Introduction
Prostate cancer varies from insignificant lesions to
locally advanced prostate tumors, even in cases without distant
metastasis (1,2). Thus, it is necessary to develop a finer
stage classification system in order to determine the most
appropriate therapy for patients with prostate cancer that require
more treatment options.
Diagnostic imaging is not as useful for prostate
cancer as it is for other malignant tumors. Although digital rectal
examination (DRE), ultrasound and magnetic resonance imaging are
commonly used to evaluate the clinical tumor stage (cT), these
diagnostic imaging modalities lack accuracy (3–5). By
contrast, the prostate-specific antigen (PSA) level is an excellent
tumor marker available for prostate cancer diagnosis (6). In addition, the grade of the tumor
(Gleason score) can be determined using a prostate needle biopsy
(7). Therefore, the disease stage is
classified using multiple factors. The risk classification of
prostate cancer corresponds with this concept, with the D'Amico
classification being the most widely used (8). According to the D'Amico criteria, for
convenience, the intermediate-risk group includes patients with
cT2b lesions, a biopsy Gleason score (bGS) of 7 or a PSA level of
>10 to ≤20 ng/ml. In the D'Amico classification, cases that do
not belong to the low-risk or high-risk groups are also assigned to
the intermediate-risk group. As a result, the breadth of cases
included in the intermediate-risk group is wide.
In the low-risk group, there is a high probability
that prostate cancer is localized in the prostate, and this group
is considered most likely to achieve a cure with radical
prostatectomy (RP) alone. By contrast, RP for patients in the
low-risk group also presents the possibility of over-treatment
(9). Conversely, in the high-risk
group, radiation therapy in combination with endocrine therapy is
common (10,11); however, there is no reason not to
treat with RP (12). There is a high
possibility, although lower than for the low-risk group, that RP
will achieve a cure in the intermediate-risk group. When
considering the control of cancer and postoperative quality of
life, the intermediate-risk group may receive the most benefit from
surgery.
Therefore, the present study assessed the outcomes
of radical prostatectomy (RP) in Japanese intermediate-risk
patients with prostate cancer who received no pre-surgical
treatment. The possibility of achieving a complete cure with RP
alone was evaluated.
Patients and methods
Patient characteristics and risk-group
classification
Patients who underwent prostate biopsies and
received a diagnosis of adenocarcinoma at the National Kyushu
Cancer Center (Fukuoka, Japan) or additional associated
institutions were assessed in the present study. Embedded
whole-mount antegrade RP tissue specimens obtained from 638
patients who underwent RP between August 1998 and May 2013 were
evaluated. The patients underwent pelvic lymph node dissection
during the same time period. Of the specimens obtained, 157
patients were excluded from the study, including 151 patients due
to a history of receiving hormonal therapy and 6 patients due to
unclear biopsy or prostatectomy specimen findings. All patients
were Japanese (median age, 66 years; range, 47–77 years), and the
PSA levels ranged between 0.623 and 39.413 ng/ml (median, 7.491
ng/ml). The median follow-up period after surgery was 54.1
months.
The patients were classified into three risk groups
according to the D'Amico criteria (3). The low-risk (stage T1c/T2a, PSA level
≤10 ng/ml and bGS of ≤6), intermediate-risk (stage T2b, bGS of 7 or
PSA level between >10 and ≤20 ng/ml) and high-risk (stage T2c,
PSA level >20 ng/ml or bGS of ≥8) groups comprised 107 (22.2%),
222 (46.2%) and 152 (31.6%) patients, respectively. The breadth of
cases included in the intermediate-risk group was wide, therefore,
the patients were subdivided according to factors for risk
classification, including the PSA level, cT status and bGS. The PSA
levels were divided into two subgroups of 0<PSA≤10 and
10<PSA≤20, the cT status was divided into two subgroups of cT1c
and cT2a, and the bGS score was divided into two subgroups of bGS 6
and bGS 7. cT was diagnosed using only DRE. Two pathologists
evaluated the degree of malignancy in the preoperative biopsy and
RP specimens according to the 2005 International Society of
Urological Pathology Consensus Conference on Gleason grading system
(13) and determined the pathological
stage based on the 2009 TNM classification (14).
Methods
The whole-organ prostate specimens obtained by RP
were fixed in 15% neutral-buffered formalin (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) for 48–96 h, and were serially
sectioned perpendicular to the rectal surface at 5-mm intervals.
