Introduction
Among urological tumors, renal cell carcinoma (RCC)
is the third leading cause of mortality (1). Worldwide, ~102,000 individuals succumb
to kidney cancer annually (2) and RCC
accounts for 2–3% of adult kidney malignancies (3). Surgical resection remains the definitive
treatment for RCC, but 20–40% of patients may relapse following
nephrectomy (4–6). The 5-year survival rate of patients with
advanced RCC is extremely poor (5–10%), due to recurrence or
distant metastasis (7,8). RCC is resistant to radiotherapy and
chemotherapy (9). Targeted therapies,
including sorafenib, sunitinib and the anti-angiogenic
multi-tyrosine kinase inhibitors everolimus and temsirolimus, have
been developed and widely used as first and second-line treatments;
however, their impact on the survival rates of patients remains
limited (10–12). The molecular mechanisms of recurrence,
metastasis and drug resistance of RCC are not yet known. Therefore,
an increased understanding of the molecular pathways of the
progression of RCC is urgently required.
MicroRNAs (miRNAs) are endogenous, conserved, small
non-coding RNA molecules (19–25 bp long) that regulate gene
expression at the post-transcriptional level by binding to the
partial sequence homology of the 3′-untranslated region of target
messenger (m)RNA, which causes translation inhibition or mRNA
degradation (13). It is known that
miRNAs control a variety of key cellular processes, including the
cell cycle and cell proliferation, differentiation and
tumorigenesis (14). Previous studies
have demonstrated that miRNAs are aberrantly expressed in various
human cancers (15,16), including RCC (9,17). miRNAs
are also vital in the initiation, development and metastasis of
cancers (18). Additionally, miRNAs
may function as oncogenes or tumor suppressor genes by specifically
regulating target gene expression (19).
Various studies have evaluated the role of miRNAs in
RCC. Yamasaki et al demonstrated that tumor suppressive
miRNA-138 (miR-138) contributes to cell migration and invasion in
RCC (20) and miRNA-218 significantly
inhibits RCC cell proliferation, migration and invasion (21). Su et al revealed that let-7d
may suppress RCC growth, metastasis and tumor macrophage
infiltration by targeting COL3A1 and chemokine ligand-7 (22). A study by Chen et al
demonstrated that miRNA-129-3p attenuates cell migration and
invasion of RCC by downregulating multiple metastasis-associated
genes, and may also act as a diagnostic and prognostic biomarker
for RCC (23). Wu et al
revealed that miRNA-133b was downregulated in RCC cell lines and
inhibited cell proliferation, migration and invasion of RCC cells
(24). These previous studies
illustrate that tumor-associated miRNAs mediate cancer molecular
pathways and may provide insights into the potential mechanisms of
RCC oncogenesis and metastasis.
The miRNA-27 (miR-27) family consists of miR-27a and
miR-27b, which are transcribed from different chromosomes and
differ by one nucleotide at the 3′ end. miR-27a is located on
chromosome 19 (25). miR-27a is
altered in several types of cancer, including colon cancer
(26), breast cancer (27), osteosarcoma (28) and gastric adenocarcinoma (29), to become an oncogene or a tumor
suppressor. A study by Shi et al demonstrated that a genetic
variant in the pre-miR-27a rs895819 is associated with a reduced
RCC risk in a Chinese population (30). However, the effects of miR-27a on RCC
have not yet been clearly elucidated. The present study evaluated
the effect of miR-27a on the human RCC 786-O cell line and a RCC
xenograft mouse model, and aimed to identify the possible mechanism
through which this effect is achieved.
Materials and methods
Cell culture
The human RCC 786-O cell line was purchased from the
Institute of Biochemistry and Cell Biology (Shanghai, China). The
786-O cells were grown in Invitrogen high-glucose Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with Gibco 10% fetal calf serum
(FCS; Thermo Fisher Scientific, Inc.) and incubated at 37°C in a
humidified atmosphere containing 5% CO2. The cells were
regularly passaged to maintain exponential growth.
