Introduction
Meningioma is a common brain tumor accounting for
~20% of all primary intracranial neoplasms (1), while schwannoma is a type of nerve
sheath tumor. Both meningiomas and schwannomas may imitate
intracranial solitary fibrous tumors (SFTs) histologically and
radiologically. SFTs are spindle-cell mesenchymal neoplasms.
Concurrence of intracranial SFTs and other tumor types is
particularly rare, and SFTs are easily misdiagnosed due to a lack
of typical symptoms and imaging features. By contrast, meningiomas,
which arise from cells covering the arachnoid layer of the dura
mater or from the intraventricular choroid plexus, present with a
typical dural tail sign upon magnetic resonance imaging (MRI)
(2,3).
Histologically, both meningiomas and SFTs are composed of
interlacing fascicles of spindle or ovoid tumor cells with
intervening collagen bands. Surgery is the first choice of therapy
for SFTs, with a good prognosis. In particular, stereotactic and
external beam radiation therapy may be recommended for postsurgical
tumor remnants and for unresectable recurrences (4). Analysis of the literature identified
~220 cases of SFTs, of which the majority were intracranial. In
decreasing frequency, intracranial tumors involved the
supratentorial and infratentorial compartments, the pontocerebellar
angle, the sellar and parasellar regions, and the cranial nerves
(4). The current study describes the
case of a patient who presented with two primary intracranial
tumors that originated from different cell types. The case report
is followed by a discussion of the pathogenesis of multiple
intracranial tumors and a brief literature review. Written informed
consent was obtained from the patient.
Case report
A 71-year-old woman was admitted to the Tianjin
Huanhu Hospital (Tianjin, China) on September 7, 2012. The patient
presented with progressive eyesight impairment, dizziness and right