Sections that were predominantly caudal and cephalic were cut in
the sagittal plane at 5-mm intervals in order to assess the bladder
neck and apical margins. The specimens were subsequently embedded
in paraffin (Merck KGaA, Darmstadt, Germany), cut into 5-µm
sections and stained with hematoxylin and eosin (Sakura Finetek
Japan, Co., Ltd., Tokyo, Japan). Extraprostatic extension (EPE) was
defined as the extension of the tumor from the prostate to the
periprostatic soft tissue. The presence of tumor cells at the
stained resection margin (RM) was defined as a positive RM. The
follow-up schedule after RP involved a PSA assay performed using
ARCHITECT® Automated Immunoassay Analyzer, ARCHITECT
Total PSA Calibrators (catalog no., 7K70-01) and ARCHITECT Total
PSA Controls (catalog no., 7k70-10) (Abbott Japan Co., Ltd., Chiba,
Japan) every 3 months for the first 2 years, followed by every 4
months for the next 3 years and every 6 months thereafter. Disease
recurrence and/or PSA failure were defined as the detection of a
serum PSA level of >0.2 ng/ml, or the use of RP if the PSA level
did not decrease to <0.2 ng/ml following surgery. A number of
patients who underwent RP were subsequently treated with radiation
and/or hormone therapy before the serum PSA level exceeded 0.2
ng/ml. Therefore, in these patients, the time point of adjuvant
therapy was defined as the date of disease recurrence. All patients
provided their written informed consent to participate in this
study, and the study protocol was approved by the ethics committee
of the National Kyushu Cancer Center.
Statistical analysis
Statistical analysis was performed using the JMP Pro
software package (version 11.0.0; SAS Institute, Inc., Cary, NC,
USA). The PSA failure-free survival rate was determined according
to the Kaplan-Meier method, and the significance of
clinicopathological parameters associated with PSA failure was
assessed using the Cox proportional hazards regression model. The
log-rank test was used to determine differences between the risk
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological characteristics
according to risk group
The clinicopathological characteristics of the three
risk groups are shown in Table I.
According to the D'Amico criteria, the low-risk group,
intermediate-risk group and high-risk group each contained 107
(22.2%), 222 (46.2%) and 152 (31.6%) patients, respectively. No
differences were observed in the age of the patients between the
groups. Based on the RP Gleason score, 9.3% (10/107), 15.3%
(34/222) and 42.1% (64/152) of the patients in the low-,
intermediate- and high-risk groups had high-grade (bGS ≥8) tumors,
respectively. A total of 17.8% (19/107), 35.1% (78/222) and 54.0%
(82/152) of patients in the low-, intermediate- and high-risk
groups had a pathological stage of ≥T3, respectively. Furthermore,
