Introduction
Medullary thyroid carcinoma (MTC) accounts for 5–10%
of all thyroid cancer cases (1). MTC
is a neuroendocrine tumor derived from parafollicular or ‘C’ cells
that secrete calcitonin (CT), which is a sensitive serum biomarker,
useful for diagnosis of MTC and postoperative follow-up. MTC may be
sporadic or familial, either in an isolated form [familial MTC
(FMTC)] or as part of multiple endocrine neoplasia (MEN) type 2A or
2B syndromes (2). Familial forms of
MTC are associated with mutations in the proto-oncogene rearranged
during transfection (RET), which are transmitted by an autosomal
dominant mode. Genetic testing enables an early diagnosis among
relatives of patients with FMTC. By contrast, the diagnosis of
familial index cases and sporadic tumors mainly relies on
fine-needle aspiration cytology (FNAC), which has a considerable
number of false-negative results that may delay the diagnosis
(3). Measurement of serum CT levels
in nodular thyroid diseases is not unanimously considered a routine
procedure. Surgery is the most effective treatment for these tumors
(4,5).
The overall cause specific mortality of these tumors
is 13.3–32.6% and 21.6–38.6% at 5 and 10 years, respectively
(6). In previously reported cases,
the main independent prognostic factors with influence on survival
have been age and stage at diagnosis (survival rates at 10 years
vary between 100% for patients in stage I and 20% for those
diagnosed at stage IV) (7).
The aim of the present study was to determine the
demographic, clinical and pathological characteristics of MTC
patients followed-up at the Portuguese Institute of Oncology
Francisco Gentil (Lisbon, Portugal).
Materials and methods
The medical files of 140 patients with MTC who were
diagnosed between 1990 and 2010 were reviewed in the present study.
Cases were identified through the Portuguese South Regional Cancer
Registry (Lisbon, Portugal) (http://www.ror-sul.org.pt/) and from the database of
the Department of Endocrinology of the Portuguese Institute of
Oncology Francisco Gentil. This study was approved by the Ethics
Committee of our center. Written informed consent was obtained from
patients submitted to genetic testing. Inclusion criterion was
histological diagnosis of MTC, which was reviewed by pathologists
at the Portuguese Institute of Oncology Francisco Gentil in those
cases of patients who had been operated at other institutions.
Familial cases subjected to prophylactic surgery and reported by
the pathologists as C-cell hyperplasia were not included in the
study. Screening of familial cases was performed by CT stimulation
with pentagastrin until 1994, and by RET mutation analysis
thereafter. Calcitonin assay was performed by different methods
over the years, from July 1988 to July 2000: ELSA-hCT (CIS bio
international, Gif-sur-Yvette, France); solid-phase two-site
immunoradiometric assay (IRMA), Reference Interval (RI) <10
pg/ml; from July 2000 to June 2006: IRMA hCT (CIS bio
international, Gif-sur-Yvette, France); IRMA, RI<10 pg/ml; from
June 2006 to present time: Calcitonin Immulite 2000 (Siemens
Healthcare Diagnostics, Llanberis, Gwynedd, United Kingdom); IRMA,
RI <8.5 pg/ml. Tumor stages were defined according to the
tumor-node-metastasis (TNM) classification (8): Stage I (T1, N0 and M0); stage II (T2, N0
and M0); stage III (T3, N0 and M0 or T1–3, N1a and M0); stage IVA
(T4a, any N and M0 or T1-T3, N1b and M0); stage IVB (T4b, any N and
M0); and stage IVC (any T, any N and M1). Biochemical cure (BC)
following surgery was considered when the CT levels measured at 3–6
months following surgery were lower than the reference values.
Recurrence was defined as the reappearance of high levels of CT or
locoregional and/or distant metastasis following a period of no
evidence of disease. Patients were classified based on their status
at the last follow-up as succumbed to MTC, succumbed to other
causes, alive without disease, alive with disease (biochemical or
locoregional and/or distant metastasis) or not available for
follow-up.