Cell transfection
A miR-27a precursor and miR-27a mimics (negative
control) were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Cells at 70–80% confluency were transfected with
miR-27a or miR-27a mimics using Invitrogen
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The cells were harvested
and assayed at various time points following transfection. Each
experiment was repeated three times.
Methylthiazol tetrazolium (MTT)
assay
The proliferative capacity of the cells was
evaluated using an MTT assay. Briefly, 786-O cells were seeded in
Costar 96-well plates (Corning Inc., Corning, NY, USA) at a density
of 4×103 cells/well and were then transfected with an
miR-27a expression vector or miR-27a control (empty vector).
Subsequent to 24 and 48 h of culture, 20 µl MTT reagent
(Sigma-Aldrich Chemie Gmbh, Munich, Germany) was added to each well
and the cells were incubated for an additional 4 h at 37°C. Optical
density was assessed by measuring the absorbance at 490 nm with a
microtiter plate reader (Model 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Each experiment contained three replicates and
was repeated at least twice. The data were expressed as the mean ±
standard deviation (SD).
Analysis of apoptotic cells
In total, 24 h subsequent to transfection, the cells
were collected and washed twice with 1X phosphate-buffered saline
(PBS; Sangon Biotech Co., Ltd., Shanghai, China) and stained using
an Annexin V-fluorescein isothiocyanate (FITC) propidium iodide
(PI) Detection kit (Nanjing KeyGen Biotechnology Co., Ltd.,
Nanjing, Jiangsu, China), following the manufacturer's protocol.
Annexin V has a high affinity for phosphatidylserine, which is
exposed on the cell surface of apoptotic cells (31). Early apoptotic cells that bind Annexin
V-FITC exhibit green staining in the plasma membrane, whereas late
apoptotic or necrotic cells, which have lost membrane integrity,
exhibit red PI staining throughout the nucleus and a halo of green
Annexin V-FITC staining on the cell surface. Each experiment
contained three replicates and was repeated at least twice. The
data are expressed as the mean ± SD.
Wound-healing assay
Cell migration was assessed using wound-healing
assays. Cells were plated in a Costar 6-well plate (Corning Inc.)
and the cell monolayer was scratched using a thin disposable p200
filtered pipette tip (Sangon Biotech Co., Ltd.). The initial gap
length (0 h) and the residual gap length 24 h subsequent to
wounding was analyzed from photomicrographs. The images were
captured using an inverted microscope (TE200; Nikon Corp., Tokyo,
Japan) Each experiment contained three replicates and was repeated
at least twice. The data were presented as the mean ± SD.
Cell invasion assay
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was added into the upper chamber of a Transwell plate (Corning
Inc., Corning, NY, USA) until it formed a thin gel layer. In total,
1×105 miR-27a-transfected 786-O cells were inoculated
into the upper compartment of the Transwell chambers, while 100
µl/well of DMEM supplemented with 10% FCS was added to the lower
chamber. Following incubation for 24 h, the cells that did not
penetrate the polycarbonate membrane at the bottom of the chamber
were completely removed with a cotton swab. The migrated cells
remaining on the bottom surface of the chamber were stained with
0.5% crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 3 min, and then washed with PBS. The
stained insert was washed thoroughly, dissolved with 33% acetic
acid (Beijing Solarbio Science & Technology Co., Ltd.) and the
absorbance was measured at 595 nm with a microtiter plate reader.
Each experiment contained three replicates and was repeated at
least twice. The data were expressed as the mean ± SD.
