
exposed to 1 mM H2O2. Statistically
significant differences between cells treated with compounds and
solvent-treated control cells are denoted by asterisks
(*P<0.0001), and were analyzed using the two-tailed Student's
t-test. For each sample, ~10,000 cells were acquired and
analyzed using CXP software. ΔΨm, mitochondrial membrane potential;
DMSO, dimethyl sulfoxide; H2O2, hydrogen
peroxide. |
Live-cell detection of intracellular
caspase-3 activation
Caspase-3, the major downstream apoptotic
executioner enzyme, is involved in the most destructive phases of
apoptosis (40,41). Also, caspase-3 activation is a
well-known biochemical marker of apoptosis (40). In the present study, the activation of
caspase-3 in living cells was analyzed via flow cytometry, using
the fluorogenic enzyme substrate NucView™ 488 Caspase-3/7. This
substrate contains the tetrapeptide sequence DEVD, which
corresponds to the cleavage site by active caspase-3 present in
poly(adenosine diphosphate-ribose) polymerase-1. Using flow
cytometry, 2a and 3e were observed to induce the conversion of
pro-caspase-3 to active caspase-3, following 6 h of treatment in
Jurkat cells (Fig. 4). A significant
(>85%; P<0.0001) induction of caspase-3 activity was detected
at CC50 concentrations of 2a and 3e, compared with
DMSO-treated and untreated cells (Fig.
4). These results indicate that 2a and 3e induced apoptosis via
caspase-3 activation, and are in agreement with the findings of the
present study, which indicated that 2a and 3e induced PS
externalization and mitochondrial depolarization. Therefore, 2a and
3e appear to activate early and middle biochemical facets of the
apoptosis cascade.
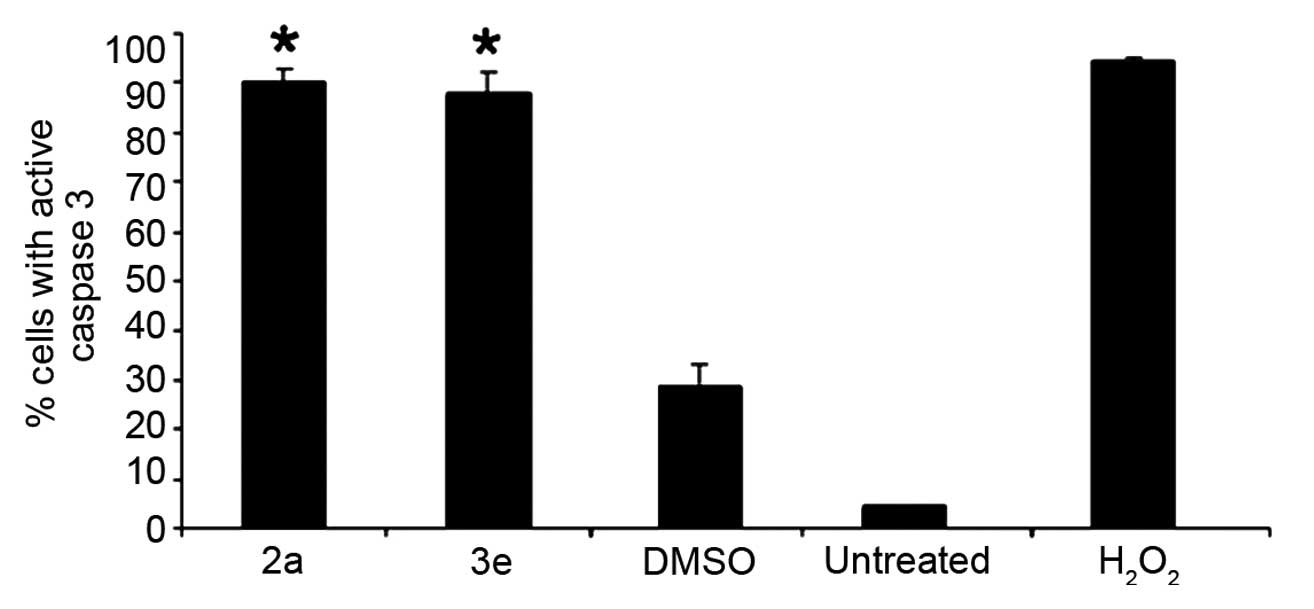 | Figure 4.Piperidones 2a and 3e elicited
caspase-3 activation in T-lymphocyte leukemia Jurkat cells. Cells
were exposed for 6 h to 50% cytotoxic concentration of 2a and 3e
(1.4 µM, as determined at 24 h). Percentages of cells with active
caspase-3, which exhibit a green fluorescence signal, are indicated
on the y axis. Cells were subjected to different treatments, as
indicated on the × axis. Each bar represents the mean value of 3
independent measurements, and the error bars represent the standard
deviation. Statistically significant differences between cells
treated with compounds and solvent-treated control cells are
denoted by asterisks (*P<0.0001), and were analyzed using the
two-tailed Student's t-test. In total, ~5,000 cells/sample
were collected and analyzed using CXP software. DMSO, dimethyl
sulfoxide; H2O2, hydrogen peroxide. |
Conclusion
The present study revealed that two novel
piperidones, 2a and 3e, exert potent and selective cytotoxicity
towards human leukemia Nalm-6, CEM and Jurkat cells. Additionally,
the T-lymphocyte leukemia Jurkat cell line exhibited increased
sensitivity to the two compounds subsequent to 24 and 48 h of
exposure. Examination of the cell death mechanism that was involved
in the observed cytotoxicity revealed that 2a and 3e elicited PS
externalization, depolarization of the mitochondrial membrane
potential and activation of caspase-3 on Jurkat cells. A key result
of the present study is that the novel 2a and 3e piperidones are