Statistical analysis was performed with SPSS version
20.0 (IBM SPSS, Armonk, NY, USA). χ2 test was used to
compare categorical variables, while one-way analysis of variance
and Tukey's test were used to compare means. Cumulative survival
curves were constructed using the Kaplan–Meier method; log rank
test was used to evaluate differences in the survival between
groups and an adjusted Cox regression model was performed to find
the independent prognostic factors with influence on survival. Age
was assessed as a categorical variable (≤45 versus >45 years) in
this analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Of the 140 patients enrolled in the study, 87
(62.1%) were diagnosed with MTC at the Portuguese Institute of
Oncology Francisco Gentil, whereas the remaining patients were
referred to the above hospital following a first surgical
intervention performed elsewhere. The average number of patients
treated per year was 6.7. In total, 80 (57.1%) patients were
females (female:male ratio, 1.3:1.0). Patients with FMTC were
significantly younger than patients with sporadic MTC (33±20
vs. 57±15 years, respectively; P<0.05). Among the
familial cases, there were 12 MEN type 2A, 1 MEN type 2B and 3
FMTC. Of these patients, 4 were index and 12 were diagnosed by
genetic/pentagastrin screening across 7 different families. The
genetic profiles and results of early thyroidectomy of the familial
cases analyzed in the present study were previously reported by
Bugalho et al (9).
The underlying conditions leading to the diagnosis
of MTC in patients not detected by screening were: Presence of
thyroid nodules in 105 cases; cervical lymph nodes in 12 cases;
distant metastases in 2 cases; increased serum CT levels in 2
cases; increased serum carcinoembryonic antigen levels in 1
patient; incidental finding in 1 patient operated for a larynx
carcinoma; and in 5 patients the reason was not specified in their
clinical files. Neck symptoms were present in 23 cases, while 15
patients complained of diarrhea, 6 of which had distant metastases.
A total of 14 patients exhibited distant metastases at diagnosis, 5
of them in the liver, 4 in the lungs, 2 in the bones, 2 in the
liver, lungs and bones, and 1 in the liver and bones.
Among the 128 (91.4%) patients not detected by
screening, 44 (34.4%) did not exhibit a preoperative suspicion of
MTC, since FNAC was either negative for MTC or not requested
and serum CT determination was not performed. FNAC and serum CT
assay sensitivities were 51.3% and 98.7%, respectively. Patients
with a negative FNAC for MTC (58 cases) had cytological findings
reported as non-diagnostic in 6 cases, benign in 13 cases and
suspicious for a different tumor in 39 cases. In total, 129 (92.1%)
patients were subjected to surgery, and total thyroidectomy with
cervical lymph node clearance was performed in 72 cases (level VI
in 6 of them), while total thyroidectomy was performed in 5
patients and hemithyroidectomy in 6 patients. Of the remaining 11
patients, 7 underwent palliative surgery, while 4 were not operated
due to advanced disease. Neck radiotherapy was performed in 21
(15.0%) patients, with a palliative intention in 18 cases and in an
adjuvant setting in 3 patients. Of the patients with advanced
disease, 9 (6.4%) were treated with classic chemotherapy and/or
off-label kinase inhibitors. The histological
characteristics of the patients are presented in Table I. Somatic mutations in the RET, Ras
and B-Raf genes were evaluated in 53 patients, and the results were
previously published by Moura et al (10,11).
 | Table I.Histological characteristics of
patients with medullary thyroid carcinoma. |
Table I.
Histological characteristics of
patients with medullary thyroid carcinoma.