hemiparesis. Routine biochemical and hematological tests were
within normal limits.
MRI (MAGNETOM Trio, A Tim System 3 Tesla; Siemens
AG, Munich, Germany) revealed two primary tumors that were in close
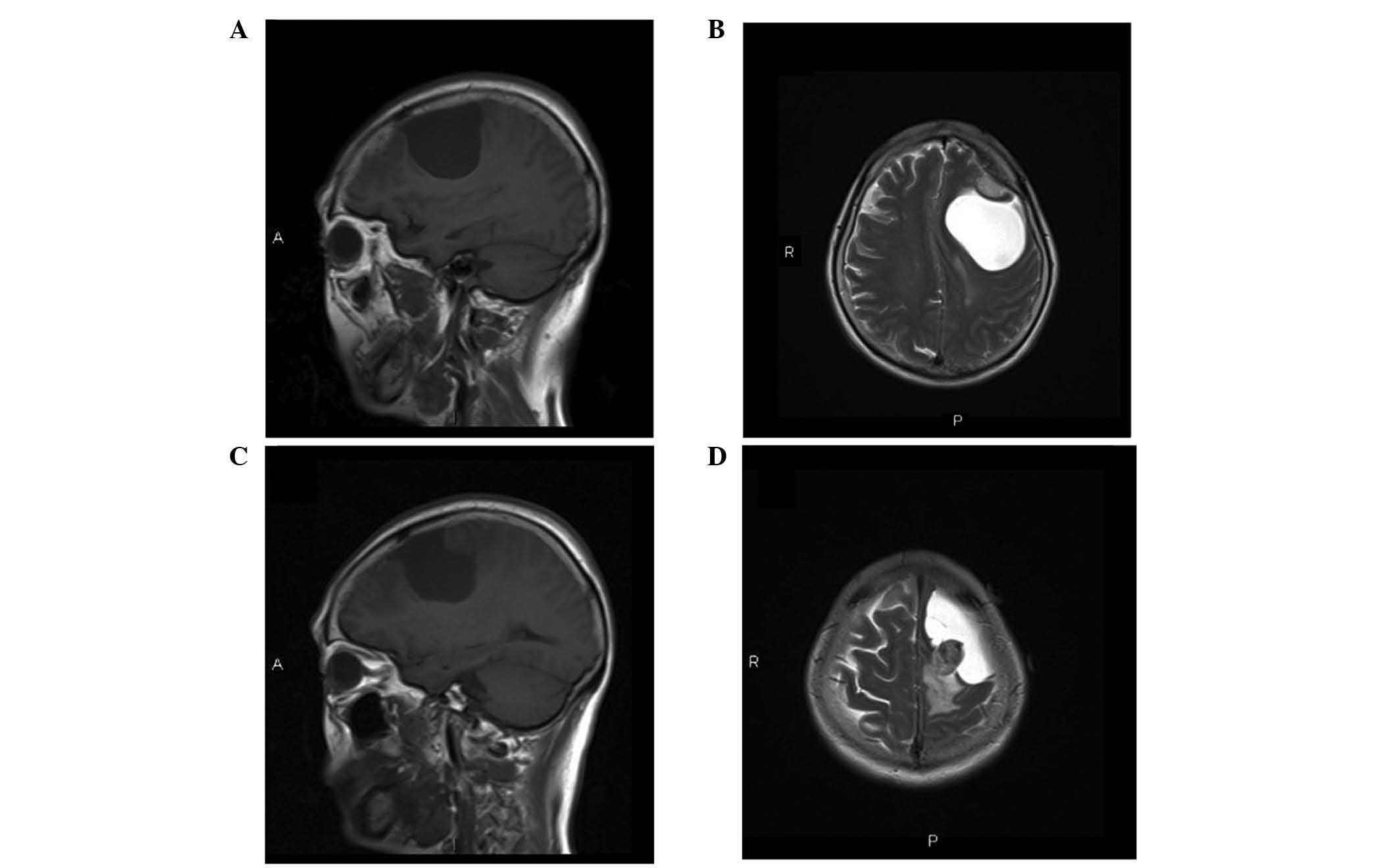
proximity (Fig. 1). The first was a
solid lesion, measuring 20×16×14 mm in size, with a clear boundary
and visible peritumoral edema. The tumor had originated from the
left frontal convex and was adhered to the dura mater, connecting
to the adjacent skull with a wide base, with associated bone
hyperplasia. The lesion was isointense to the brain parenchyma on
T1- and T2-weighted images (Fig. 1A and
B). The tumor demonstrated intense and homogeneous enhancement
following the intravenous administration of gadolinium (Fig. 2A). The radiological and clinical
features were highly indicative of a meningioma. The second lesion
was located in close proximity to the first lesion, and was cystic
and solid with an irregular shape, measuring 45×46×66 mm in size.
The cystic region of the mass exhibited hypointensity on
T1-weighted images and hyperintensity on T2-weighted images. The
solid region of the mass exhibited isointensity to adjacent brain
tissue on T1-weighted images and iso- or hyperintensity on
T2-weighted images (Fig. 1C and D).
In addition, the mass demonstrated intense and homogeneous
enhancement following the intravenous administration of gadolinium
(Fig. 2A). The clinical features were
suggestive of a hemangiopericytoma or astrocytoma.
The patient underwent a left temporoparietal
craniectomy, and complete excision of each tumor was achieved. A
well-defined, 20×16×14 mm, solid tumor, which was located in the
left frontal convex, was extirpated along with the attached dura
mater. Following excision, the tumors were placed in normal saline
and sent to the Department of Pathology in Tianjin Huanhu Hospital
for pathological, histological and immunohistochemical analysis.
