Introduction
In 2000, Bednarek et al identified a novel
tumor suppressor gene termed WW domain-containing oxidoreductase
(WWOX) (1). The WWOX
gene maps at the 16p23.3–24.1 chromosomal region and spans the
second most common fragile site in the human genome, FRA16D
(1). Since its discovery,
WWOX/WWOX has been shown to exhibit reduced or no expression
in a range of neoplastic diseases of varying locations, such as the
lungs, breasts, bladder, ovaries, esophagus and pancreas (2–7). Moreover,
abnormal WWOX/WWOX expression profiles had been reported to
be associated with a poor prognosis in carcinomas (8–10).
Therefore, WWOX is expected to be a potential target for the
gene-targeted therapy of human carcinomas.
The role of WWOX/WWOX in leukemia has also
been reported, suggesting that WWOX may exert antineoplastic
activity in human hematopoietic malignancies (11–16). Our
previous studies have demonstrated that WWOX exerts a role
as an tumor suppressor gene in leukemia, inhibiting cell
proliferation and promoting apoptosis via binding with p73 and
triggering of the mitochondrial pathway (14,15).
However, the clinical characterization of WWOX in leukemia
remains unclear thus far. In order to obtain more information on
the role of WWOX with regard to the occurrence, development
and therapeutic effect of leukemia, the present study evaluated the
expression of WWOX in 182 primary leukemia patients and 5 leukemic
cell lines. Additionally, the study attempted to assess the
association between WWOX expression and clinical features.
It was demonstrated that a low expression level of WWOX is
present in leukemia, and that WWOX expression varies among
the different phases of leukemia. Moreover, WWOX expression
was shown to have an association with the treatment outcome. These
findings may aid in providing a better understanding of the role of
WWOX in leukemia.
Materials and methods
Agents
RPMI-1640, Dulbecco's modified Eagle's medium (DMEM)
and fetal bovine serum (FBS) was purchased from Thermo Fisher
Scientific, Inc., (Waltham, MA, USA) and Lymphocyte Separation
Medium was obtained from TBD Sciences (Tianjin, China). The reverse
transcription-polymerase chain reaction (RT-PCR) kit,
radioimmunoprecipitation assay (RIPA) lysis buffer, TRIzol reagent
and 2X PCR Master Mix were all purchased from Thermo Fisher
Scientific, Inc. The 4′,6-diamidino-2-phenylindole (DAPI) and
fluorescein isothiocyanate (FITC)-conjugated polyclonal goat
anti-rabbit immunoglobulin G (IgG) antibodies (cat. no. P0186) were
obtained from the Beyotime Institute of Biotechnology (Shanghai,
China), while the polyclonal rabbit anti-human WWOX (cat. no.
ab189410) and monoclonal mouse anti-human β-actin (cat. no.
sc-8432) antibodies were purchased from Abcam (Cambridge, MA, USA)
and Santa Cruz Biotechnology Inc. (Dallas, TX, USA),
respectively.
Patients and samples
Between October 2010 and June 2012, blood samples
were collected from the diagnosed leukemia cases at Fujian Medical
University Union Hospital (Fujian, China) and 182 cases were
enrolled in the study. Among these patients, 101 were male. The
mean age ± standard deviation of the total patients was 32.0±11.68
years (range, 6–76 years). The cohort included 61 cases of acute
lymphoblastic leukemia (ALL), 89 cases of acute myelogenous
leukemia (AML) and 32 cases of chronic myelogenous leukemia (CML),
which were diagnosed based on morphology, histopathology, leukocyte
differentiation antigens and the French-American-British
classification (17). Mononuclear
cells purified from 43 healthy volunteers were used as paired
controls. Prior consent was obtained from the patients for the use
of these clinical materials for research purposes and study
approval was granted by the project approval authorities (Fujian
Medical University & Fujian Provincial Department of Science
and Technology).
