Introduction
Ovarian cancer is the leading cause of gynecologic
cancer-related mortality worldwide, as the majority of patients
present with advanced disease at diagnosis (1). The standard treatment for ovarian cancer
is surgical cytoreduction and systemic chemotherapy, typically
paclitaxel and platinum (2). Although
improvement in median survival has been observed in recent decades,
the majority of patients eventually succumb to recurrent,
progressive disease due to resistance to chemotherapy (3). A combination of paclitaxel and
carboplatin has widely been used as the first-line chemotherapy for
patients with ovarian cancer. Paclitaxel acts specifically during
the G2-M phase of the cell cycle by inducing abnormal spindles and
disruption of microtubule dynamics, thereby blocking cell cycle
progression. Despite its initial effectiveness as a cancer
therapeutic agent, in the majority cases patients eventually become
insensitive to paclitaxel-based chemotherapy and relapse (4). With the increasing emergence of
paclitaxel resistance, the identification of suitable biomarkers
for predicting chemosensitivity to paclitaxel may be key for
improving the therapeutic outcome of patients with ovarian
cancer.
Cathepsin L (CTSL), a lysosomal endopeptidase
expressed in most eukaryotic cells, is a member of the papain-like
family of cysteine proteinases (5).
CTSL has a major role in antigen processing, tumor invasion and
metastasis, bone resorption, and turnover of intracellular and
secreted proteins involved in growth regulation (6). Increased CTSL levels have been
identified in multiple tumor types and associated with short
survival of several types of cancer. In addition to its
well-established roles in development, growth and carcinogenesis,
CTSL has been implicated in drug resistance (7–9). In a
recent study, CTSL was overexpressed in ovarian cancer (10); however, no research regarding the
association between CTSL with paclitaxel resistance in ovarian
cancer has been performed thus far. In the present study, we
hypothesized that high CTSL would be associated with intrinsic
clinical drug resistance, manifesting as decreased time to disease
progression/recurrence in patients with ovarian cancer.
Materials and methods
Cell lines
SKOV3 human ovarian adenocarcinoma and SKOV3/TAX
paclitaxel-resistant human ovarian adenocarcinoma cells were
purchased from the Cell Bank of the Chinese Academy of Sciences
Institute (Shanghai, China). SKOV3 and SKOV3/TAX cells were
cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum and 100 U/ml
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All cell lines were maintained at 37°C in
humidified incubator with 5% CO2. Cell culture medium
was changed every 3–5 days depending on cell density. For routine
passage, cells were split at a ratio of 1:4 when they reached
85–90% confluency.
Western blotting analysis
Cell samples were solubilized in sodium dodecyl
sulfate (SDS) lysis buffer (Beijing Solarbio Science and
Technology, Co., Ltd., Beijing, China) and the protein
concentrations were detected using the BCA protein assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Equal quantities
of protein sample (30 mg/lane) were separated by electrophoresis
through 9.0% resolving SDS-polyacrylamide gel and then transferred
to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The non-specific binding sites were blocked by immersing
the membrane into 5% non-fat milk in Tris-buffered saline with 0.5%
Tween-20 (TBS-T) solution (Beijing Solarbio Science and Technology,
Co., Ltd.) for 1 h, and then incubating the membrane with a primary
mouse monoclonal anti-CTSL antibody (catalog no., sc-135859;
dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 2 h at room temperature (RT). After washing 3 times with TBS-T,
the membranes were incubated with a HRP-conjugated secondary sheep
anti-mouse IgG antibody (catalog no., NA9310-1ML; dilution, 1:1,000
in TBS-T; GE Healthcare Life Sciences, Chalfont, UK) for 1 h at RT.
The membranes were washed and proteins were detected using an
enhanced chemiluminescence system (GE Healthcare), according to the
manufacturer's instructions. Mouse monoclonal anti-GAPDH antibody
(catalog no., sc-365062; dilution, 1:5,000; Santa Cruz
Biotechnology, Inc.) was used to confirm equal loading of lysates.
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used to analyze the gray value.