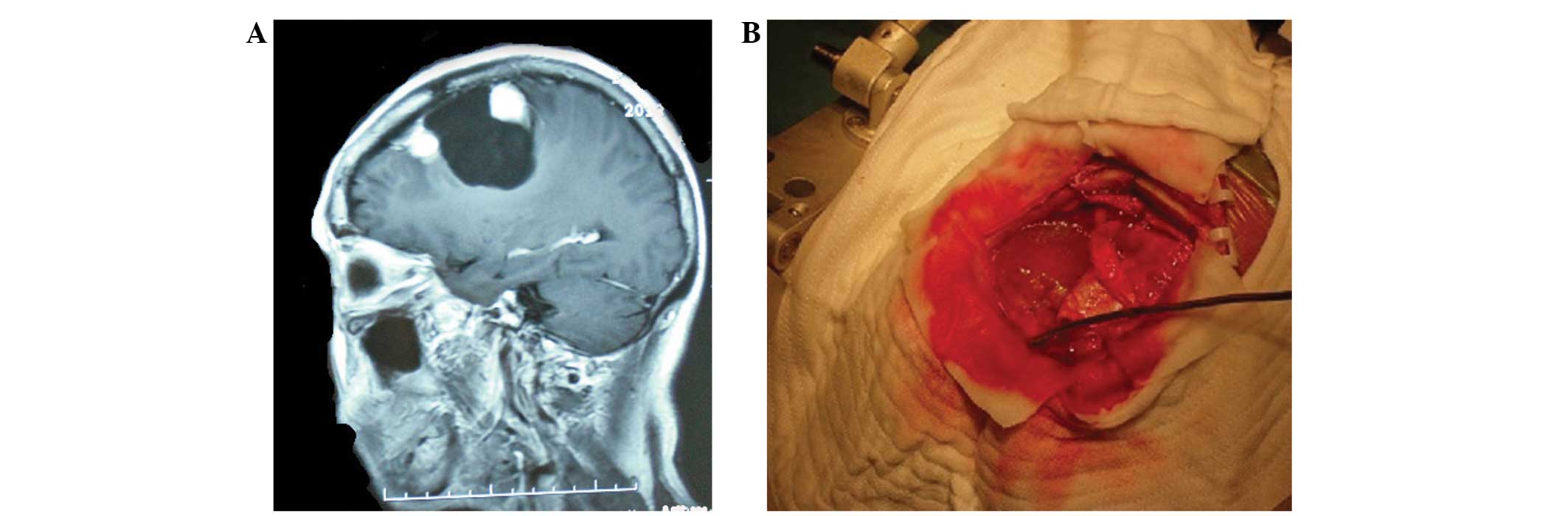
Pathological examination confirmed that this mass was a secretory
meningioma. The second solid mass was encapsulated, contained
yellow cystic liquid and was located in close proximity to the
meningioma (Fig. 2B). This lesion was
located in a capsule wall, measured 45×46×66 mm in size and was
separated from the dura mater. Pathological examination confirmed a
diagnosis of an SFT.
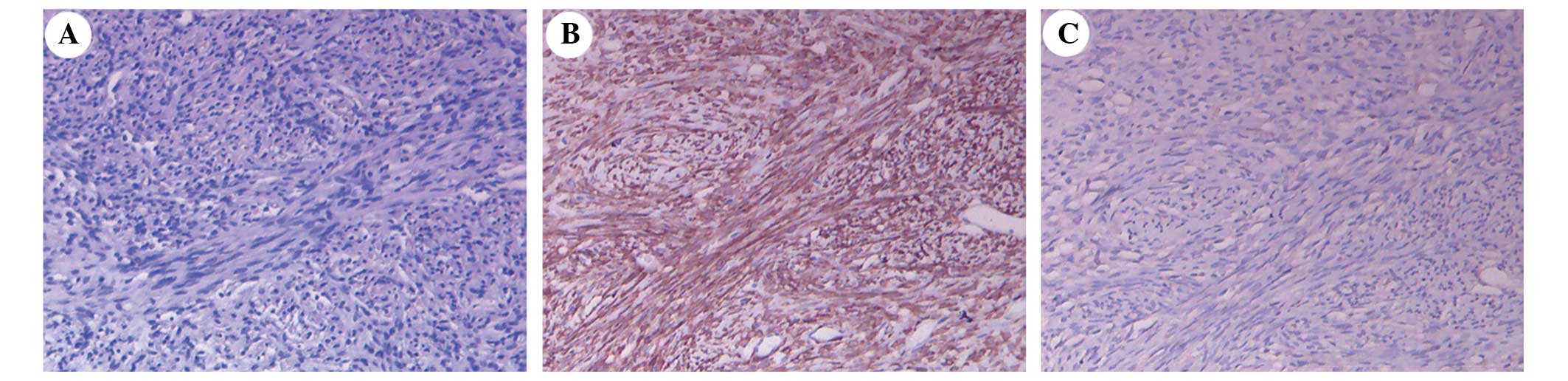
Following histological analysis of the specimens, it
was noted that the SFT was composed of proliferating spindle cells
(Fig. 3A). Immunohistochemistry
determined that the SFT cells were positive for cluster of
differentiation (CD)34, vimentin, B-cell lymphoma 2 (Bcl-2)
(Fig. 3B) and CD117, and negative for
epithelial membrane antigen (EMA) (Fig.