lymph node involvement was observed in 1 patient in the low-risk
group, 5 patients in the intermediate-risk group and 7 patients in
the high-risk group.
 | Table I.Clinicopathological characteristics
according to the risk group classification. |
Table I.
Clinicopathological characteristics
according to the risk group classification.
|
| Risk group |
|---|
|
|
|
|---|
| Characteristic | Low | Intermediate | High |
|---|
| Total patients,
n | 107 | 222 | 152 |
| Median age (range),
years | 66 (47–77) | 66 (57–76) | 67 (48–77) |
| cT, n (%) |
|
|
|
| 1c | 84 (78.5) | 157 (70.7) | 77 (50.7) |
| 2a/b | 23 (21.5) | 65 (29.3) | 47 (30.9) |
| 2c | – | – | 21 (13.8) |
| 3 | – | – | 7 (4.6) |
| Preoperative PSA, n
(%) |
|
|
|
| ≤10
ng/ml | 107 (100) | 151 (68.1) | 83 (54.6) |
| 10≤20
ng/ml | – | 71 (31.9) | 36 (23.7) |
| >20
ng/ml | – | – | 33 (21.7) |
| Biopsy Gleason score,
n (%) |
|
|
|
| ≤6 | 107 (100) | 19 (8.6) | 8 (5.2) |
| 7 | – | 203 (91.4) | 27 (17.8) |
| ≥8 | – | – | 117 (77.0) |
| RP Gleason score, n
(%) |
|
|
|
| ≤6 | 45 (42.1) | 18 (8.2) | 6 (3.4) |
| 7 | 52 (48.6) | 170 (76.5) | 82 (53.9) |
| ≥8 | 10 (9.3) | 34 (15.3) | 64 (42.1) |
| pT, n (%) |
|
|
|
|
2a/b | 18 (16.8) | 27 (12.2) | 9 (5.9) |
| 2c | 70 (65.4) | 117 (52.7) | 61 (40.1) |
| 3a | 17 (15.9) | 71 (32.0) | 64 (42.1) |
| 3b | 2 (1.9) | 7 (3.1) | 18 (11.9) |
| EPE, n (%) | 17 (15.9) | 66 (29.7) | 65 (42.8) |
| Positive RM, n
(%) | 13 (12.1) | 45 (20.3) | 35 (23.0) |
| sv, n (%) | 2 (1.9) | 7 (3.2) | 18 (11.8) |
| pN, n (%) | 1 (0.9) | 5 (2.3) | 7 (4.6) |
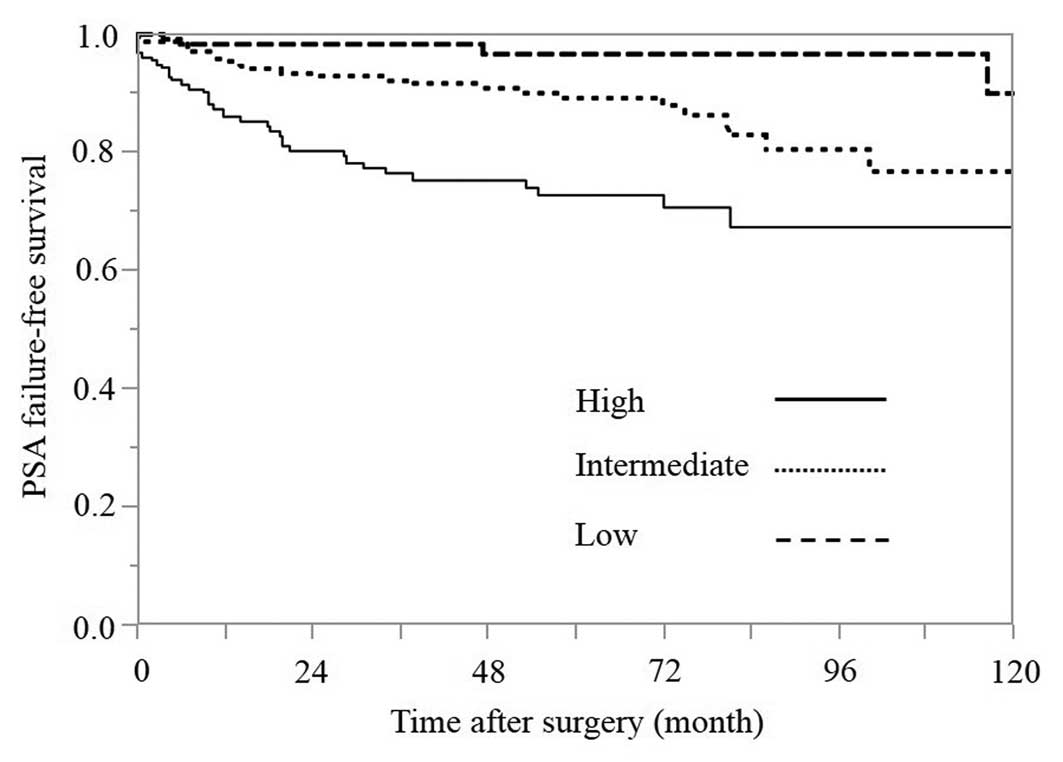
PSA failure-free survival rates
according to the risk group classification
The 5-year PSA failure-free survival rates in the
low-, intermediate- and high-risk groups were 96.5, 88.9 and 72.6%,
respectively (Fig. 1). The difference
between the low- and intermediate-risk groups was statistically
significant, according to the log-rank test (P=0.0113). In
addition, the difference between the intermediate- and high-risk
groups was statistically significant, according to the log-rank
test (P=0.0004).
Correlations between
clinicopathological characteristics and PSA failure in the
intermediate-risk group
The correlations between clinicopathological
characteristics and PSA failure in the intermediate-risk group are
shown in Table II. According to Cox
proportional hazards analysis in this group, cT was the only
preoperative variable that was a significant predictor of PSA
failure (P<0.001), whereas the postoperative variables of RP
Gleason score, pathological tumor stage, EPE, seminal vesicle
invasion and positive lymph nodes were found to be significant
predictors, based on the univariate analysis.
 | Table II.Correlations between the
clinicopathological characteristics and PSA failure in the
intermediate-risk group. |
Table II.