promising experimental anti-leukemia cytotoxins. The piperidones
primarily inflict programmed cell death in human T-lymphocyte
leukemic cells via the intrinsic/mitochondrial/caspase-3 apoptotic
pathway, which indicates that additional studies regarding these
compounds are required. Future studies on the drugs discussed in
the present study may include the development of novel
piperidone-derived analogues based on the structure of the
aforementioned two lead compounds, with the aim of improving
potency and selective cytotoxicity against malignant cells in
anti-cancer drug design strategies.
Acknowledgements
Funding for the present study was provided by the
National Institute of General Medical Sciences Support of
Competitive Research [(a component of the National Institutes of
Health (NIH); Bethesda, MD, USA] (grant no. 1SC3GM103713-03), the
Canadian Institutes of Health Research (Ottawa, ON, Canada) and the
Saskatchewan Health Research Foundation (Saskatoon, SK, Canada).
The authors would like to thank Ms. Gladys Almodovar for the
critical review of the manuscript and cell culture expertise, and
the staff members of the Cytometry, Screening and Imaging Core
Facility of the Border Biomedical Research Center at The University
of Texas at El Paso (El Paso, TX, USA), which was supported by the
National Institute on Minority Health and Health Disparities (a
component of the NIH) via the Research Center in Minority
Institutions program (grant no. 5G12MD007592-22). Mrs. Yoshira M.
Ayala-Marin was supported by the National Institutes of General
Medical Sciences Research Initiative for Scientific Enhancement
grant (no. R25GM069621-12).
References
|
1
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2011. National Cancer
Institute. Surveillance, Epidemiology, and End Results Program.
National Institutes of Health (Bethesda, MD, USA). 2013.http://seer.cancer.gov/csr/1975_2012Accessed.
February. 2016
|
|
2
|
American Cancer Society. Cancer
Infographics Gallery (Atlanta, GA, USA). 2015.https://.cancer.org/infographicsAccessed. March.
2016
|
|
3
|
Inklekofer AM and Younes A: Precision
therapy for lymphoma - current state and future directions. Nat Rev
Clin Oncol. 11:585–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diaz T, Navarro A, Ferrer G, Gel B, Gaya
A, Artells R, Bellosillo B, Garcia-Garcia M, Serrano S, Martínez A
and Monzo M: Lestaurtinib inhibition of the Jak/STAT signaling
pathway in hodgkin lymphoma inhibits proliferation and induces
apoptosis. PLoS One. 6:e188562011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hart S, Goh KC, Novotny-Diermayr V, Hu CY,
Hentze H, Tan YC, Madan B, Amalini C, Loh YK, Ong LC, et al:
SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the
treatment of myeloid and lymphoid malignancies. Leukemia.
25:1751–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burington B, Yue P, Shi X, Advani R, Lau
JT, Tan J, Stinson S, Stinson J, Januario T, de Vos S, et al: CD40
pathway activation status predicts response to CD40 therapy in
diffuse large B cell lymphoma. Sci Transl Med. 3:74ra222011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arrondeau J, Gan HK, Razak AR, Paoletti X
and Le Tourneau C: Development of anti-cancer drugs. Discov Med.