3C) and S-100, with a Ki-67 proliferation labeling index of
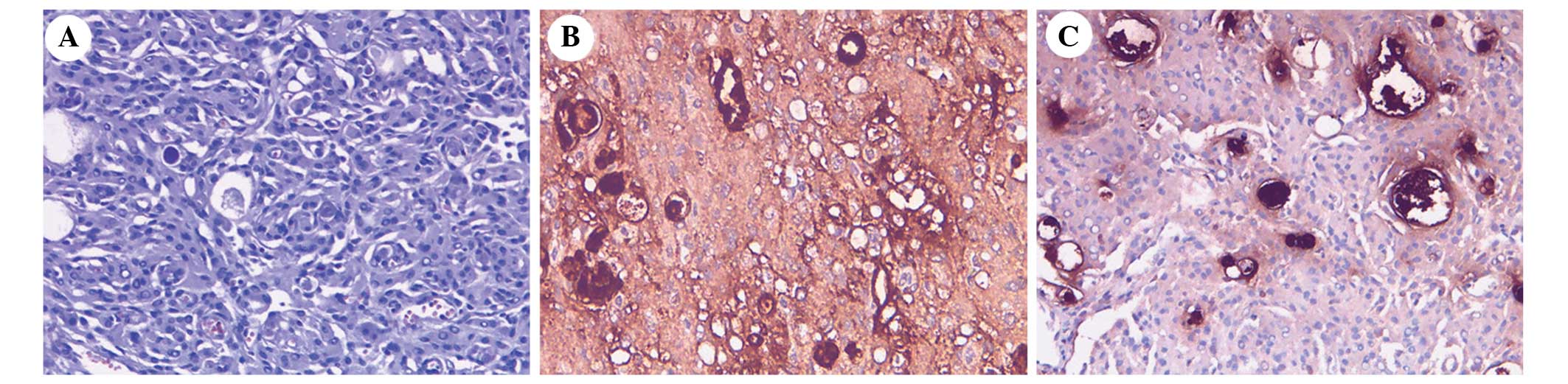
~2.5%. Histological examination of the secretory meningioma
demonstrated evidence of multifocal epithelial cell differentiation
and an intraepithelial microcavity containing eosinophil
pseudopsammoma bodies (Fig. 4A).
Immunohistochemistry determined that the secretory meningioma cells
were positive for EMA (Fig. 4B),
vimentin and carcinoembryonic antigen (Fig. 4C), with a Ki-67 proliferation labeling
index of ~2.3%. Periodic acid-Schiff staining was positive. No
complications appeared following surgery. The patient was
followed-up at 4 and 8 months and every 12 months subsequent to
surgery. At the 8-month follow-up, there were no signs of
recurrence.
Discussion
Multiple primary intracranial neoplasms were first
described in 1938 (5), and since
then, an increasing number of cases have been reported. However,
the majority of cases report the incidence of common intracranial
tumors, including glioma and meningioma (6). The current study introduces a case that
presented with the co-occurrence of mixed intracranial tumors. The
tumors consisted of a secretory meningioma, a relatively uncommon
subtype of meningioma, and an intracranial SFT, which is extremely
rare. To the best of our knowledge, this is the first case of its
type to be reported in the literature.