| Histological
characteristics | Patients |
|---|
| Tumor size, cm (mean
± standard deviation) | 3.6±2.3 |
| Side, % |
|
| Right
lobe | 48 |
| Left
lobe | 35 |
| Right and
left lobes | 17 |
| Multifocality, % | 23 |
| Angioinvasion, % | 43 |
| Extrathyroidal
extension, % | 44 |
| Lymph node
metastasis, % |
|
|
Total | 43 |
| N1 | 5 |
| N1a | 5 |
| N1b | 90 |
| Associated
histological diagnosis |
|
| Total,
% | 33 |
|
Follicular hyperplasia, n | 19 |
| C-cell
hyperplasia, n | 10 |
| Papillary
carcinoma, n | 4 |
|
Follicular carcinoma, n | 3 |
|
Lymphocytic thyroiditis,
n | 8 |
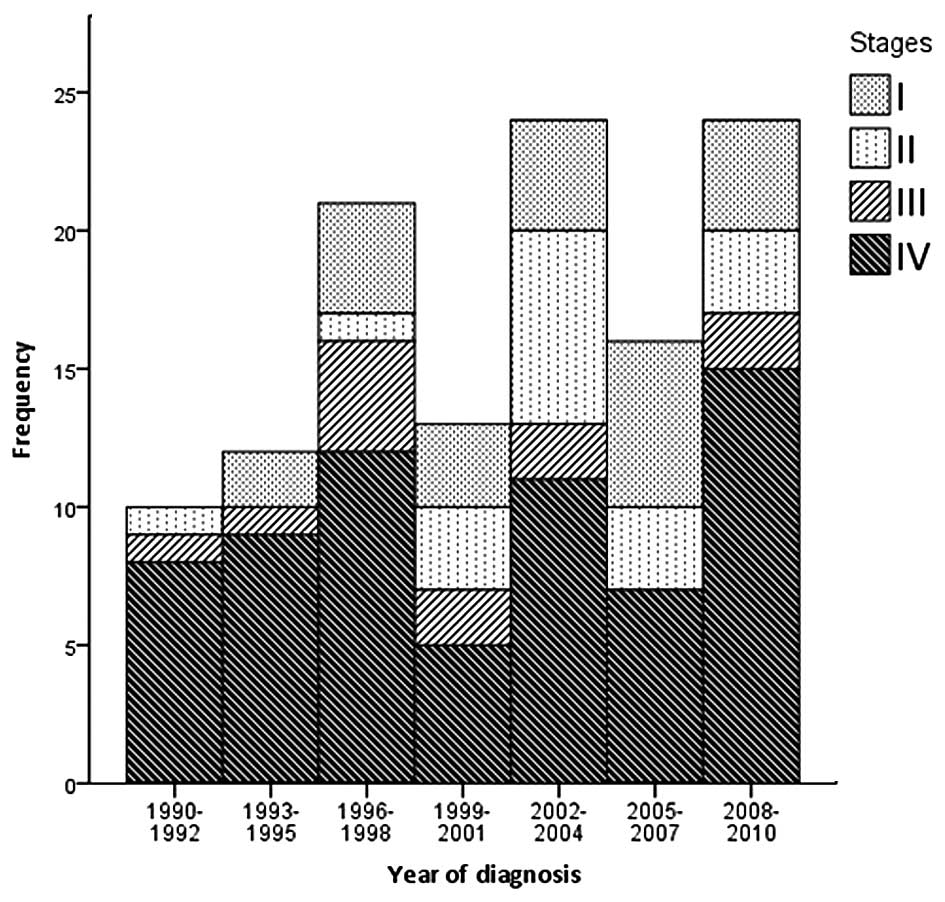
Staging at diagnosis was possible in 120 patients
(Fig. 1), and demonstrated that 23
(19.2%) patients were in stage I, 18 (15.0%) in stage II, 12
(10.0%) in stage III and 67 (55.8%) in stage IV (of which, 42 were
in stage IVA, 11 in stage IVB and 14 in stage IVC) (P<0.05). The
majority of the sporadic cases [72 (68.6%)] and all familial index
cases presented with advanced stage (III–IV) whilst 72.7% (8
patients) of familial cases, detected by screening, were diagnosed
at early stages of disease (stages I and II) (P=0.008). Among 116
patients with serum CT evaluation following primary surgery, BC was
achieved in 46 patients (39.7%). Of these, only 3 patients (6.5%)
relapsed, 1 of them with biochemical recurrence and 2 with
recurrence in the thyroidectomy surgical bed.
The median follow-up time was 5 years. At the last
follow-up, 32 (22.9%) patients had succumbed to MTC, while 4 (2.9%)
had deceased due to other causes, 38 (27.1%) were in remission and
38 (27.1%) were alive with disease. The remaining 28 patients
(20.0%) were not available for follow-up. In the group of patients
that were alive with disease, biochemical evidence of disease (high
serum CT levels) was present in 17 (44.7%) cases, while structural
disease (locorregional disease, metastatic cervical lymph nodes or
a mass in thyroidectomy surgical bed) was detected in 15 patients
(39.5%) and distant metastasis in 6 patients (15.8%).