Vector construction and
transfection
A CTSL small hairpin RNA (shRNA) plasmid was
purchased from Santa Cruz Biotechnology, Inc. (catalog no.,
sc-40685-SH; Dallas, TX, USA). Vector transfection was performed
according to the manufacturer's protocol. SKOV3 and SKOV3/TAX cells
were transfected with CTSL shRNA plasmid or empty vector (Control
shRNA Plasmid-A; catalog no., sc-108060; Santa Cruz Biotechnology,
Inc.) to knock down the expression of CTSL. Transfection was
performed for 48 h, according to the manufacturer's protocol.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction assay
Cells were seeded into 96-well plates at 2,000
cells/well. Each sample had four replicates. The cells were
incubated with 0.2% MTT (Sigma-Aldrich) for 4 h at 37°C.
Subsequently, 100 ml dimethylsulfoxide (Beijing Solarbio Science
and Technology, Co., Ltd.) per well was added to the culture cells
to dissolve the crystals, and cells were counted every day for 3
days by reading the absorbance at 490 nm (Synergy NEO; Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Annexin V assay of
chemoresistance
After 48 h of shRNA transfection, the cells were
exposed to 100 nm paclitaxel (6 mg/ml; Laboratório Químico
Farmacêutico Bergamo Ltda., São Paulo, Brazil) for 24, 48 and 72 h.
Then, cells were harvested, washed twice with ice-cold
phosphate-buffered saline (Beijing Solarbio Science and Technology,
Co., Ltd.) and suspended in Annexin V binding buffer (BD
Biosciences, San Diego, CA, USA). The indicated amount of propidium
iodide and Annexin V-fluorescein isothiocyanate (BD Biosciences)
was added to the suspension and incubated for 20 min at RT in the
dark. Subsequently, fluorescence was measured on a flow cytometer
(BD FACSVerse 6 color; BD Biosciences).
Statistical analysis
Data analyses were performed using SPSS statistical
software (version 15.0; SPSS, Inc., Chicago, IL, USA). Results are
presented as the mean ± standard deviation of three independent
experiments. Comparisons of the percentage of viable cells and the
number of apoptotic cells among groups were performed using the
two-tailed Student's t-test. P<0.05 was used to indicate a
statistically significant difference.
Results
CTSL expression in SKOV3 and SKOV3/TAX
cell lines
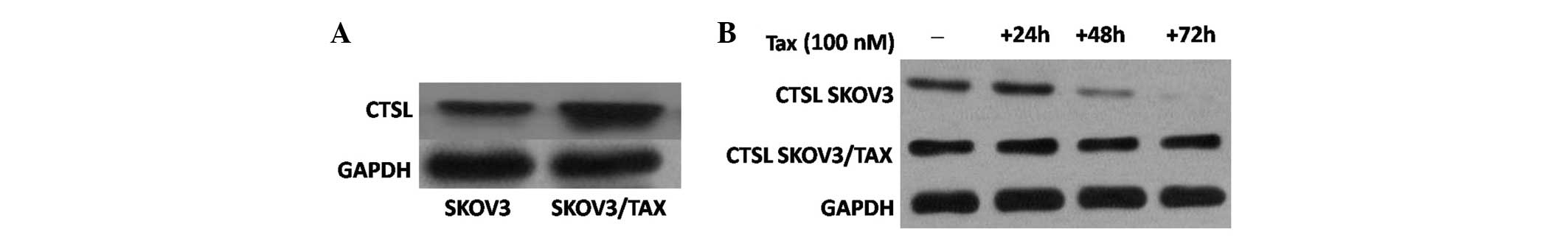
The results demonstrated that CTSL is highly
expressed in SKOV3/TAX cells compared with SKOV3 cells (Fig. 1A). Considering this, SKOV3 and
SKOV3/TAX cells were used to analyze the effect of paclitaxel
treatment on the expression of CTSL. Cells were treated with
paclitaxel (100 nM), and harvested at 24, 48 and 72 h. Notably,
immunoblotting demonstrated CTSL expression to be decreased at 48
and 72 h in SKOV-3 cells. However, CTSL expression remained
relatively constant at high levels in SKOV3/TAX cells upon
paclitaxel treatment (Fig. 1B). Thus,
paclitaxel treatment downregulates the expression of CTSL in SKOV-3
cells but not in paclitaxel-resistant SKOV3/TAX cells, suggesting a
role of CTSL in mediating paclitaxel resistance in ovarian cancer
cells.