Cell lines and cell culture
Human Jurkat (acute T-lymphoblastic leukemia), K562
(chronic myelogenous leukemia in erythroid crisis), HL-60 (acute
myelogenous leukemia), HL-60/ADR (HL-60 resistance to doxorubicin),
K562/ADR (K562 resistance to doxorubicin) and 293a cell lines were
all purchased from the cell bank of the Chinese Academy of Medical
Sciences (Beijing, China). The cells were maintained in RPMI-1640
supplemented with 10% FBS, and cultured at 37°C in a 5% CO2
humidified atmosphere. The human 293a cell line was maintained in
DMEM containing 10% FBS and was utilized as a positive control.
RT-PCR analysis
Mononuclear cells were purified with the aid of
Lymphocyte Separation Medium. Total RNA was extracted with TRIzol
reagent and was reverse transcribed into cDNA using a RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
Briefly, total RNA (1–2 µg), hexamer primer (1 µl) and ddH2O were
added to a total volume of 12 µl, and incubated at 65°C for 5 min.
Subsequently, 10 mM dNTP mix (2 µl), 5X reaction buffer (4 µl), 1
µl RNase inhibitor and 1 µl Reverse Transcriptase were added,
followed by incubation at 25°C for 5 min, 45°C for 1 h and 70°C for
5 min. The primer sequences for WWOX and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used
as previously described (14): WWOX
forward, 5′-CACGCATTTTAGAAGAATGG-3′ and reverse,
5′-GACAGCAGCACAGTACACG-3′; and GAPDH forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′. cDNA was then amplified by PCR using
the 2X Green Master Mix (Thermo Fisher Scientific, Inc). The
reaction system (25 µl) contained 2 µl cDNA template, 12.5 µl
Master Mix (2X), 8.5 µl ddH2O and 2 µl primers (20 µM). The cDNA
template was replaced with ddH2O for the negative control. PCR was
performed using an Applied Biosystems 2720 Thermal Cycler (Thermo
Fisher Scientific, Inc.), under the recommended conditions of an
initial denaturation for 5 min at 94°C, followed by 30 cycles of
94°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec, and a final
extension for 7 min at 72°C. Quantified data were normalized to
GAPDH. Each PCR experiment was performed twice.
Western blotting analysis
Cell lysates were prepared using RIPA protein lysis
buffer, and the protein extracts were quantified and subjected to
electrophoresis on a 10–12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis gel. The proteins were transferred onto
polyvinylidene difluoride membranes and blocked in Tris-buffered
saline containing 5% skimmed milk powder. The primary antibodies
were used at 1:1,000 dilutions for both the rabbit anti-WWOX and
mouse monoclonal anti-β-actin antibodies.
Immunofluorescence staining assay
In brief, cell monolayers were fixed with 4%
paraformaldehyde and incubated at 4°C overnight with rabbit
anti-human WWOX (1:500). FITC-conjugated goat anti-rabbit IgG was
diluted to 1:1,000. DAPI was used to stain the nuclei. The stained
cells were washed with PBS and observed with a fluorescence
microscope (Olympus, Tokyo, Japan) at ×400 magnification.
Statistical analysis
Data are presented as the mean ± standard deviation
and all statistical analyses were performed using SPSS 13.0 (SPSS
Inc., Chicago, IL, USA) software. The χ2 test or
corrected χ2 test was used to analyze the association
between the WWOX-positive rate and the clinical features.
P<0.05 (confidence level >95%) was considered to indicate a
statistically significant difference.
Results
Expression of WWOX in primary leukemia
cases
The expression level of WWOX mRNA and the
WWOX protein was examined in the leukemia patients via RT-PCR and
western blotting analysis. As shown in Fig. 1A, D and E, the expression of
WWOX/WWOX was reduced or lost in the leukemia cases when
compared with that in the normal paired controls (P<0.000); the
positive rates of WWOX for the AML, ALL and CML patients
were 47.19% (42/89), 50.81% (31/61) and 34.38% (11/32),
respectively, which was significant (AML, P<0.000, χ2=23.15;
ALL, P<0.000, χ2=18.23; CML, P<0.000, χ2=26.19) compared with
the normal controls (90.70%, 39/43) (Fig.