Western blot analysis
The miR-27a-transfected and miR-27a-mimic 780-O
cells were washed with cold PBS and 300 µl radioimmunoprecipitation
buffer (Beyotime Institute of Biotechnology, Shanghai, China) was
added to each group of cells. The cell lysates were agitated at 4°C
for 30 min and centrifuged at 14,000 × g for 10 min. Subsequently,
the total protein concentrations in the supernatant were analyzed
using a bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
western blot analysis, 50 µg proteins were separated by 12%
[weight/volume (w/v)] sodium dodecylsulphate-polyacrylamide gel
electrophoresis. The separated proteins were transferred onto a
polyvinylidene fluoride (PVDF) membrane and were blocked with 5%
(w/v) skim milk in blocking buffer containing 10 mM
Tris-hydrochloric acid (pH 7.6), 100 mM sodium chloride and 0.1%
(v/v) Tween 20 (all obtained from Sangon Biotech Co., Ltd.) for 2 h
at room temperature. The following primary antibodies were added
and incubated overnight on a shaker (SK-O/L180-E Analog Orbital
Shaker & Linear Shaker; Dragon Laboratory Instruments Ltd.,
Beijing, China) at 4°C: Rabbit polyclonal anti-human epidermal
growth factor receptor (EGFR; dilution, 1:500; catalog no., BS1101;
Nanjing Bioworld Biotech Co., Ltd., Nanjing, Jiangsu, China),
rabbit polyclonal anti-human cyclin D1 (dilution, 1:500; catalog
no., BS2436; Nanjing Bioworld Biotech Co., Ltd), rabbit polyclonal
anti-human cyclin-dependent kinase 2 (CDK2; dilution, 1:500;
catalog no., 2546; Cell Signaling Technology, Inc., Danvers, MA,
USA), rabbit polyclonal anti-human p27 (dilution, 1:500; catalog
no., BS4143; Nanjing Bioworld Biotech Co., Ltd) and rabbit
polyclonal anti-human β-actin (dilution, 1:500; catalog no., 4970;
Cell Signaling Technology, Inc.). Subsequently, the PVDF membranes
were incubated with secondary antibody (goat anti-rabbit
immunoglobulin G; dilution, 1:2,000; catalog no., BS13278; Nanjing
Bioworld Biotech Co., Ltd.) for 1 h at room temperature. The
semi-quantification of proteins was evaluated using a Gel Imaging
Analysis System (Tanon 2500R; Tanon Science and Technology, Co.,
Ltd., Shanghai, China). Each western blot analysis was repeated
three times.
Mouse tumor xenograft model
All mice in the present study were kept under
pathogen-free conditions and were maintained in accordance with the
National Institutes of Health guidelines for the care and use of
laboratory animals (32), with the
approval of the Institutional Animal Care and Use Committee of the
Jiangsu Provincial Academy of Chinese Medicine (Xuzhou, Jiangsu,
SCXK 2010-0003). To evaluate the role of miR-27a in tumor
formation, 12 nude BALB/c mice (between 5 and 6-weeks-old,
purchased from Vital River Lab Animal Technology Co., Ltd.,
Beijing, China) were injected subcutaneously in the right flank
with 3×106 786-0 cells. Once palpable tumors developed,
the tumor volume was measured with a caliper every other day, using
the following formula: Tumor volume = (length × width2)
/ 2. When the tumor volume reached an average volume of 100
mm3, the mice were randomly divided into a miR-27a group
(n=6) and a control group (n=6). Mice in the miRNA-27a group were
injected with miR-27a mimic, and the control group mice were
injected with non-coding miR-27a mimic. miR-27a or miR-27a
non-coding mimics were repeatedly administered by intratumoral
injections every other day for 30 days.