Survival analysis
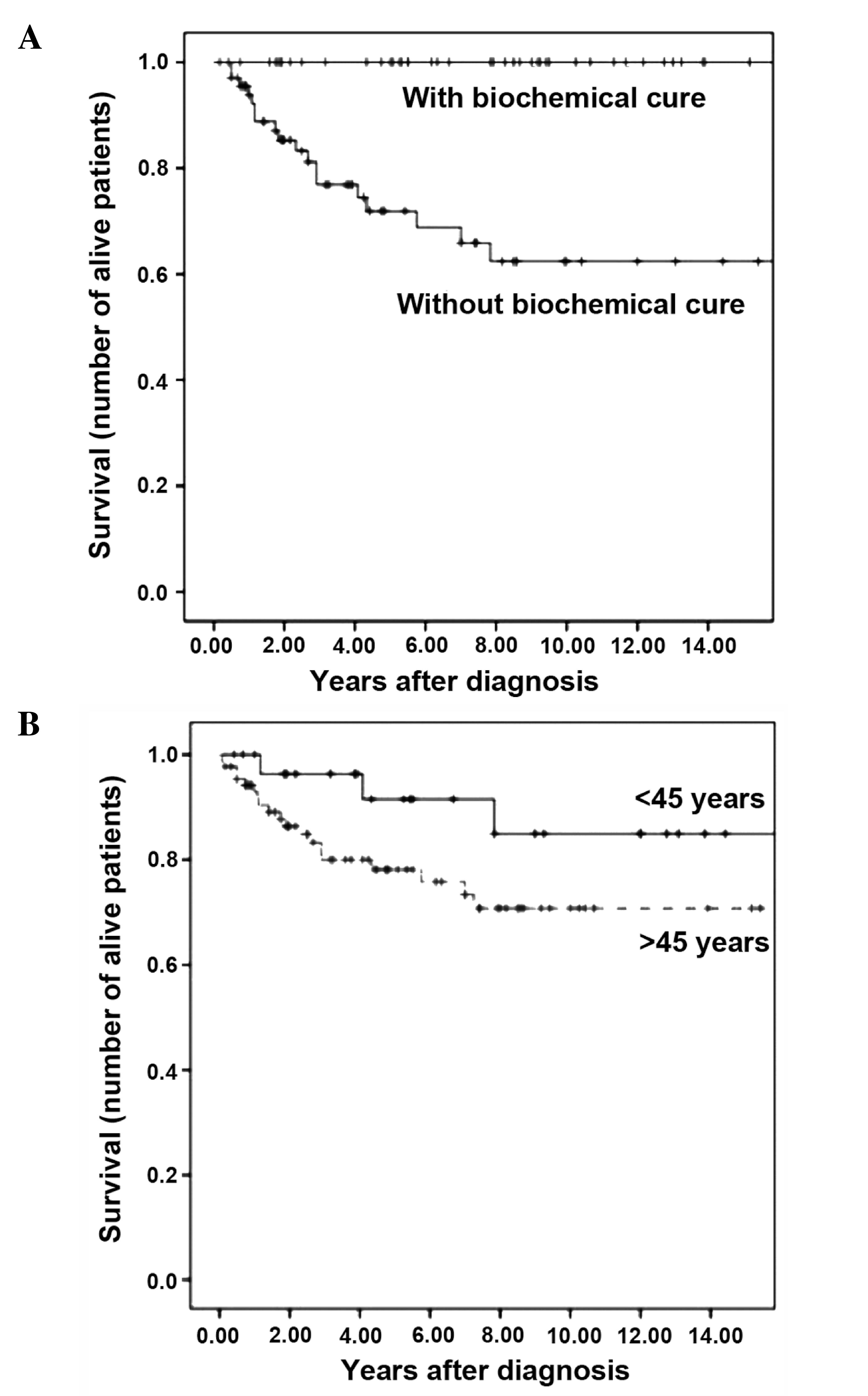
The survival rates at 5 and 10 years were 79.3 and
73.6%, respectively, while median survival was not reached. When
survival rate was analyzed for tumor stage at diagnosis, only 1
patient in stage II was observed to succumb to MTC, while no
patients in stages I or III succumbed to MTC. The survival rates at
5 and 10 years for patients diagnosed with stage IV MTC were 58.6
and 49.0%, respectively. The patient in stage II who succumbed to
MTC had been subjected to total thyroidectomy plus unilateral
cervical lymph node dissection. His initial TNM staging was T2N0M0,
and a RET somatic mutation in codon 918 was also detected in the
tumor. Biochemical evidence of disease persisted, and 8 months
following surgery, liver and bone metastases were detected. The
serum CT levels of the patient during his last follow-up were
97,706 pg/ml. The patient succumbed to disease 4 months later. None
of the patients who achieved BC succumbed to MTC, and the survival
rates at 5 and 10 years for patients with postoperative
hypercalcitoninemia were 71.9 and 62.4%, respectively. Patients
detected by genetic screening exhibited a 100.0% survival rate at 5
and 10 years, while sporadic MTC patients exhibited a survival rate
at 5 and 10 years of 78.3 and 71.4%, respectively. For familial
index cases, the survival rate at 5 and 10 years was 75.0 and
37.5%, respectively.