1B). Notably, the WWOX-positive rates of newly diagnosed
and relapsed cases in AML, ALL and CML were significantly lower
than those of remission cases, accompanied by a P-value of <0.05
or <0.01 (Fig. 1B; AML, P=0.008;
ALL, P=0.036; CML, P=0.011). Nevertheless, the WWOX-positive
rates of remission cases in AL (involving ALL and AML) and CML
exhibited no significance with the normal controls (AL, P=0.299;
CML, P=0.051; corrected χ2=3.763 or 3.811). The
quantified data of WWOX levels for AML, ALL and CML are
displayed in Fig. 1C. As is shown,
the expression levels of WWOX in AML, ALL and CML were
distinctly reduced when compared with the normal paired controls
(AML, P<0.000; ALL, P=0.025; CML, P<0.000). Significantly,
WWOX expression were relatively elevated in the remission
cases (AML, P=0.022; ALL, P=0.010; CML, P=0.002; all vs. newly
diagnosed cases), yet silenced in the relapsed or CML in blastic
phase (CML-BP) cases (P<0.000 vs. remission cases).
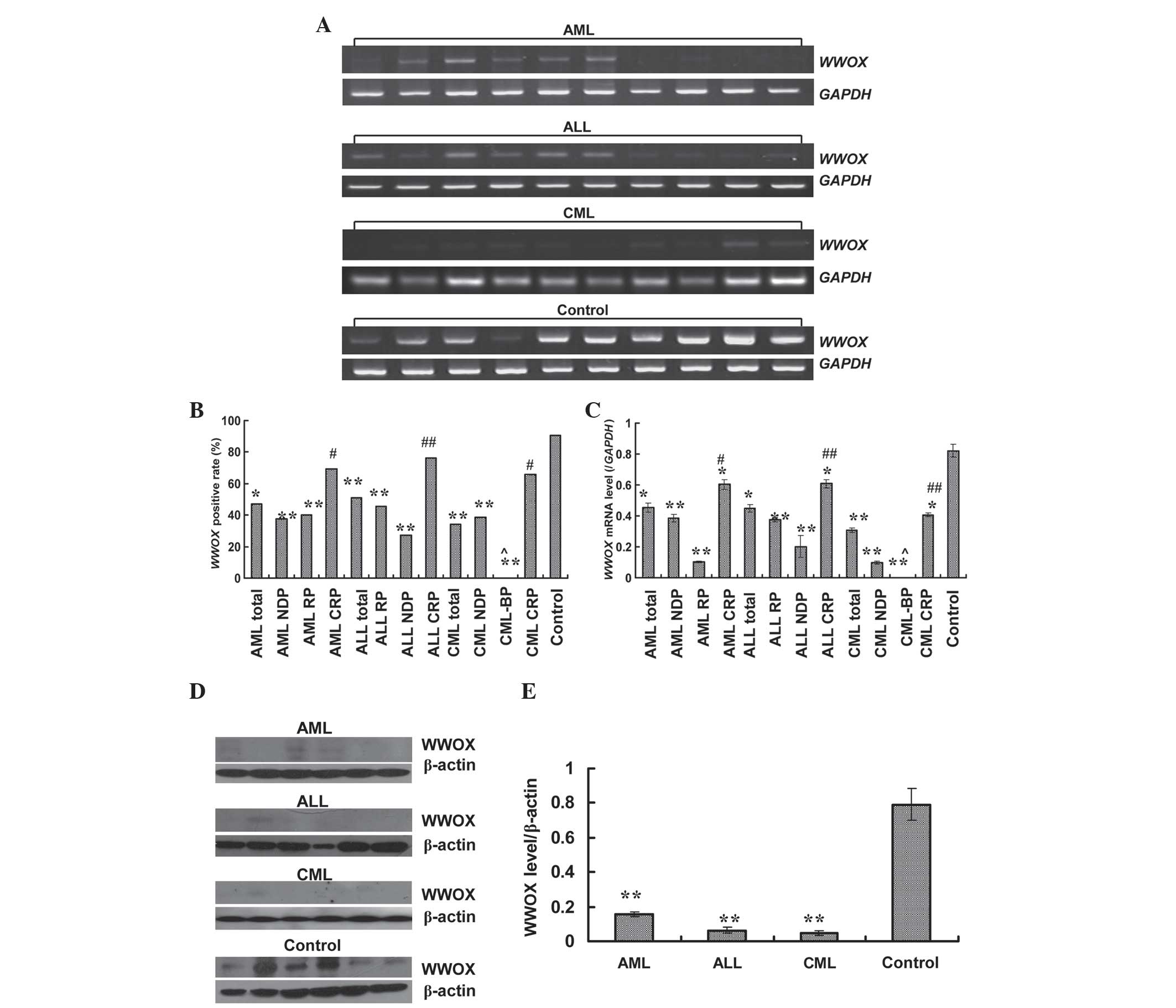 | Figure 1.Expression of WWOX in primary
leukemia cases. (A) Expression of WWOX mRNA analyzed by
reverse transcription-polymerase chain reaction. (B)
WWOX-positive rate in different types or stages of leukemia.