Although various theories have been proposed to
explain the occurrence of multiple primary intracranial neoplasms
of diverse germinal origins in the same individual, none of these
have yet been proven. The concurrence of the tumors could be
considered as purely coincidental. The majority of reported cases
have presented with common intracranial tumors that were not in a
close juxtaposition (7). If one tumor
is close to or intermixed with another, there may be an association
between them. The present study proposes that an initial tumor may
form and function as an irritating agent, subsequently inducing and
stimulating the excessive growth of a second lesion (8). It is generally considered that the
relatively slow growth of benign stimulation induced the malignant
tumor. With regard to the current case, it was hypothesized that
the meningioma functioned as a stimulus source, which subsequently
induced the SFT.
Other theories have been proposed stating that there
may be certain unidentified carcinogens serving as stimuli, which
result in the development of tumors in different tissues (9), or that residual embryonic structures may
instead form the basis of multiple lesions (10).
It has also been hypothesized that common genes may
be implicated in the development and progression of concurrent
tumors. According to Black et al (11), deletion of chromosome 22 in patients
with type 2 neurofibromatosis, and in up to 50% of solitary
meningiomas, is associated with the appearance of multiple
meningiomas (11). Previously, a
meningioma-associated tumor suppressor gene was identified on the
long arm of chromosome 14, determined as N-myc downstream-regulated
gene 2, which was commonly inactivated in clinically aggressive
meningiomas (12). However, only 1
case of an SFT of the central nervous system (CNS) has been
detected by DNA analysis and flow cytometry, and 2 cases have been
detected by molecular analyses (4).
Therefore, further research is required to draw reliable
conclusions.
Currently, no etiological association has been
identified between meningiomas and SFTs. A review of the literature
demonstrated that there have been no cases reported that are
similar to the present case. The theory of stimulation may account
for this pattern of tumoral linkage, but an increased number of
similar cases in the future may enable identification of a
potential association between such tumors.
With regard to the present case, a pre-operative
diagnosis was challenging. According to the clinical and imaging
features alone, the lesions were diagnosed as meningioma and
hemangiopericytoma or astrocytoma. As the diagnosis of SFT proved
to be difficult, it is necessary to include a brief literature
review for intracranial SFT in the present study.
An SFT is a rare, mesenchymal neoplasm, which was
first described as a pleural lesion by Klemperer and Rabin in 1931
(13). SFTs of the meninges were
originally described by Carneiro et al in 1996 (14). The origin of SFTs has been a subject
of controversy; they are typically dura-based, but may also present
as intraventricular masses arising from cranial nerves or
ubiquitous CD34-positive, dendritic, fibroblastic cells, which do
not have an apparent association with the meninges (15,16). The
World Health Organization classification of tumors of the CNS
states that mesenchymal, non-meningothelial tumors originate from
submesothelial, mesenchymal, fibroblast-like cells as opposed to
developing from the mesothelium itself (17). The spine and posterior fossa are the
most frequent locations for SFTs to develop (18). These tumors primarily occur following
the third decade of life, with patient ages ranging from 33–75
years (19), and demonstrate a slight
female preference, with a male to female ratio of 1:1.5 (19,20).
There are no reliable neuroradiological signs of an
SFT, therefore, the pre-operative diagnosis is challenging. SFTs
are generally isointense on T1-weighted MRI and hyperintense on
T2-weighted MRI. Cystic lesions commonly exhibit peripheral
enhancement (21). In the present
case, the SFT appeared isointense to adjacent brain tissue on
T1-weighted MRI and iso- or hyperintense on T2-weighted images.
Following intravenous contrast administration, the tumor exhibited
homogeneous enhancement.