Statistical analysis
Statistical analysis was performed using Student's
t-test (SPSS software version 19; IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-27a transfection inhibits
proliferation and induces apoptosis in human RCC 786-O cells
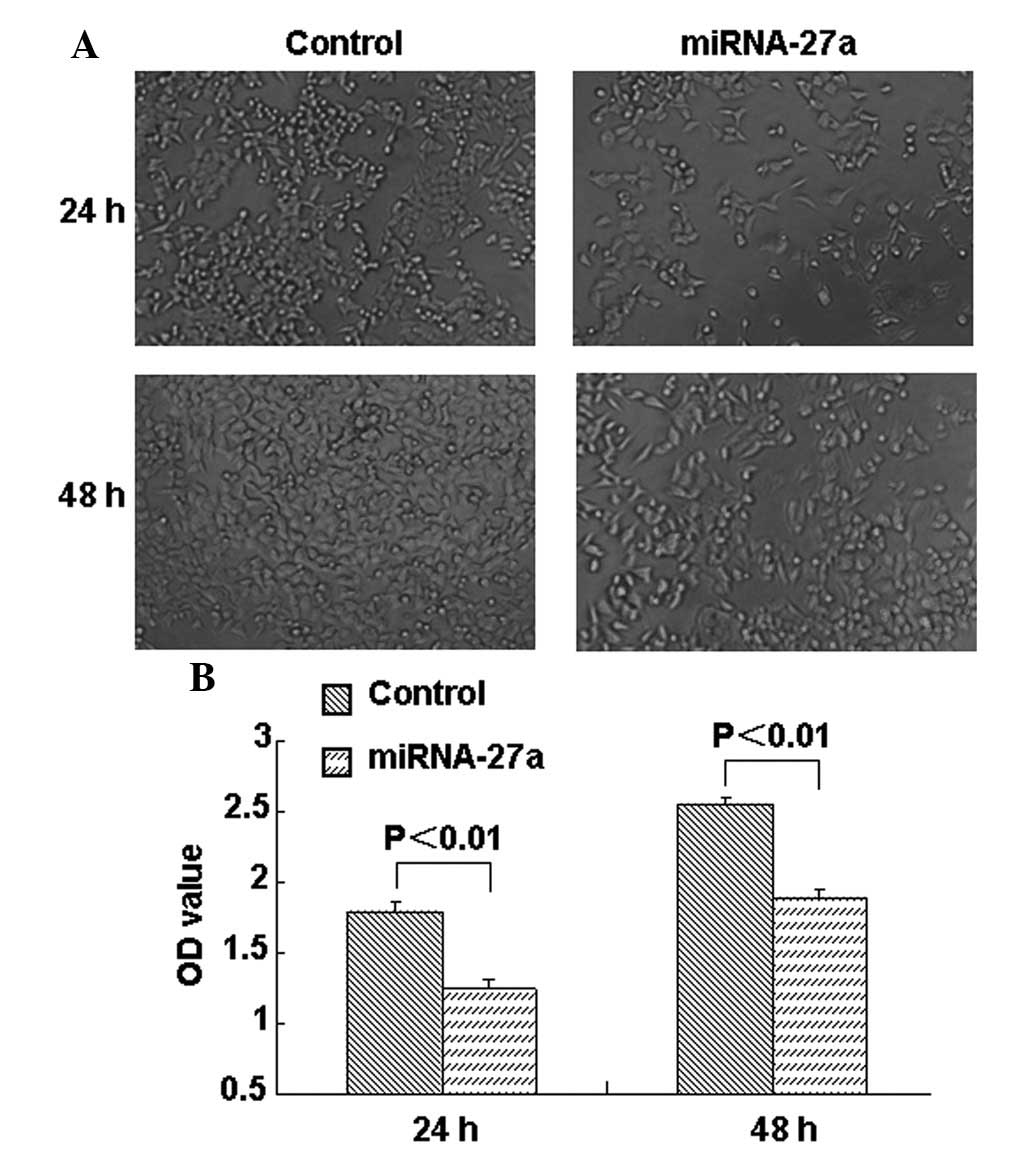
The present study examined the effect of miR-27a on
RCC cell growth. As shown in Fig. 1A,
the growth of miR-27a-transfected cells was decreased compared with
the growth of the control group cells. MTT assays were performed at