CTSL may affect the proliferation
ability of ovarian cancer in vitro
To investigate the effect of CTSL on the
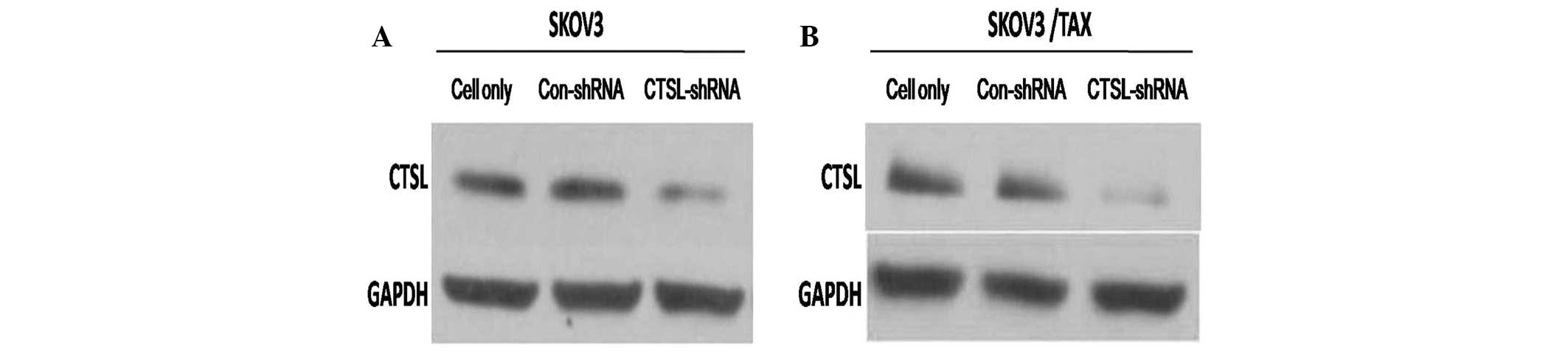
proliferation ability of ovarian cancer cells, stable SKOV3 and
SKOV3/TAX cell lines with downregulation of CTSL by shRNA were
established (SKOV3-CTSL-shRNA and SKOV3/TAX-CTSL-shRNA,
respectively). As indicated in Fig. 2A
and B, the expression level of CTSL was markedly decreased in
SKOV3-CTSL-shRNA and SKOV3/TAX-CTSL-shRNA cells compared with
control cells (SKOV3-Con-shRNA and SKOV3/TAX-Con-shRNA,
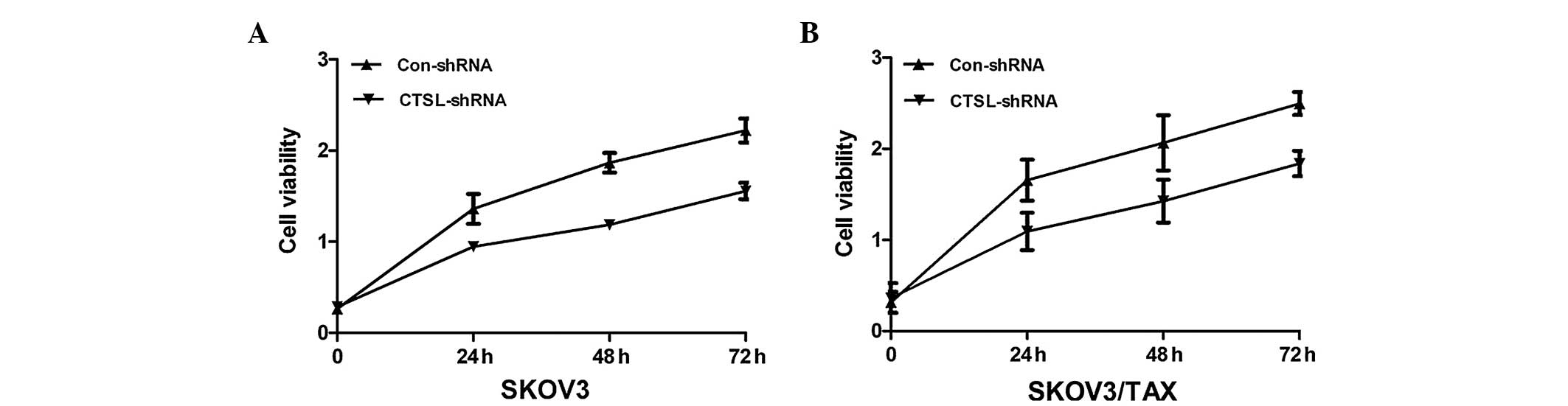
respectively). Next, the impact of CTSL silencing on cell
proliferation was investigated. The results of the MTT assay
demonstrated that knocking down CTSL in SKOV3 and SKOV3/TAX cells
decreased cell proliferation (Fig.