In the current case, radiological evaluation could
not provide an accurate diagnosis, and detailed histopathological
and immunohistochemical examinations were required. Histologically,
SFTs are composed of interlacing fascicles of spindle to ovoid
tumor cells, with intervening bands of collagen (21). Immunohistochemically, the tumor cells
demonstrate strong positivity for CD34, vimentin and the
antiapoptotic marker Bcl-2, and are typically negative for EMA and
S-100 protein. By contrast, meningiomas are usually positive for
EMA and negative for CD34 (22). In
the present case, the immunohistochemical findings were consistent
with the features of SFTs.
Regarding the treatment of SFTs, surgery is the
preferred choice of management. The tumors are typically
well-circumscribed and therefore amenable to gross total resection.
Radiotherapy, including external beam radiation therapy or
gamma-knife radiosurgery, is administered in cases that experience
incomplete (partial or subtotal) resection, or in certain cases
with malignant histology or recurrence (23). If the proliferation rate is high, the
chemotherapeutic agent, toremifene, may also be administered
(23). In the present case, the tumor
was totally resected and no further treatment was required.
Due to the limited available data, the clinical
behavior of these tumors is unpredictable. Although the majority of
SFTs behave in a benign manner, recurrence, cerebrospinal fluid
dissemination and malignant variants with distal metastasis have
been reported (24). With regard to
recurrence, the Ki-67/MIB-1 labeling index (>5%) is a useful
marker of the risk of recurrence and tumor grade in the
prognostication of SFTs of the CNS. Although the Ki-67
proliferation labeling index was particularly low (~2.5%) in the
present case, long-term follow-up is essential to detect any signs
of recurrence.
In conclusion, to the best of our knowledge, the
current case is the first of its type to report of an SFT with
concurrent meningioma. Despite SFT being rare, it should be
considered in the neuroimaging differential diagnosis.
Immunohistochemical examination is particularly important in aiding
the differentiation between SFT and the more prevalent meningioma
and schwannoma, which may imitate SFT histologically and
radiologically. Surgical removal is considered as the optimal
therapeutic strategy in managing this rare entity. As such lesions
typically exhibit benign histological behavior, generally no
adjuvant post-operative therapy is required; however, long-term
follow-up is essential to detect any signs of possible recurrence.
The possibility of the coexistence of multiple tumors at two sites
should be taken into consideration. In order to understand the
mechanisms underlying the development of multiple intracranial
tumors, further research and a greater number of case studies are
required.
References
|
1
|
Walker AE, Robins M and Weinfeld FD:
Epidemiology of brain tumors: The national survey of intracranial
neoplasms. Neurology. 35:219–226. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang ZY, Qiu K, Ma YH, Wang XT, Bao JJ,
Zhang ZF and Liu XZ: Intracranial solitary fibrous tumors: A report
of two cases and a review of the literature. Oncol Lett.
11:1057–1060. 2016.PubMed/NCBI
|
|
3
|
Thway K, Ng W, Noujaim J, Jones RL and
Fisher C: The current status of solitary fibrous tumor: Diagnostic
features, variants, and genetics. Int J Surg Pathol. Jan
25–2016.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Bisceglia M, Galliani C, Giannatempo G,
Lauriola W, Bianco M, D'angelo V, Pizzolitto S, Vita G, Pasquinelli
G, Magro G and Dor DB: Solitary fibrous tumor of the central
nervous system: A 15-year literature survey of 220 cases (August
1996-July 2011). Adv Anat Pathol. 18:356–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cushing H and Eisenhardt L: Meningiomas:
Their Classification, Regional Behaviour, Life History and Surgical
End Results. Charles C Thomas. Springfield, IL: 1938.
|
|
6
|
Lee EJ, Chang CH, Wang LC, Hung YC and
Chen HH: Two primary brain tumors, meningioma and glioblastoma
multiforme, in opposite hemispheres of the same patient. J Clin
Neurosci. 9:589–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Russell DS and Rubinstein LJ: Pathology of
Tumors of the Nervous System (5th). Edward Arnold. London:
1989.
|
|
8
|
Spallone A, Santoro A, Palatinsky E and
Giunta F: Intracranial meningiomas associated with glial tumours: A
review based on 54 selected literature cases from the literature
and 3 additional personal cases. Acta Neurochir (Wien).