(C) Expression level of WWOX in different types or stages of
leukemia, normalized to GAPDH. (D) Expression of WWOX protein
examined by western blotting. (E) Expression level of WWOX in
different types of leukemia, quantified to β-actin. Data shown are
the mean ± standard deviation (n=182 for reverse
transcription-polymerase chain reaction, and n=18 for western
blotting). *P<0.05 and **P<0.01 vs. normal volunteers;
#P<0.05 and ##P<0.01 vs. newly
diagnosed cases; and ^P<0.01 vs. remission cases. WWOX, WW
domain-containing oxidoreductase; AML, acute myelogenous leukemia;
ALL, acute lymphoblastic leukemia; CML, chronic myelogenous
leukemia; NDP, newly diagnosed patients; CRP, complete remission
patients; RP, relapsed patients; CML-BP, chronic myelogenous
leukemia in blastic phase; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
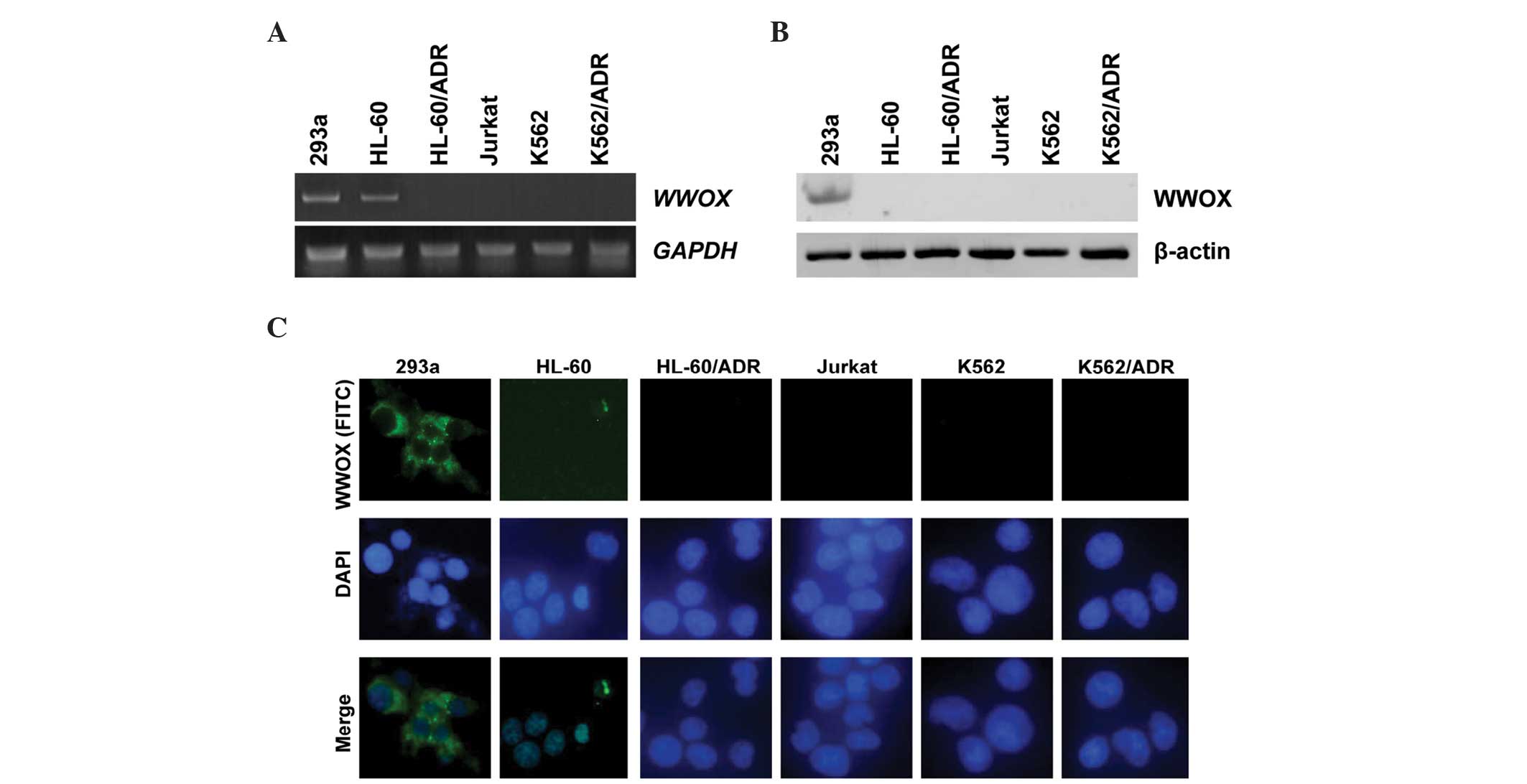
Expression of WWOX in leukemia cell
lines
Expression status of WWOX mRNA and WWOX
protein in the leukemia cell lines was similar to that in the
leukemia patients. As indicated in Fig.
2A, 4/5 leukemic cell lines (80%) showed absent or reduced
WWOX expression; Jurkat, K562, HL-60/ADR and K562/ADR cell
lines were WWOX-negative or exhibited a low expression
level, and only HL-60 cells exhibited a high expression level of
WWOX similar to the 293a controls (Fig. 2A). Western blotting and
immunofluorescence assays showed that endogenous WWOX protein in
all leukemic cells was undetectable, whereas the 293a cells yielded
a positive expression level of WWOX protein (Fig. 2B and C).