3), suggesting that overexpression of CTSL may be involved in
the development of ovarian cancer.
CTSL silencing promotes apoptosis
induced by paclitaxel treatment in the resistant SKOV3/TAX cell
line
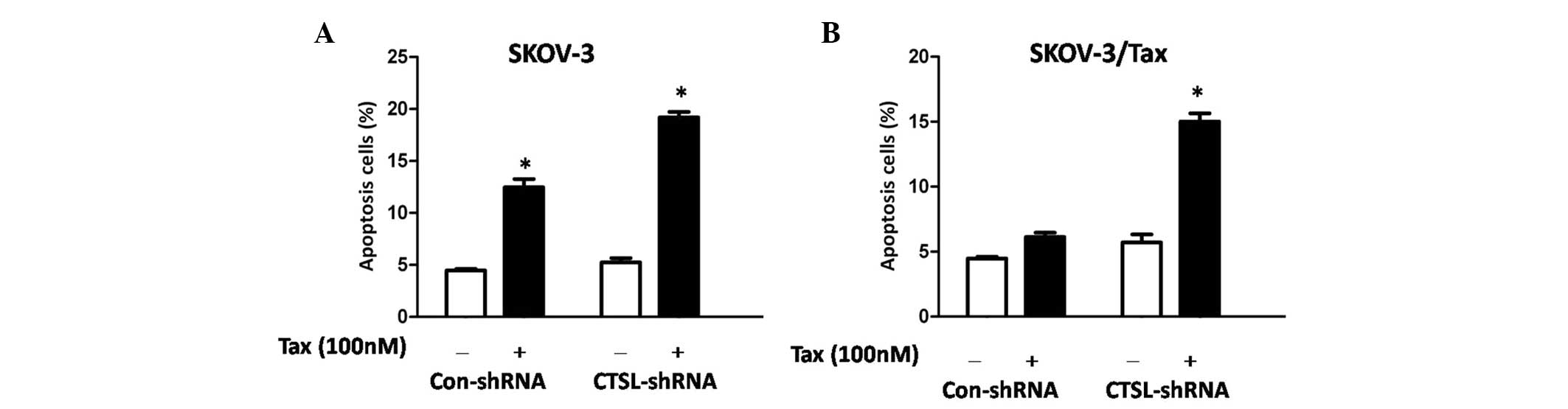
To clarify the possible mechanisms involved in CTSL
knockdown sensitizing cells to paclitaxel, an Annexin V apoptosis
assay was performed. To this end, SKOV-3 and SKOV3/TAX cells were
transiently transfected with control and CTSL shRNA, and cultured
in the presence or absence of paclitaxel (100 nM) for 48 h. The
results revealed that paclitaxel induced significantly potentiated
apoptosis in SKOV-3 cells transfected with CTSL or control shRNA,
compared with equivalent untreated cells (P=0.031 and P=0.012,
respectively; Fig. 4A). Notably, it
was observed that CTSL silencing significantly potentiated
apoptosis induced by paclitaxel in SKOV3/TAX cells compared with
SKOV3/TAX-Con-shRNA cells (P=0.016; Fig.
4B), suggesting that CTSL contributes to paclitaxel resistance
in ovarian cancer cells and that CTSL silencing may enhance
paclitaxel-mediated cell apoptosis. Depletion of CTSL in the SKOV-3
cells has no additive effect to paclitaxel treatment (Fig. 4A). This is likely due to the fact that
paclitaxel functions through downregulating CTSL expression in
SKOV-3 cells, as revealed by the western blot analysis.
Discussion
The majority of women with epithelial ovarian cancer
present with advanced disease (stages III or IV) at the time of
diagnosis. Current standard treatment of ovarian cancer, in both
early and advanced stages, consists of complete cytoreductive
surgery followed by chemotherapy, typically based on platinum and
paclitaxel (11). However, the
development of chemoresistance presents a major impediment for
successful treatment. Of those patients with ovarian cancer,
>70% experience clinical remission after initial treatment,
however, 60–75% of patients eventually relapse within 2 years of
treatment (12). As paclitaxel is by
far the most widely used microtubule-stabilizing agent in clinical
treatment, the present study focused on for further characterizing
paclitaxel-resistant cell lines.