Univariate analysis was initially performed with the
129 patients who underwent surgery with a curative intent. The
following adverse prognostic factors for survival were identified:
Age >45 years (P=0.026), advanced stage at diagnosis (P=0.0001)
and absence of BC (P=0.00001). When patients detected by
genetic/pentagastrin screening were excluded from the analysis, age
was no longer a prognostic factor (P=0.077), but stage of disease
remained a strong predictor of survival (P<0.001) (Fig. 2). Multivariate analysis, performed
with the initial 129 patients, revealed that only the absence of BC
(P=0.031; 95% confidence interval, 0.001–0.691) had a negative
impact on prognosis.
Discussion
The incidence of MTC among the present patients
remained stable throughout the years, although a high proportion of
advanced cases was observed, probably due to the absence of routine
serum CT determination in the management of thyroid nodules. At
diagnosis, MTC was frequently revealed in an advanced stage of
disease, as observed by other authors (6,12). A
diagnosis of MTC, either in sporadic or in familial index cases, is
usually suspected by FNAC and/or by detection of high serum CT
levels in patients with thyroid nodules. In the present study, the
sensitivities of FNAC and serum CT analysis for the diagnosis of
MCT were 51.3 and 98.7%, respectively. Among the patients analyzed
in the present study, there was only 1 patient whose levels of
preoperative CT (determined at another institution) were below the
reference value. Following total thyroidectomy, the CT levels of
this patient (measured at the Portuguese Institute of Oncology
Francisco Gentil) were elevated, thus ruling out the possibility of
a non-secreting tumor. Routine CT determination in thyroid nodules
is a controversial issue (2,13). For instance, Elisei et al
(3), performed CT determination in
10,864 patients with thyroid nodules, and detected 1 case of MTC in
every 250 patients screened. The authors verified that CT
determination enabled a diagnosis of MTC at an early stage with
10-year survival rates higher than those observed in MCT cases who
were not detected by screening. In the present study, in 67.2%
(39/58) of patients with a negative FNAC, the cytological findings
were suspicious for carcinoma. Thus, FNAC provided an indication
for surgery in ~2/3 of patients with negative-MTC FNAC. However, in
the present cohort, 22% of the patients would have not been
subjected surgery due to a benign FNAC result. This value was
higher than that previously published by the present authors in a
smaller cohort (14), which was
similar to the value described by Elisei et al (3), but lower than that reported by
Papaparaskeva et al (15),
whereby FNAC was positive for MTC in 89.0% (81/91) of the cases
analyzed.
Of the 140 patients included in the present study,
only 11% represented familial cases, which indicates a prevalence
significantly lower than that reported by others (6,16–19). However, the present study only
included patients with MTC confirmed by histology, which may have
underestimated the total number of familial cases. Patients
detected by genetic screening were diagnosed at younger ages than
sporadic cases, which is in agreement with the literature (6,19,20), and were also diagnosed at earlier
stages of the disease (6,19,20),
presenting 5 and 10-year survival rates of 100.0%. This underlines
the importance of early screening for mutation carriers. It also
supports the idea that germline mutations should be investigated in
sporadic cases, particularly in those with multifocality or C-cell
hyperplasia, since ≤10% of these patients represent hereditary MTC
index cases (9,12). In agreement with previous studies
(21,22), mutation in the codon 634 in the RET
gene was the most prevalent among the present cohort. In previous
studies published by the present authors (10,11), it
was demonstrated that patients with 918 and 883 somatic mutations
were associated with aggressive disease and higher number of lymph
nodes metastases, higher percentage of multifocality, persistent
hypercalcitoninemia following surgery and more advanced stages of
disease at presentation than those with other RET mutations.