Association between WWOX-positive rate
and clinical features
The χ2 test or corrected χ2
test was utilized to analyze the association between the
WWOX-positive rate and the clinical features. The clinical
characteristics of the newly diagnosed patient cohort are
summarized in Table I. There were no
significant differences between WWOX positive rate and age,
gender, leukocytes or immature cells in the newly diagnosed
patients (all P>0.05; see Table I
for exact P-values).
 | Table I.Association between
WWOX-positive rate and clinical features. |
Table I.
Association between
WWOX-positive rate and clinical features.
| Parameters | n | WWOX-positive,
n | Positive rate,
% | χ2 | P-value |
|---|
| Age, years |
|
|
≤40 | 61 | 26 | 42.62 | 0.531 | 0.446 |
|
>40 | 38 | 14 | 36.84 |
|
|
| Gender |
|
|
Male | 59 | 24 | 40.68 | 0.005 | 0.946 |
|
Female | 40 | 16 | 40.00 |
|
|
| Immature cells |
|
|
≤50% | 47 | 16 | 34.04 | 1.504 | 0.220 |
|
>50% | 52 | 24 | 46.15 |
|
|
| Leukocytes |
|
|
≤5×1010 | 57 | 23 | 40.35 | <0.000 | 0.990 |
|
>5×1010 | 42 | 17 | 40.48 |
|
|
Association between remission rate and
WWOX expression
In order to trace whether WWOX expression was
correlated with the therapeutic effect, a total of 99 newly
diagnosed patients were followed up. A total of 94 peripheral blood
samples were collected and 59.57% (56/94) were revealed to be
WWOX-negative prior to treatment. The data indicated that
the remission rate of the 56 WWOX-negative hospitalized
cases was 35.71% (20/56), compared with 76.32% (29/38) in the
WWOX-positive cases (χ2=14.96; P<0.000).