The first observed function of CTSL in cancer
progression was its ability to promote cancer metastasis (13). An early experimental study revealed
that the metastatic capability of kidney and testicular tumor cells
was correlated with CTSL activity (14). Furthermore, the finding that CTSL
contributes to anti-apoptosis is also a well-accepted observation
experimentally. Upon lysosomal membrane damage, CTSL is released
into the cytosol where it cleaves BH3-interacting domain death
agonist, disrupting the mitochondrial membrane and inducing
apoptosis (15). CTSL is
anti-apoptotic regardless of whether the induction of apoptosis
triggers the intrinsic or extrinsic pathway, or even an indirect
pathway via autophagy, upon treatment with arsenic trioxide
(16). By contrast, other authors
have highlighted the role of CTSL in conferring resistance towards
chemotherapeutics and thus mediating an anti-apoptotic effect
(17,18). However, to date, little is known
regarding the involvement of CTSL in drug-resistant ovarian
carcinoma cells.
To determine an association between CTSL and
chemoresistance, the present study employed a pair of established
paclitaxel-sensitive and -resistant cell lines to analyze the
effect of paclitaxel on CTSL. First, it was demonstrated that CTSL
was more highly expressed in SKOV3/TAX cells compared with SKOV3
cells. Furthermore, CTSL expression decreased after 48 and 72 h of
paclitaxel treatment in SKOV-3 cells, but remained relatively
constant at high levels in SKOV3/TAX cells upon paclitaxel
treatment, suggesting that the expression of CTSL mediated the
resistance to paclitaxel in ovarian cancer cells. Thus, elevated
levels of CTSL appear to correlate with lower drug
susceptibility.
Previous studies have addressed the role of CTSL in
conferring resistance towards chemotherapeutics and, thus,
mediating an anti-apoptotic effect. In addition, CTSL silencing may
inhibit the induction of the intrinsic apoptotic pathway in cancer
cells via reduced CTSL nuclear activity, involving indirect p53
regulation of caspase 3/7 expression (19). CTSL was shown to be a critical
molecule in paclitaxel resistance, however, the mechanism remained
unclear. In the present study, SKOV-3 and SKOV3/TAX cells were
transiently transfected with control and CTSL shRNA for 48 h, and
cultured in the presence or absence of paclitaxel (100 nM) for 48
h. The results showed that paclitaxel induced significantly
potentiated apoptosis in SKOV-3 cells transfected with either CTSL
or control shRNA. Notably, it was identified that CTSL silencing
significantly potentiated apoptosis induced by paclitaxel in
SKOV3/TAX cells with CTSL knockdown compared with SKOV3/TAX
transfected with control shRNA, suggesting that CTSL silencing may
enhance paclitaxel-mediated cell apoptosis.
Kenig et al partially revealed the mechanism
of this apoptotic induction, demonstrating that CTSL inhibition
lowers the apoptotic threshold of glioblastoma cells by
upregulating p53 and caspase 3/7 expression (20).
The current findings demonstrate that CTSL knockdown
can enhance the sensitivity of ovarian cancer cells to paclitaxel.
Thus, CTSL should be explored as a candidate therapeutic target for
modulating paclitaxel sensitivity in ovarian cancer.
References
|
1
|
Chen WQ, Zhang SW, Zou XN and Zhao P:
Cancer incidence and mortality in China, 2006. Chin J Cancer Res.
23:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, Friedlander M, et al: Rethinking ovarian cancer:
Recommendations for improving outcomes. Nat Rev Cancer. 11:719–725.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brouwer-Visser J, Lee J, McCullagh K,
Cossio MJ, Wang Y and Huang GS: Insulin-like growth factor 2
silencing restores taxol sensitivity in drug resistant ovarian
cancer. PLoS One. 9:e1001652014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao F, Siu MK, Jiang L, Tam KF, Ngan HY,
Le XF, Wong OG, Wong ES, Gomes AR, Bella L, et al: Overexpression
of forkhead box protein M1 (FOXM1) in ovarian cancer correlates
with poor patient survival andcontributes to paclitaxel resistance.