Correlations between the
clinicopathological characteristics and PSA failure in the
intermediate-risk group.
| Variable | Hazard ratio | P-value | 95% CI |
|---|
| Univariate
analysis |
|
|
|
| Age,
<70 vs. ≥70 yearsa | 1.180 | 0.705 | 0.518–3.022 |
| PSA,
0–10 vs. >10–20 ng/mla | 1.625 | 0.242 | 0.709–3.551 |
| cT, 1c
vs. 2a/ba | 10.274 | <0.001 | 4.350–28.227 |
| RP
Gleason score, ≤7 vs. ≥8 | 3.188 | 0.010 | 1.354–7.020 |
| pT, 2
vs. 3 | 3.627 | 0.001 | 1.653–8.516 |
| EPE, 0
vs. 1 | 3.240 | 0.003 | 1.470–7.240 |
| RM, 0
vs. 1 | 1.198 | 0.703 | 0.438–2.816 |
| sv, 0
vs. 1 | 6.378 | 0.006 | 1.834–17.017 |
| pN, 0
vs. 1 | 18.860 | <0.001 | 4.207–62.156 |
| Multivariate
analysis |
|
|
|
| Age,
<70 vs. ≥70 yearsa | 1.655 | 0.245 | 0.718–4.279 |
| PSA,
0–10 vs. >10–20 ng/mla | 1.237 | 0.636 | 0.494–2.872 |
| cT, 1c
vs. 2a/ba | 11.481 | <0.001 | 4.754–32.310 |
| RP
Gleason score, ≤7 vs. ≥8 | 1.728 | 0.237 | 0.684–4.074 |
| pT, 2
vs. 3 | 2.349 | 0.068 | 1.001–5.717 |
| EPE, 0
vs. 1 | 2.252 | 0.061 | 1.033–5.063 |
| RM, 0
vs. 1 | 1.614 | 0.334 | 0.581–3.875 |
| sv, 0
vs. 1 | 8.538 | 0.003 | 2.383–24.473 |
| pN, 0
vs. 1 | 2.914 | 0.199 | 0.529–12.559 |
In the multivariate analysis, statistically
significant differences were observed for the cT stage (P<0.001)
and seminal vesicle invasion (P=0.003) in the patients with and
without PSA failure.
PSA failure-free survival rates
according to PSA subgroups in the intermediate-risk group (Fig. 2)
Based on the range of the PSA level, the
intermediate-risk group was divided into two subgroups with values
of 0<PSA≤10 and 10<PSA≤20 ng/ml. A value of 10<PSA≤20
ng/ml was identified in 31.9% (71/222) of the patients. The 5-year
PSA failure-free survival rates in the 0<PSA≤10 and 10<PSA≤20
ng/ml groups were 91.7 and 82.5%, respectively, although the
difference between these groups was not statistically significant
according to the log-rank test (P=0.2266).
PSA failure-free survival rates
according to the bGS subgroups in the intermediate-risk group
(Fig. 3)
Based on the bGSs, the intermediate-risk group was
divided into two subgroups of bGS 6 and 7. A bGS of 6 was
identified in only 8.6% (19/222) of the patients, and there were no
cases of PSA failure (100% 5-year PSA failure-free survival). The
5-year PSA failure-free survival rate in the bGS 7 group was 87.9%,
and the difference between the bGS 6 and bGS 7 groups was not
statistically significant according to the log-rank test
(P=0.1329).
PSA failure-free survival rates based
on the primary Gleason pattern in the bGS 7 subgroup within the
intermediate-risk group (Fig. 4)
In the intermediate-risk group, a bGS of 7 was
identified in 91.4% (203/222) of the patients, and bGSs of 3+4 and
4+3 were identified in 128 and 74 patients, respectively. Only 1
patient had a bGS of 5+2. The 5-year PSA failure-free survival
rates in the bGS 3+4 and 4+3 groups were 92.7 and 79.9%,
respectively, and the difference between the bGS 3+4 and 4+3 groups
was statistically significant according to the log-rank test
(P=0.0298).
PSA failure-free survival rates
according to the cT subgroups in the intermediate-risk group
(Fig. 5)
The cT was diagnosed using only DRE. In the
intermediate-risk group, a cT2a/b status was identified in 29.3%
(65/222) of the patients. The 5-year PSA failure-free survival
rates in the cT1c and cT2a/b groups were 94.0 and 76.4%,
respectively, and the difference between the cT1c and cT2a/b groups
was statistically significant according to the log-rank test
(P<0.0001).