24 and 48 h post-transfection, and it was found that the
proliferation of 786-O cells was significantly suppressed at 24 and
48 h subsequent to miR-27a transfection compared with the control
group (Fig. 1B). The present study
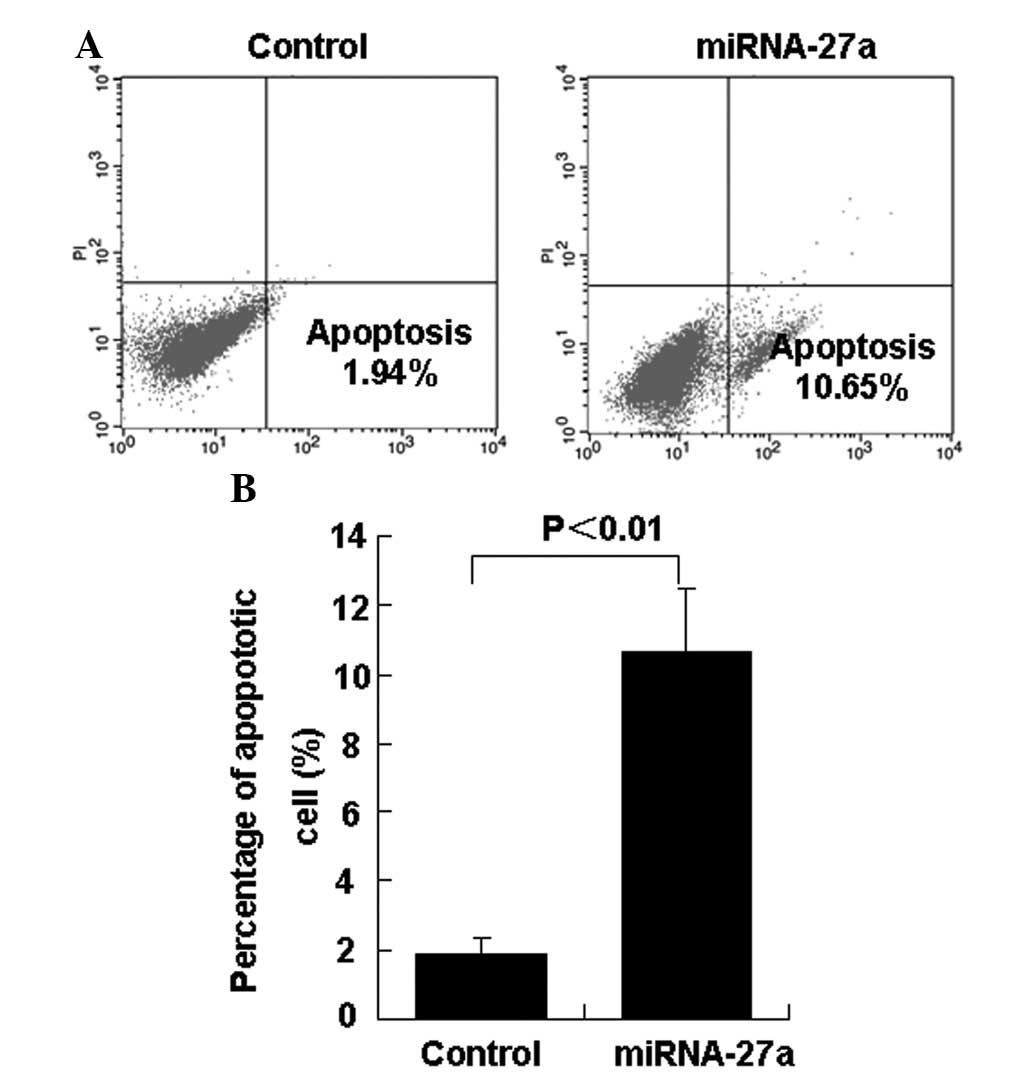
investigated the effect of miR-27a on the percentage of apoptotic
786-O cells. In total, 24 h subsequent to the transfection of
miR-27a into 786-O cells, the percentage of apoptotic cells was
significantly decreased in the miR-27a transfection group compared
with the control group (Figs. 2A and
B).