10:355–362. 2010.PubMed/NCBI
|
|
8
|
Masood A, Sher T, Paulus A, Miller KC,
Chitta KS and Chanan-Khan A: Targeted treatment for chronic
lymphocytic leukemia. Onco Targets Ther. 4:169–183. 2011.PubMed/NCBI
|
|
9
|
Pedroza DA, De Leon F, Varela-Ramirez A,
Lema C, Aguilera RJ and Mito S: The cytotoxic effect of
2-acylated-1,4-naphthohydroquinones on leukemia/lymphoma cells.
Bioorg Med Chem. 22:842–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martínez A, Carreon T, Iniguez E,
Anzellotti A, Sánchez A, Tyan M, Sattler A, Herrera L, Maldonado RA
and Sánchez-Delgado RA: Searching for new chemotherapies for
tropical diseases: Ruthenium-clotrimazole complexes display high in
vitro activity against Leishmania major and Trypanosoma
cruzi and low toxicity toward normal mammalian cells. J Med
Chem. 55:3867–3877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robles-Escajeda E, Lerma D, Nyakeriga AM,
Ross JA, Kirken RA, Aguilera RJ and Varela-Ramirez A: Searching in
mother nature for anti-cancer activity: Anti-proliferative and
pro-apoptotic effect elicited by green barley on leukemia/lymphoma
cells. PLoS One. 8:e735082013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez A, Rajapakse CS, Sánchez-Delgado
RA, Varela-Ramirez A, Lema C and Aguilera RJ:
Arene-Ru(II)-chloroquine complexes interact with DNA, induce
apoptosis on human lymphoid cell lines and display low toxicity to
normal mammalian cells. J Inorg Biochem. 104:967–977. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robles-Escajeda E, Martínez A,
Varela-Ramirez A, Sánchez-Delgado RA and Aguilera RJ: Analysis of
the cytotoxic effects of ruthenium-ketoconazole and
ruthenium-clotrimazole complexes on cancer cells. Cell Biol
Toxicol. 29:431–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elie BT, Levine C, Ubarretxena-Belandia I,
Varela-Ramírez A, Aguilera RJ, Ovalle R and Contel M: Water soluble
phosphane-gold(I) complexes. Applications as recyclable catalysts
in a three-component coupling reaction and as antimicrobial and
anticancer agents. Eur J Inorg Chem. 2009:3421–3430. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaik N, Martínez A, Augustin I,
Giovinazzo H, Varela-Ramírez A, Sanaú M, Aguilera RJ and Contel M:
Synthesis of apoptosis-inducing iminophosphorane organogold(III)
complexes and study of their interactions with biomolecular
targets. Inorg Chem. 48:1577–1587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santiago-Vazquez Y, Das S, Das U,
Robles-Escajeda E, Ortega NM, Lema C, Varela-Ramírez A, Aguilera
RJ, Balzarini J, De Clercq E, et al: Novel
3,5-bis(arylidene)-4-oxo-1-piperidinyl dimers: Structure-activity
relationships and potent antileukemic and antilymphoma
cytotoxicity. Eur J Med Chem. 77:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pati HN, Das U, Sharma RK and Dimmock JR:
Cytotoxic thiol alkylators. Mini Rev Med Chem. 7:131–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen EX and Moore MJ: Principles of
Medical Pharmacology. Kalant H, Grant DM and Mitchell J: (7th).
Saunders-Elsevier. (Toronto, Canada). 7782007.
|
|
19
|
Das U, Sharma RK and Dimmock JR:
1,5-diaryl-3-oxo-1,4- pentadienes: A case for antineoplastics with
multiple targets. Curr Med Chem. 16:2001–2020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das U, Pati HN, Sakagami H, Hashimoto K,
Kawase M, Balzarini J, De Clercq E and Dimmock JR:
3,5-Bis(benzylidene)-1-[3-(2-
hydroxyethylthio)propanoyl]piperidin-4-ones: A novel cluster of
potent tumor-selective cytotoxins. J Med Chem. 54:3445–3449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pandeya SN and Dimmock JR: Chemical
parameters in drug design. An Introduction to Drug Design. New Age
International. (New Delhi, India). 901997.
|
|
22
|
Hossain M, Das U, Umemura N, Sakagami H,
Balzarini J, De Clercq E, Kawase M and Dimmock JR: Tumour-specific
cytotoxicity and structure-activity relationships of novel
1-[3-(2-methoxyethylthio)propionyl]-3,5-bis(benzylidene)-4-pip
eridones. Bioorg Med Chem (In press).
|
|
23
|
Schneider U, Schwenk HU and Bornkamm G:
Characterization of EBV-genome negative ‘null’ and ‘T’ cell lines
derived from children with acute lymphoblastic leukemia and
leukemic transformed non-Hodgkin lymphoma. Int J Cancer.