Discussion
RP is selected as a treatment for prostate cancer to
achieve a cure by resecting the cancer lesion. However, depending
on the patient's clinical condition, it may be difficult to resect
the prostate cancer completely and/or distant metastasis may
develop even if the entire local prostate tumor is successfully
resected. This treatment is considered to be successful if the
postoperative PSA level remains stable at a low value. Therefore,
it is understood that RP should be performed in cases with a low
likelihood of PSA recurrence. However, surgical intervention may
result in overtreatment when the indication for surgery is limited
to only patients whose clinical condition is not likely to lead to
PSA failure, as other treatments are expected to have equivalent
outcomes, and certain types of prostate cancer may be monitored
with active surveillance or a ‘watchful waiting’ strategy.
According to the 2012 version of the clinical
practice guidelines for prostate cancer in Japan (15), RP is recommended for patients who are
not expected to develop PSA recurrence, such as those with a bGS
≤7, a PSA level of <10 ng/ml and a cT of cT1c-T2c. This
guideline is based on the results of a variety of prognostic
studies with large RP samples (16–19),
although these studies were retrospective. However, there is no
definitive reason as to why RP is should not be used, even in
high-risk cases of PSA recurrence (20–23). In
other words, a complete cure with surgery alone is possible in
these cases.
In patients with prostate cancer, as in other
malignancies, it is important to assess the degree of malignancy
and determine the prognosis in order to select the appropriate
treatment. Risk classification, the grouping of patients based on
several clinical factors, is widely used in the clinical setting.
Several pre-treatment risk classification models for prostate
cancer have been proposed to date, with the D'Amico classification
being the most widely applied (8).
However, in the D'Amico classification, cases that do not belong to
the low-risk or high-risk groups are assigned to the
intermediate-risk group. Hence, the range of cases included in the
intermediate-risk group is wide.
Therefore, the present study aimed to assess the
outcomes of RP in intermediate-risk Japanese patients who received
no pre-surgical treatment in order to investigate the possibility
of achieving a complete cure with RP alone. According to the
D'Amico criteria, indicated in Table
I, the low-, intermediate- and high-risk group each contained
107 (22.2%), 222 (46.2%) and 152 (31.6%) patients, respectively.
Based on the bGS, high-grade (bGS ≥8) tumors were only noted in
patients in the high-risk group (77.0%; 117/152); by comparison,
high-grade RP Gleason scores were observed in 9.3% (10/107), 15.3%
(34/222) and 42.1% (64/152) of the patients in the low-,
intermediate- and high-risk groups, respectively, which emphasizes
that high-grade Gleason scores may be present in a proportion of
patients of in the intermediate-risk group.
The present study determined the cT classification
based on only the results of the DRE, in accordance with the
original study by D'Amico et al (8). The PSA failure-free survival rates for
each risk group are shown in Fig. 1.
The 5-year PSA failure-free survival rates in the low-,
intermediate- and high-risk groups were 96.5, 88.9 and 72.6%,
respectively (Fig. 1). The difference
between the intermediate- and high-risk groups (P=0.0004), and the
difference between the intermediate- and low-risk groups (P=0.0113)
were statistically significant according to the log-rank test. The
results indicate that the rate of a complete cure with RP alone in
the intermediate-risk group was lower than that observed in the
low-risk group and higher than that observed in the high-risk
group.
In addition, correlations between
clinicopathological characteristics and PSA failure were examined
in the intermediate-risk group (Table
II). There were no cases of PSA failure among the bGS 6 cases,
therefore, the preoperative variable of bGS was not analyzed in the
univariate or multivariate Cox proportional hazards regression
model. According to the results of multivariate analysis, cT was
the only significant predictor in the patients with and without PSA
failure (P<0.001) among the preoperative variables. Univariate
and multivariate analyses did not reveal any other statistically
significant differences in the preoperative variables, including
the preoperative PSA level (P=0.242), which is a component of the
risk profile in the D'Amico risk classification. The postoperative
variables of RP Gleason score, pathological tumor stage, EPE and
positive lymph nodes were found to be significant predictors based
on the univariate analysis (P=0.010, P=0.001, P=0.003 and
P<0.001, respectively), while the only postoperative variable
identified to be a significant predictor in the univariate and
multivariate analyses was seminal vesicle invasion (P=0.006 and
P=0.003, respectively). However, the intermediate-risk group
included only cases that did not belong to the low- or high-risk
groups; as a result, the breadth of cases included in the
intermediate-risk group was unexpectedly wide. Therefore,
additional analyses were performed in the intermediate-risk group,
including factors used for risk stratification, such as the PSA
level, bGS and cT status.