Moreover, 73.68% (14/19) WWOX-negative cases exhibited
restored WWOX expression following treatment, and the mean
level of WWOX expression was 0.2180±0.1251 vs.
0.5457±0.3859, prior to and following treatment, respectively.
Additionally, WWOX expression in the originally positive
cases yielded a value of 0.3932±0.2083 prior to treatment, which
was elevated to a level of 0.8696±0.3216 after corresponding
chemotherapies, which included intensive chemotherapy for AML and
vincristine, daunorubicin, cyclophosphamide, L-asparaginase, and
prednisone chemotherapy for ALL (data not shown).
Discussion
In the present study, the expression of
WWOX/WWOX in 182 primary leukemia patients and 5 leukemia
cell lines was evaluated, and mRNA and protein expression were
found to be significantly reduced or absent in the two sources.
Notably, the WWOX-positive rates of newly diagnosed and
relapsed cases were significantly lower than those of remission
cases, indicating a prognostic role of WWOX expression in
leukemia. Moreover, WWOX expression was correlated with the
treatment effect, which further suggests that the WWOX gene
has a potential to be a biomarker or target for the therapy of
leukemia.
Leukemia is an oligoclonal or monoclonal disease and
the carcinogenesis is generally a complicated multipathway process,
including the activation of oncogenes and/or the inactivation of
tumor suppressor genes (18).
WWOX is a tumor suppressor gene that maps to the common
fragile site FRA16D and is involved in carcinogenesis and cancer
progression in a number of carcinoma types (1,19,20). The bulk of the data documented have
shown that the expression of WWOX/WWOX was lost or reduced
in various types of solid cancers (2–10).
Similarly, the aberration or absence of WWOX/WWOX expression
in primary hematopoietic malignancies has also been reported
(11–16). A recently published study detected the
expression of WWOX/WWOX in a small cohort of 38 primary
leukemia patients and observed low expression levels (14). Chen et al (13) recently validated the expression of
WWOX in ALL, and observed that the mRNA expression of
WWOX, fragile histidine triad and tumor protein p73 was
significantly lower in the ALL samples compared with the controls.
Correspondingly, the current study showed that WWOX/WWOX
expression was reduced or lost in a range of different leukemia
types and cell lines in comparison to the normal controls. The
present findings are consistent with the published studies.
Reduced WWOX/WWOX expression is associated
with more aggressive phenotypes or the development of carcinomas
(21–26). Guler et al (22) reported that WWOX protein expression
was more frequently reduced in high-grade lesions, and Donati et
al (26) presented similar
results. In addition, other studies showed that the expression of
WWOX/WWOX exhibited an inverse correlation with clinical or
clinicopathological stage, implying that WWOX/WWOX
expression varies during the development of carcinomas (8,26,27). For instance, Nunez et al
(27) evaluated the WWOX protein
expression levels in ovarian carcinomas and observed that the
WWOX-negative rate in stage I patients was 23%, which
increased to 48% in stage IV patients. Another study by Huang et
al (8) also demonstrated that the
downregulation of WWOX/WWOX was an unfavorable predictor for
overall survival and cumulative recurrence rates. In agreement with
these reported studies, the present study observed that the
WWOX-positive rates of newly diagnosed and relapsed cases in
AML, ALL and CML were significantly lower than those of remission
cases; WWOX expression was reduced or silenced in newly
diagnosed, relapsed and CML-BP cases, but was relatively elevated
when remission was induced. These data strongly demonstrated that
WWOX may play an important role in the development of
cancer. Nevertheless, data from the study by Nunez et al
showed no statistical difference between the relapsing and
non-relapsing cases of ovarian carcinoma with regard to WWOX
expression (27). Hence, further
larger studies are required to confirm these findings.
Another noteworthy finding of the present study was
the association that was found between WWOX level and the curative
effect among patients. In the present data, the remission rate in
expression-negative and -positive hospitalized patients was 35.71
vs. 76.32%. Moreover, WWOX-negative cases exhibited restored
WWOX expression following treatment, and the mean expression
level in the WWOX-positive patients was relatively elevated
after receiving clinical therapy, indicating that WWOX has
the potential to be a candidate predictive marker for treatment.