19:621–626. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hurwitz R, Hozier J, LeBien T, Minowada J,
Gajl-Peczalska K, Kubonishi I and Kersey J: Characterization of a
leukemic cell line of the pre-B phenotype. Int J Cancer.
23:174–180. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foley GE, Lazarus H, Farber S, Uzman BG,
Boone BA and McCarthy RE: Continuous culture of human lymphoblasts
from peripheral blood of a child with acute leukemia. Cancer.
18:522–529. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nunes LM, Robles-Escajeda E,
Santiago-Vazquez Y, Ortega NM, Lema C, Muro A, Almodovar G, Das U,
Das S, Dimmock JR, et al: The gender of cell lines matters when
screening for novel anti-cancer drugs. AAPS J. 16:872–874. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lema C, Varela-Ramirez A and Aguilera RJ:
Differential nuclear staining assay for high-throughput screening
to identify cytotoxic compounds. Curr Cell Biochem. 1:1–14.
2011.PubMed/NCBI
|
|
28
|
Robles-Escajeda E, Das U, Ortega NM, Parra
K, Francia G, Dimmock JR, Varela-Ramirez A and Aguilera RJ: A novel
curcumin-like dienone induces apoptosis in triple-negative breast
cancer cells. Cell Oncol. Feb 26–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
29
|
Varela-Ramirez A, Costanzo M, Carrasco YP,
Pannell KH and Aguilera RJ: Cytotoxic effects of two organotin
compounds and their mode of inflicting cell death on four mammalian
cancer cells. Cell Biol Toxicol. 27:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fadok VA, de Cathelineau A, Daleke DL,
Henson PM and Bratton DL: Loss of phospholipid asymmetry and
surface exposure of phosphatidylserine is required for phagocytosis
of apoptotic cells by macrophages and fibroblasts. J Biol Chem.
276:1071–1077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Williamson P and Schlegel RA: Back and
forth: The regulation and function of transbilayer phospholipid
movement in eukaryotic cells. Mol Membr Biol. 11:199–216. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soares MM, King SW and Thorpe PE:
Targeting inside-out phosphatidylserine as a therapeutic strategy
for viral diseases. Nat Med. 14:1357–1362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boersma HH, Kietselaer BL, Stolk LM,
Bennaghmouch A, Hofstra L, Narula J, Heidendal GA and
Reutelingsperger CP: Past, present, and future of annexin A5: From
protein discovery to clinical applications. J Nucl Med.
46:2035–2050. 2005.PubMed/NCBI
|
|
34
|
Fadok VA, Voelker DR, Campbell PA, Cohen
JJ, Bratton DL and Henson PM: Exposure of phosphatidylserine on the
surface of apoptotic lymphocytes triggers specific recognition and
removal by macrophages. J Immunol. 148:2207–2216. 1992.PubMed/NCBI
|
|
35
|
Lavrik I, Golks A and Krammer PH: Death
receptor signaling. J Cell Sci. 118:265–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang L, Boise LH, Dent P and Grant S:
Potentiation of 1-beta-D-arabinofuranosylcytosine-mediated
mitochondrial damage and apoptosis in human leukemia cells (U937)
overexpressing bcl-2 by the kinase inhibitor 7-hydroxystaurosporine
(UCN-01). Biochem Pharmacol. 60:1445–1456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Petit PX, Lecoeur H, Zorn E, Dauguet C,
Mignotte B and Gougeon ML: Alterations in mitochondrial structure
and function are early events of dexamethasone-induced thymocyte
apoptosis. J Cell Biol. 130:157–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Solary E, Droin N, Bettaieb A, Corcos L,
Dimanche-Boitrel MT and Garrido C: Positive and negative regulation
of apoptotic pathways by cytotoxic agents in hematological
malignancies. Leukemia. 14:1833–1849. 2000. View Article : Google Scholar : PubMed/NCBI
|