First, the patients were divided into two groups
based on the PSA values: 0<PSA≤10 and 10<PSA≤20 ng/ml
(Fig. 2). Values of 0<PSA≤10 and
10<PSA≤20 ng/ml were identified in 68.1% (151/222) and 31.9%
(71/222) of the patients, respectively. Pathological organ-confined
disease is known to occur in 80% of patients with a PSA level
<4.0 ng/ml, 66% of those with a PSA level between 4.0 and 10.0
ng/ml and <50% of those with a PSA level >10.0 ng/ml
(24,25). Therefore, it is expected that the rate
of organ-confined disease decreases as the PSA value increases and,
thus, the risk of recurrence increases. Compared with that observed
in the low-risk group, the intermediate-risk group demonstrated a
wide range of PSA values according to the D'Amico risk
classification. However, the 5-year PSA failure-free survival rates
in the 0<PSA≤10 and 10<PSA≤20 ng/ml groups were 91.7 and
82.5%, respectively, and the difference between the 0<PSA≤10 and
10<PSA≤20 ng/ml groups was not statistically significant
according to the log-rank test (P=0.2266).
Next, the patients were divided into two groups
based on the bGS: bGS 6 and bGS 7 (Fig.
3). bGS 6 was identified only in 8.6% (19/222) of the patients
and bGS 7 was identified in 91.4% (203/222) of the patients. There
were no episodes of PSA failure in the bGS 6 group (PSA
failure-free survival rate, 100%), and the 5-year PSA failure-free
survival rate in the bGS 7 group was 87.9%. The difference between
the bGS 6 and 7 groups was not statistically significant according
to the log-rank test (P=0.1329). This result is considered to be a
contributory factor to the lack of PSA failure among bGS 6 cases.
Furthermore, the bGS 7 pattern is additionally considered to be a
contributory factor, as the intermediate-risk group consisted
largely of patients with a bGS 7 score. According to the bGS 7
pattern in the intermediate-risk group, the bGS 3+4 and 4+3
subgroups contained 128 (63.1%) and 74 (36.5%) patients, and the
bGS 5+2 subgroup contained 1 patient. In the present study, among
all of the bGS7 cases, excluding the single case of bGS 5+2, the
5-year PSA failure-free survival rates in the bGS 3+4 and 4+3
groups were 92.7 and 79.9%, respectively (Fig. 4). The difference between the bGS 3+4
and 4+3 groups was statistically significant according to the
log-rank test (P=0.0298).
Although numerous grading systems exist for
evaluating prostate adenocarcinoma, the Gleason grading system is
the most widely accepted (26). The
Gleason system is based on the glandular pattern of the tumor, as
identified at relatively low magnification; cytological features
have no role in the grade of the tumor. The primary (predominant)
and secondary (second most prevalent) architectural patterns are
identified and assigned a grade between 1 and 5, with 1 being the
most differentiated and 5 being the least differentiated. As both
the primary and secondary patterns are influential for predicting
the prognosis, the Gleason sum score is obtained by adding the
primary and secondary grades. It is important to recognize Gleason
pattern 4 tumors, as tumors with this pattern are associated with a
significantly worse prognosis than those with pure Gleason pattern
3 (27,28). It has also been demonstrated in RP
specimens that tumors with a Gleason score of 4+3=7 exhibit a worse
prognosis than those with a Gleason score of 3+4=7 (29). These descriptions are consistent with
the observations made in the present study.
Finally, the patients in the present study were
divided into two groups based on the cT status: cT1c and cT2a/b.
cT1c and cT2a/b was identified in 70.7% (157/222) and 29.3%
(65/222) of the patients, respectively. Numerous imaging modalities
are applied for staging prostate cancer; however, no technique is
reliably sensitive for detecting extraprostatic disease. The
inability to image microscopic disease limits the accuracy of
current modalities (30). In the
current study, the cT classification was determined based only on
the results of DRE, in accordance with the original study by
D'Amico et al (8). The 5-year
PSA failure-free survival rates in the clinical cT1c and cT2a/b
groups were 94.0 and 76.4%, respectively (Fig. 5), and the difference between the cT1c
and cT2a/b groups was statistically significant according to the
log-rank test (P<0.0001). An abnormal DRE was recently reported
to be associated with an increased risk of detecting high-grade
(Gleason score >7) prostate cancer lesions in a screened
population (31–34). These descriptions are consistent with
the observations obtained in the present study.