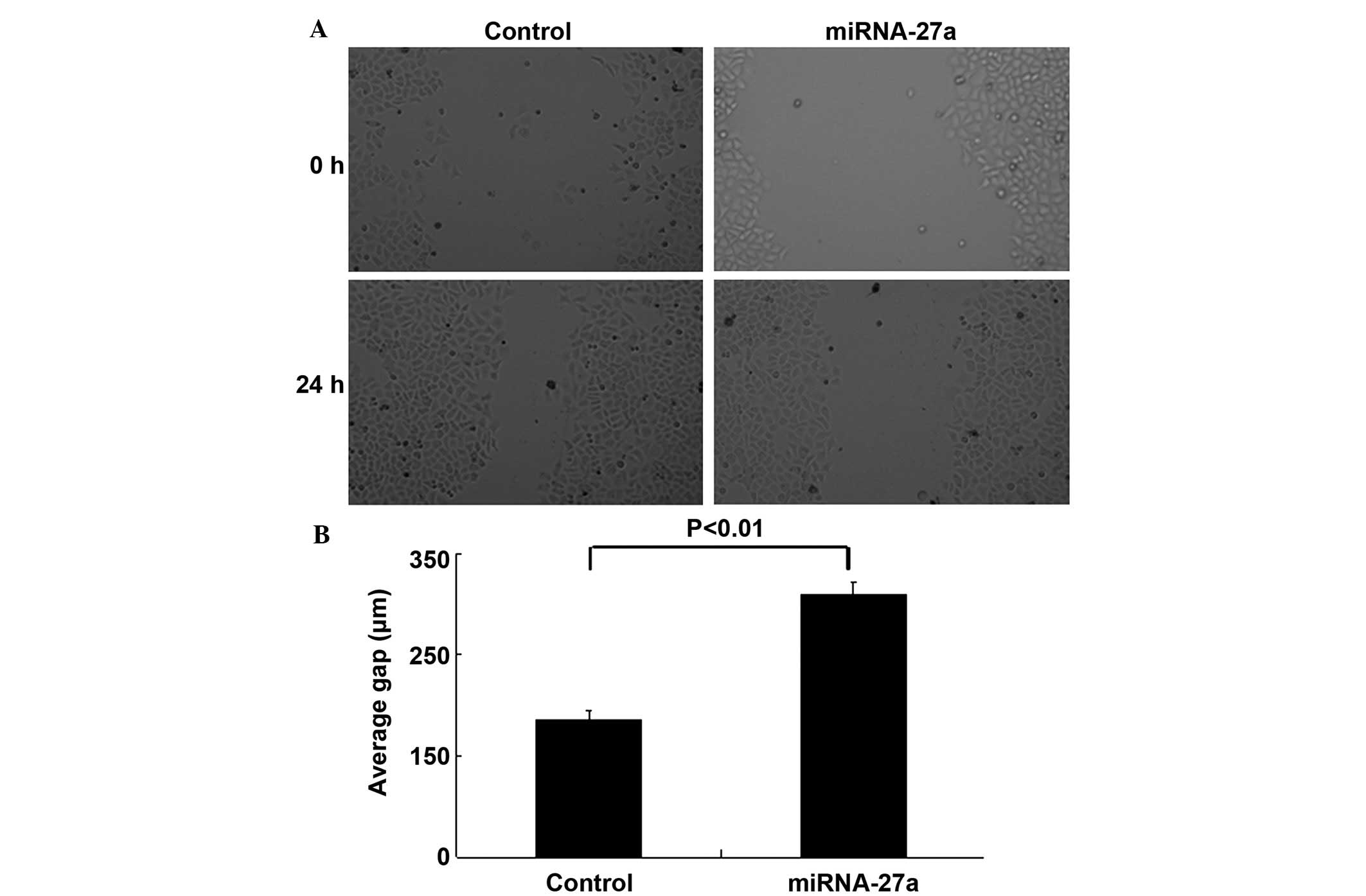
Overexpression of miR-27a blocks RCC
cell migration and invasion
The wound-healing and Transwell invasion assays were
performed to determine whether miR-27a was involved in the
migratory and invasive behaviors of 786-O cells. The wound-healing
assay demonstrated that inhibition of cell migration occurred in