Supporting evidence from another study similarly showed that WWOX
protein expression was significantly elevated in the
5-Aza-CdR-treated ovarian cancer group (28). Recently published data indicated that
in the treated breast cancer cases with a tumor expressing moderate
or strong WWOX protein, recurrence-free survival was improved
(29). Additionally, a previous study
conducted by Guler et al (30)
revealed that reduced or absent WWOX expression was associated with
tamoxifen resistance in breast cancer, hinting of the potential of
WWOX in predicting tamoxifen response.
In summary, the current study suggested that the
loss or silencing of WWOX/WWOX expression may contribute to
the occurrence and development of leukemia, and that detecting the
expression level of WWOX/WWOX may aid in the development of
future treatment approaches for leukemia. Despite these promising
results, the number of cases examined in the present study may not
be sufficient, and the study did not evaluate the expression of
WWOX in chronic lymphoblastic leukemia, multiple myeloma and
other hematopoietic malignancies. Even though no significant
association was observed between the WWOX-positive rate and
clinical features in leukemia, additional prospective and
retrospective studies are warranted.
Acknowledgements
This study was supported by the National Clinical
Key Specialty Construction Program of China and the Provincial
Natural Science Foundation of Fujian (grant no. 2010J01181) and
Professor Foundation of Fujian Medical University (grant no.
JS08009). The authors would like to thank Dr Jianda Hu (Fujian
Medical University Union Hospital, Fujian, China) for the
collection of samples.
References
|
1
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3–24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
2
|
Iliopoulos D, Guler G, Han SY, Johnston D,
Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R and Huebner K:
Fragile genes as biomarkers: Epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lan C, Chenggang W, Yulan B, Xiaohui D,
Junhui Z and Xiao W: Aberrant expression of WWOX protein in
epithelial ovarian cancer: A clinicopathologic and
immunohistochemical study. Int J Gynecol Pathol. 31:125–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ekizoglu S, Muslumanoglu M, Dalay N and
Buyru N: Genetic alterations of the WWOX gene in breast cancer. Med
Oncol. 29:1529–1535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo W, Wang G, Dong Y, Guo Y, Kuang G and
Dong Z: Decreased expression of WWOX in the development of
esophageal squamous cell carcinoma. Mol Carcinog. 52:265–274. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of human
hepatoma cell line SMMC-7721. World J Gastroenterol. 18:3020–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakayama S, Semba S, Maeda N, Matsushita
M, Kuroda Y and Yokozaki H: Hypermethylation-mediated reduction of
WWOX expression in intraductal papillary mucinous neoplasms of the
pancreas. Br J Cancer. 100:1438–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang C, Tian Y, Peng R, et al:
Down-regulation of WWOX is associated with poor prognosis in
patients with intrahepatic cholangiocarcinoma after Curative
Resection. J Gastroenterol Hepatol. 30:421–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Göthlin Eremo A, Wegman P, Stål O,
Nordenskjöld B, Fornander T and Wingren S: Wwox expression may
predict benefit from adjuvant tamoxifen in randomized breast cancer
patients. Oncol Rep. 29:1467–1474. 2013.PubMed/NCBI
|
|
10
|
Cancemi L, Romei C, Bertocchi S, Tarrini
G, Spitaleri I, Cipollini M, Landi D, Garritano S, Pellegrini G,
Cristaudo A, et al: Evidences that the polymorphism Pro-282-Ala
within the tumor suppressor gene WWOX is a new risk factor for
differentiated thyroid carcinoma. Int J Cancer. 129:2816–2824.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishii H, Vecchione A, Furukawa Y,
Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M,
Saito Y, et al: Expression of FRA16D/WWOX and FRA3B/FHIT genes in
hematopoietic malignancies. Mol Cancer Res. 1:940–947.