In summary, the present study retrospectively
assessed the outcomes of performing RP alone in Japanese patients
with intermediate-risk prostate cancer based on the D'Amico risk
classification. Patients classified into the intermediate-risk
group with cT2a/b stage, based on positive DRE findings, are not
considered to be likely to achieve a complete cure with RP surgery
alone. Furthermore, the current findings for intermediate-risk
group RP patients demonstrated that tumors with a bGS of 4+3=7 are
associated with poorer PSA failure-free survival rates than those
with a bGS of 3+4=7 in the bGS 7 group. Therefore, for patients
meeting these criteria in the intermediate-risk group, RP surgery
alone is likely to be insufficient, and other additional treatments
may be considered subsequent to RP.
References
|
1
|
Cooperberg MR, Broering JM and Carroll PR:
Time trends and local variation in primary treatment of localized
prostate cancer. J Clin Oncol. 28:1117–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilt TJ, Brawer MK, Jones KM, Barry MJ,
Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, et al:
Prostate Cancer Intervention versus Observation Trial (PIVOT) Study
Group: Radical prostatectomy versus observation for localized
prostate cancer. N Engl J Med. 367:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rifkin MD: MRI of the prostate. Crit Rev
Diagn Imaging. 31:223–262. 1990.PubMed/NCBI
|
|
4
|
Tempany CM, Zhou X, Zerhouni EA, Rifkin
MD, Quint LE, Piccoli CW, Ellis JH and McNeil BJ: Staging of
prostate cancer: Results of Radiology Diagnostic Oncology Group
project comparison of three MR imaging techniques. Radiology.
192:47–54. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf JS Jr, Cher M, Dall'era M, Presti JC
J, Hricak H and Carroll PR: The use and accuracy of cross-sectional
imaging and fine needle aspiration cytology for detection of pelvic
lymph node metastases before radical prostatectomy. J Urol.
153:993–999. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oesterling JE, Jacobsen SJ, Klee GG,
Pettersson K, Piironen T, Abrahamsson PA, Stenman UH, Dowell B,
Lövgren T and Lilja H: Free, complexed and total serum prostate
specific antigen: The establishment of appropriate reference ranges
for their concentrations and ratios. J Urol. 154:1090–1095. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) consensus conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Amico AV, Whittington R, Malkowicz SB,
Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA,
Kaplan I, Beard CJ and Wein A: Biochemical outcome after radical
prostatectomy, external beam radiation therapy, or interstitial
radiation therapy for clinically localized prostate cancer. JAMA.
280:969–974. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith JA Jr: Radical prostatectomy for low
risk carcinoma of the prostate. World J Urol. 26:443–446. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolla M, Collette L, Blank L, Warde P,
Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg
C, et al: Long-term results with immediate androgen suppression and
external irradiation in patients with locally advanced prostate
cancer (an EORTC study): a phase III randomised trial. Lancet.
360:103–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Amico AV, Manola J, Loffredo M, Renshaw
AA, DellaCroce A and Kantoff PW: 6-month androgen suppression plus
radiation therapy vs radiation therapy alone for patients with
clinically localized prostate cancer: a randomized controlled
trial. JAMA. 292:821–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pierorazio PM, Ross AE, Lin BM, Epstein
JI, Han M, Walsh PC, Partin AW, Pavlovich CP and Schaeffer EM:
Preoperative characteristics of high-Gleason disease predictive of
favourable pathological and clinical outcomes at radical
prostatectomy. BJU Int. 110:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) consensus conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: Urological Tumors. TNM Classification of Malignant Tumors
(7th). Wiley-Blackwell. (Oxford). 243–248. 2009.
|
|
15
|
The Japanese Urological Association (eds):
Surgical Therapy. In: Clinical Practice Guidelines for Prostate
Cancer in Japan. 2012 version. Kanehara-shuppan Press. (Tokyo).