Patients with Ras mutations exhibited an intermediate behavior
between these two groups. Therefore, these results suggest that
analyzing tumor DNA for RET and Ras mutations may be important,
since their impact in patient prognosis may differ.
Specific survival at 5 and 10 years in the present
cohort was 79.3 and 73.6%, respectively, which was similar to
previous reports (16,19,20,22,23).
Another possible adverse prognostic factor identified in the
present study was age >45 years. However, when patients detected
by genetic/pentagastrin screening were omitted in the univariate
analysis, age was no longer a prognostic factor for survival,
contrarily to disease stage, which remained a significant factor.
This is due to the fact that patients detected by genetic screening
were significantly younger than index or sporadic cases and
exhibited a survival rate at 5 and 10 years of 100.0%. Therefore,
the influence of age on prognosis should be carefully assessed in
cohorts with a high number of hereditary MTC cases, such as those
available in the literature (4,6). This is
important considering that there are certain studies (6) who defend that age should be considered
in the staging of MTCs, as it happens in thyroid cancer of the
follicular epithelium.
In the present study, postoperative CT was the only
prognostic factor observed to exert a significant impact on
survival, as previously described by other authors (20). In fact, of the 40.0% of patients who
achieved BC following the first surgical procedure, only 3 patients
(6.5%) relapsed. Therefore, complete resection should be performed,
if feasible.
The present cohort consisted of a considerable
number of MTC patients followed-up in a single institution (and
therefore, with uniform criteria for collecting data) and with a
median follow-up time similar to other published studies (6,16).
However, the current study presents certain limitations: i) It is a
retrospective analysis that included patients referred from other
institutions; thus, it was not possible to retrieve certain
information for these patients. In addition, the surgical protocols
for these patients may have varied across institutions, which may
have influenced the results in terms of cure, survival and
recurrence; ii) since the studied period was long, it is likely
that the surgical protocols varied for each tumor stage over the
years.
In conclusion, the present study confirms that the
majority of MTCs are detected in advanced stages of disease, and
that CT determination is more sensitive than FNAC in the diagnosis
of these types of carcinoma. Furthermore, the present study
demonstrated that the annual incidence of these tumors has remained
stable for the past two decades, contrarily to the ‘epidemic’
increase that has been observed for papillary thyroid carcinomas.
Once subjected to primary surgery treatment, if BC is achieved,
relapse of MTC is rarely observed. To the best of our knowledge,
the present study is the first to report that age is only capable
of acting as a potential prognostic factor if patients detected by
genetic screening are considered in the analysis, since these
patients are usually diagnosed earlier and at initial stages of the
disease.
References
|
1
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995. Cancer.
83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kloos RT, Eng C, Evans DB, Francis GL,
Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M
and Wells SA Jr: American Thyroid Association Guidelines Task
Force: Medullary thyroid cancer: Management guidelines of the
American Thyroid Association. Thyroid. 19:565–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elisei R, Bottici V, Luchetti F, Di Coscio
G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A
and Pacini F: Impact of routine measurement of serum calcitonin on
the diagnosis and outcome of medullary thyroid cancer: Experience
in 10,864 patients with nodular thyroid disorders. J Clin
Endocrinol Metab. 89:163–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans DB, Fleming JB, Lee JE, Cote G and
Gagel RF: The surgical treatment of medullary thyroid carcinoma.
Semin Surg Oncol. 16:50–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fleming JB, Lee JE, Bouvet M, Schultz PN,
Sherman SI, Sellin RV, Friend KE, Burgess MA, Cote GJ, Gagel RF and
Evans DB: Surgical strategy for the treatment of medullary thyroid
carcinoma. Ann Surg. 230:697–707. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kebebew E, Ituarte PH, Siperstein AE, Duh
QY and Clark OH: Medullary thyroid carcinoma: Clinical
characteristics, treatment, prognostic factors, and a comparison of
staging systems. Cancer. 88:1139–1148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leboulleux S, Baudin E, Travagli JP and
Schlumberger M: Medullary thyroid carcinoma. Clin Endocrinol (Oxf).
61:299–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greene FL, Page DL, Fleming ID, Fritz A
and Balch DM: AJCC Cancer Staging Manual (6th). Springer Verlag.