2003.PubMed/NCBI
|
|
12
|
Ishii H and Furukawa Y: Alterations of
common chromosome fragile sites in hematopoietic malignancies. Int
J Hematol. 79:238–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Zhang H, Li P, Yang Z, Qin L and
Mo W: Gene expression of WWOX, FHIT and p73 in acute lymphoblastic
leukemia. Oncol Lett. 6:963–969. 2013.PubMed/NCBI
|
|
14
|
Cui Z, Lin D, Cheng F, Luo L, Kong L, Xu
J, Hu J and Lan F: The role of the WWOX gene in leukemia and its
mechanisms of action. Oncol Rep. 29:2154–2162. 2013.PubMed/NCBI
|
|
15
|
Lin D, Cui Z, Kong L, Cheng F, Xu J and
Lan F: p73 participates in WWOX-mediated apoptosis in leukemia
cells. Int J Mol Med. 31:849–854. 2013.PubMed/NCBI
|
|
16
|
Zhang H, Kong L, Cui Z, Du W, He Y, Yang
Z, Wang L and Chen X: The WWOX gene inhibits the growth of U266
multiple myeloma cells by triggering the intrinsic apoptotic
pathway. Int J Mol Med. 34:804–809. 2014.PubMed/NCBI
|
|
17
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Odenike O and Rowley JD:
Leukaemogenesis: More than mutant genes. Nat Rev Cancer. 10:23–36.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aqeilan RI and Croce CM: WWOX in
biological control and tumorigenesis. J Cell Physiol. 212:307–310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gardenswartz A and Aqeilan RI: WW
domain-containing oxidoreductase's role in myriad cancers: Clinical
significance and future implications. Exp Biol Med (Maywood).
239:253–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo W, Dong Z, Dong Y, Guo Y, Kuang G and
Yang Z: Genetic and epigenetic alterations of WWOX in the
development of gastric cardia adenocarcinoma. Environ Mol Mutagen.
54:112–123. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guler G, Uner A, Guler N, Han SY,
Iliopoulos D, McCue P and Huebner K: Concordant loss of fragile
gene expression early in breast cancer development. Pathol Int.
55:471–478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diniz MG, Borges ER, Pimenta FJ, De
Mesquita Netto AC, De Marco L, Gomez RS and Gomes CC: Evidence of
molecular alterations in the tumour suppressor gene WWOX in benign
and malignant bone related lesions of the jaws. Oncol Rep.
25:499–502. 2011.PubMed/NCBI
|
|
24
|
Maeda N, Semba S, Nakayama S, Yanagihara K
and Yokozaki H: Loss of WW domain-containing oxidoreductase
expression in the progression and development of gastric carcinoma:
Clinical and histopathologic correlations. Virchows Arch.
457:423–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Li P, Yang Z and Mo WN: Expression
of fragile histidine triad (FHIT) and WW-domain oxidoreductase gene
(WWOX) in nasopharyngeal carcinoma. Asian Pac J Cancer Prev.
14:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donati V, Fontanini G, Dell'Omodarme M,
Prati MC, Nuti S, Lucchi M, Mussi A, Fabbri M, Basolo F, Croce CM
and Aqeilan RI: WWOX expression in different histologic types and
subtypes of non-small cell lung cancer. Clin Cancer Res.
13:884–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nunez MI, Rosen DG, Ludes-Meyers JH, Abba
MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB and
Aldaz CM: WWOX protein expression varies among ovarian carcinoma
histotypes and correlates with less favorable outcome. BMC Cancer.
5:642005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan H, Yu N and Tong J: Effects of
5-Aza-2′-deoxycytidine on the methylation state and function of the
WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett.
6:845–849. 2013.PubMed/NCBI
|
|
29
|
Göthlin Eremo A, Wegman P, Stål O,
Nordenskjöld B, Fornander T and Wingren S: Wwox expression may
predict benefit from adjuvant tamoxifen in randomized breast cancer
patients. Oncol Rep. 29:1467–1474. 2013.PubMed/NCBI
|
|
30
|
Guler G, Iliopoulos D, Guler N, Himmetoglu
C, Hayran M and Huebner K: Wwox and Ap2gamma expression levels
predict tamoxifen response. Clin Cancer Res. 13:6115–6121.. 2007.
View Article : Google Scholar : PubMed/NCBI
|