115–117. 2012.
|
|
16
|
Roehl KA, Han M, Ramos CG, Antenor JA and
Catalona WJ: Cancer progression and survival rates following
anatomical radical retropubic prostatectomy in 3,478 consecutive
patients: Long-term results. J Urol. 172:910–914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Porter CR, Kodama K, Gibbons RP, Correa R
Jr, Chun FK, Perrotte P and Karakiewicz PI: 25-year prostate cancer
control and survival outcomes: A 40-year radical prostatectomy
single institution series. J Urol. 176:569–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isbarn H, Wanner M, Salomon G, Steuber T,
Schlomm T, Köllermann J, Sauter G, Haese A, Heinzer H, Huland H and
Graefen M: Long-term data on the survival of patients with prostate
cancer treated with radical prostatectomy in the prostate-specific
antigen era. BJU Int. 106:37–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suardi N, Porter CR, Reuther AM, Walz J,
Kodama K, Gibbons RP, Correa R, Montorsi F, Graefen M, Huland H, et
al: A nomogram predicting long-term biochemical recurrence after
radical prostatectomy. Cancer. 112:1254–1263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carver BS, Bianco FJ Jr, Scardino PT and
Eastham JA: Long-term outcome following radical prostatectomy in
men with clinical stage T3 prostate cancer. J Urol. 176:564–568.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gontero P, Marchioro G, Pisani R,
Zaramella S, Sogni F, Kocjancic E, Mondaini N, Bonvini D, Tizzani A
and Frea B: Is radical prostatectomy feasible in all cases of
locally advanced non-bone metastatic prostate cancer? Results of a
single-institution study. Eur Urol. 51:922–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Poppel H and Joniau S: An analysis of
radical prostatectomy in advanced stage and high-grade prostate
cancer. Eur Urol. 53:253–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miocinovic R, Berglund RK, Stephenson AJ,
Jones JS, Fergany A, Kaouk J and Klein EA: Avoiding androgen
deprivation therapy in men with high-risk prostate cancer: The role
of radical prostatectomy as initial treatment. Urology. 77:946–950.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Catalona WJ, Smith DS and Ornstein DK:
Prostate cancer detection in men with serum PSA concentrations of
2.6 to 4.0 ng/ml and benign prostate examination. Enhancement of
specificity with free PSA measurements. JAMA. 277:1452–1455. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rietbergen JB, Hoedemaeker RF, Kruger AE,
Kirkels WJ and Schröder FH: The changing pattern of prostate cancer
at the time of diagnosis: Characteristics of screen detected
prostate cancer in a population based screening study. J Urol.
161:1192–1198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974.PubMed/NCBI
|
|
27
|
McNeal JE, Villers AA, Redwine EA, Freiha
FS and Stamey TA: Histologic differentiation, cancer volume, and
pelvic lymph node metastasis in adenocarcinoma of the prostate.
Cancer. 66:1225–1233. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Epstein JI, Pizov G and Walsh PC:
Correlation of pathologic findings with progression after radical
retropubic prostatectomy. Cancer. 71:3582–3593. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan TY, Partin AW, Walsh PC and Epstein
JI: Prognostic significance of Gleason score 3+4 versus Gleason
score 4+3 tumor at radical prostatectomy. Urology. 56:823–827.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wein AJ, Kavoussi LR, Novick AC, Partin
AW, Peters CA and Ramchandani P: Prostate cancer tumor markers.
Campbell-Walsh urology tenth edition review. McDougal SW: (10th).
Elsevier Saunders. (Philadelphia, PA). 2748–2770. 2011.
|
|
31
|
Gosselaar C, Roobol MJ, Roemeling S and
Schröder FH: The role of the digital rectal examination in
subsequent screening visits in the European randomized study of
screening for prostate cancer (ERSPC), Rotterdam. Eur Urol.
54:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borden LS Jr, Wright JL, Kim J,
Latchamsetty K and Porter CR: An abnormal digital rectal
examination is an independent predictor of Gleason > or =7
prostate cancer in men undergoing initial prostate biopsy: A
prospective study of 790 men. BJU Int. 99:559–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okotie OT, Roehl KA, Han M, Loeb S, Gashti
SN and Catalona WJ: Characteristics of prostate cancer detected by
digital rectal examination only. Urology. 70:1117–1120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thompson IM, Ankerst DP, Chi C, Goodman
PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL and Coltman CA Jr:
Assessing prostate cancer risk: Results from the prostate cancer
prevention trial. J Natl Cancer Inst. 98:529–534. 2006. View Article : Google Scholar : PubMed/NCBI
|