110:133–139. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myerson PG: Multiple tumors of the brain
of diverse origin. J Neuropathol Exp Neurol. 1:406–415. 1942.
View Article : Google Scholar
|
|
10
|
Andrioli GC, Zuccarello M, Scanarini M and
d'Avella D: Concurrent primary intracranial tumours of different
histogenesis. Acta Neuropathol Suppl. 7:111–115. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Black P, Morokoff A, Zauberman J, Claus E
and Carroll R: Meningiomas: Science and surgery. Clin Neurosurg.
54:91–99. 2007.PubMed/NCBI
|
|
12
|
Lusis EA, Watson MA, Chicoine MR, Lyman M,
Roerig P, Reifenberger G, Gutmann DH and Perry A: Integrative
genomic analysis identifies NDRG2 as a candidate tumor suppressor
gene frequently inactivated in clinically aggressive meningioma.
Cancer Res. 65:7121–7126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klemperer P and Rabin CB: Primary
neoplasms of the pleura. Arch Pathol. 11:385–412. 1931.
|
|
14
|
Carneiro SS, Scheithauer BW, Nascimento
AG, Hirose T and Davis DH: Solitary fibrous tumor of the meninges:
A lesion distinct from fibrous meningioma. A clinicopathologic and
immunohistochemical study. Am J Clin Pathol. 106:217–224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alapatt JP, Ajaya KA, Govindan A, Rajeev
MP and Radhakrishnan M: Solitary fibrous tumor of the tentorium: A
case report. Turk Neurosurg. 22:454–457. 2012.PubMed/NCBI
|
|
16
|
Badion ML, Lim CC, Teo J, Ong PL and Hui
F: Solitary fibrous tumor of the hypoglossal nerve. AJNR Am J
Neuroradiol. 24:343–345. 2003.PubMed/NCBI
|
|
17
|
Louis DN, Ohgaki H, Wiesler OD and Cavenee
WK: WHO Classification of Tumours of the Central Nervous System
(4th). IARC. Lyon: 2007.
|
|
18
|
Caroli E, Salvati M, Orlando ER, Lenzi J,
Santoro A and Giangaspero F: Solitary fibrous tumors of the
meninges: Report of four cases and literature review. Neurosurg
Rev. 27:246–251. 2004.PubMed/NCBI
|
|
19
|
Metellus P, Bouvier C, Guyotat J, Fuentes
S, Jouvet A, Vasiljevic A, Giorgi R, Dufour H, Grisoli F and
Figarella-Branger D: Solitary fibrous tumors of the central nervous
system: Clinicopathological and therapeutic considerations of 18
cases. Neurosurgery. 60:715–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deniz K, Kontas O, Tucer B and Kurtsoy A:
Meningeal solitary fibrous tumor: Report of a case and literature
review. Folia Neuropathol. 43:178–185. 2005.PubMed/NCBI
|
|
21
|
Mekni A, Kourda J, Hammouda KB, Tangour M,
Kchir N, Zitouna M and Haouet S: Solitary fibrous tumour of the
central nervous system: Pathological study of eight cases and
review of the literature. Pathology. 41:649–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki SO, Fukui M, Nishio S and Iwaki T:
Clinicopathological features of solitary fibrous tumor of the
meninges: An immunohistochemical reappraisal of cases previously
diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol
Int. 50:808–817. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reames DL, Mohila CA and Sheehan JP:
Treatment of intracranial solitary fibrous tumors with gamma knife
radiosurgery: Report of two cases and review of literature.
Neurosurgery. 69:E1023–E1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyashita K, Hayashi Y, Fujisawa H,
Hasegawa M and Yamashita J: Recurrent intracranial solitary fibrous
tumor with cerebrospinal fluid dissemination. Case report. J
Neurosurg. 101:1045–1048. 2004. View Article : Google Scholar : PubMed/NCBI
|