PLoS One. 9:e1134782014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goulet B, Baruch A, Moon NS, Poirier M,
Sansregret LL, Erickson A, Bogyo M and Nepveu A: A cathepsin L
isoform that is devoid of a signal peptide localizes to the nucleus
in S phase and processes the CDP/Cux transcription factor. Mol
Cell. 14:207–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruan J, Zheng H, Fu W, Zhao P, Su N and
Luo R: Increased expression of cathepsin L: A novel independent
prognostic marker of worse outcome in hepatocellular carcinoma
patients. PLoS One. 9:e1121362014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zajc I, Hreljac I and Lah T: Cathepsin L
affects apoptosis of glioblastoma cells: A potential implication in
the design of cancer therapeutics. Anticancer Res. 26:3357–3364.
2006.PubMed/NCBI
|
|
8
|
Zheng X, Chu F, Chou PM, Gallati C, Dier
U, Mirkin BL, Mousa SA and Rebbaa A: Cathepsin L inhibition
suppresses drug resistance in vitro and in vivo: A putative
mechanism. Am J Physiol Cell Physiol. 296:C65–C74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Primon M, Huszthy PC, Motaln H, Talasila
KM, Torkar A, Bjerkvig R and Lah Turnšek T: Cathepsin L silencing
enhances arsenic trioxide mediated in vitro cytotoxicity and
apoptosis in glioblastomaU87MG spheroids. Exp Cell Res.
319:2637–2648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Wei L, Shen G, He B, Gong W, Min
N, Zhang L, Duan Y, Xie J, Luo H and Gao X: Cathepsin L is involved
in proliferation and invasion of ovarian cancer cells. Mol Med Rep.
11:468–474. 2015.PubMed/NCBI
|
|
11
|
Jiang L, Siu MK, Wong OG, Tam KF, Lu X,
Lam EW, Ngan HY, Le XF, Wong ES, Monteiro LJ, et al: iASPP and
chemoresistance in ovarian cancers: Effects on paclitaxel-mediated
mitotic catastrophe. Clin Cancer Res. 17:6924–6933. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deraco M, Baratti D, Laterza B, Balestra
MR, Mingrone E, Macrì A, Virzì S, Puccio F, Ravenda PS and Kusamura
S: Advanced cytoreduction as surgical standard of care and
hyperthermic intraperitoneal chemotherapy as promising treatment in
epithelial ovarian cancer. Eur J SurgOncol. 37:4–9. 2011.
View Article : Google Scholar
|
|
13
|
Chauhan SS, Goldstein LJ and Gottesman MM:
Expression of cathepsin L in human tumors. Cancer Res.
51:1478–1481. 1991.PubMed/NCBI
|
|
14
|
Zhang W, Wang S, Wang Q, Yang Z, Pan Z and
Li L: Overexpression of cysteine cathepsin L is a marker of
invasion and metastasis in ovarian cancer. Oncol Rep. 31:1334–1342.
2014.PubMed/NCBI
|
|
15
|
Stoka V1, Turk B, Schendel SL, Kim TH,
Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson
M, et al: Lysosomal protease pathways to apoptosis. Cleavage of
bid, not pro-caspases, is the most likely route. J Biol Chem.
276:3149–3157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Yang L, Rong JG, Ni Y, Gu WW, Luo Y,
Ishidoh K, Katunuma N, Li ZS and Zhang HL: Inhibition of cysteine
cathepsin B and L activation in astrocytes contributes to
neuroprotection against cerebral ischemia via blocking the
tBid-mitochondrial apoptotic signaling pathway. Glia. 62:855–880.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wille A, Gerber A, Heimburg A, Reisenauer
A, Peters C, Saftig P, Reinheckel T, Welte T and Bühling F:
Cathepsin L is involved in cathepsin D processing and regulation of
apoptosis in A549 human lung epithelial cells. Biol Chem.
385:665–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walz M, Kellermann S, Bylaite M, Andrée B,
Rüther U, Paus R, Kloepper JE, Reifenberger J and Ruzicka T:
Expression of the human Cathepsin L inhibitor hurpin in mice: Skin
alterations and increased carcinogenesis. Exp Dermatol. 16:715–723.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levicar N, Dewey RA, Daley E, Bates TE,
Davies D, Kos J, Pilkington GJ and Lah TT: Selective suppression of
cathepsin L by antisense cDNA impairs human brain tumor cell
invasion in vitro and promotes apoptosis. Cancer Gene Ther.
10:141–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenig S, Frangež R, Pucer A and Lah T:
Inhibition of cathepsin L lowers the apoptotic threshold of
glioblastoma cells by up-regulating p53 and transcription of
caspases 3 and 7. Apoptosis. 16:671–682. 2011. View Article : Google Scholar : PubMed/NCBI
|