Chicago: 2003.
|
|
9
|
Bugalho MJ, Domingues R, Santos JR,
Catarino AL and Sobrinho L: Mutation analysis of the RET
proto-oncogene and early thyroidectomy: Results of a Portuguese
cancer centre. Surgery. 141:90–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moura MM, Cavaco BM, Pinto AE, Domingues
R, Santos JR, Cid MO, Bugalho MJ and Leite V: Correlation of RET
somatic mutations with clinicopathological features in sporadic
medullary thyroid carcinomas. Br J Cancer. 100:1777–1783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moura MM, Cavaco BM, Pinto AE and Leite V:
High prevalence of RAS mutations in RET-negative sporadic medullary
thyroid carcinomas. J Clin Endocrinol Metab. 96:E863–E868. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marsh DJ, Learoyd DL and Robinson BG:
Medullary thyroid carcinoma: Recent advances and management update.
Thyroid. 5:407–424. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gharib H, Papini E, Paschke R, Duick DS,
Valcavi R, Hegedüs L and Vitti P: AACE/AME/ETA Task Force on
Thyroid Nodules: American Association of Clinical Endocrinologists,
Associazione Medici Endocrinologi, and European Thyroid Association
medical guidelines for clinical practice for the diagnosis and
management of thyroid nodules: Executive summary of
recommendations. J Endocrinol Invest. 33(Suppl 5): S51–S56.
2010.
|
|
14
|
Bugalho MJ, Santos JR and Sobrinho L:
Preoperative diagnosis of medullary thyroid carcinoma: Fine needle
aspiration cytology as compared with serum calcitonin measurement.
J Surg Oncol. 91:56–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papaparaskeva K, Nagel H and Droese M:
Cytologic diagnosis of medullary carcinoma of the thyroid gland.
Diagn Cytopathol. 22:351–358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cupisti K, Wolf A, Raffel A, Schott M,
Miersch D, Yang Q, Eisenberger CF, Röher HD and Knoefel WT:
Long-term clinical and biochemical follow-up in medullary thyroid
carcinoma: A single institution's experience over 20 years. Ann
Surg. 246:815–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wells SA Jr and Franz C: Medullary
carcinoma of the thyroid gland. World J Surg. 24:952–956. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kameyama K and Takami H: Medullary thyroid
carcinoma: Nationwide Japanese survey of 634 cases in 1996 and 271
cases in 2002. Endocr J. 51:453–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modigliani E, Cohen R, Campos JM,
Conte-Devolx B, Maes B, Boneu A, Schlumberger M, Bigorgne JC,
Dumontier P, Leclerc L, et al: Prognostic factors for survival and
for biochemical cure in medullary thyroid carcinoma: Results in 899
patients. The GETC Study Group. Groupe d'etude des tumeurs à
calcitonine. Clin Endocrinol (Oxf). 48:265–273. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grozinsky-Glasberg S, Benbassat CA,
Tsvetov G, Feinmesser R, Peretz H, Shimon I and Lapidot M:
Medullary thyroid cancer: A retrospective analysis of a cohort
treated at a single tertiary care center between 1970 and 2005.
Thyroid. 17:549–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rohmer V, Vidal-Trecan G, Bourdelot A,
Niccoli P, Murat A, Wemeau JL, Borson-Chazot F, Schvartz C, Tabarin
A, Chabre O, et al: Groupe Français des Tumeurs Endocrines:
Prognostic factors of disease-free survival after thyroidectomy in
170 young patients with a RET germline mutation: A multicenter
study of the Groupe Français d'Etude des Tumeurs Endocrines. J Clin
Endocrinol Metab. 96:E509–E518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frank-Raue K, Buhr H, Dralle H, Klar E,
Senninger N, Weber T, Rondot S, Höppner W and Raue F: Long-term
outcome in 46 gene carriers of hereditary medullary thyroid
carcinoma after prophylactic thyroidectomy: Impact of individual
RET genotype. Eur J Endocrinol. 155:229–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dottorini ME, Assi A, Sironi M, Sangalli
G, Spreafico G and Colombo L: Multivariate analysis of patients
with medullary thyroid carcinoma. Prognostic significance and
impact on treatment of clinical and pathologic variables. Cancer.
77:1556–1565. 1996. View Article : Google Scholar : PubMed/NCBI
|