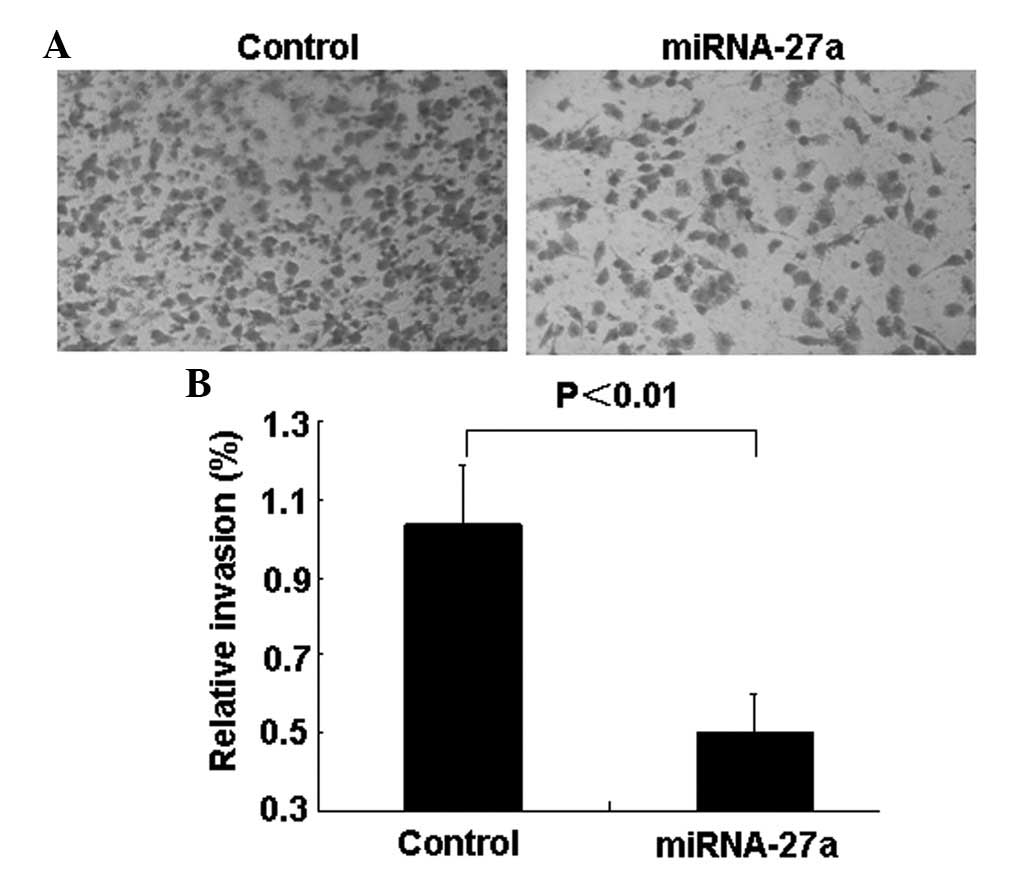
miR-27a transfectants compared with control transfectants (Figs. 3A and B). Transwell invasion assays
demonstrated that the number of invading cells was significantly
decreased in miR-27a-transfected cells compared with control group
cells (Figs. 4A and B).
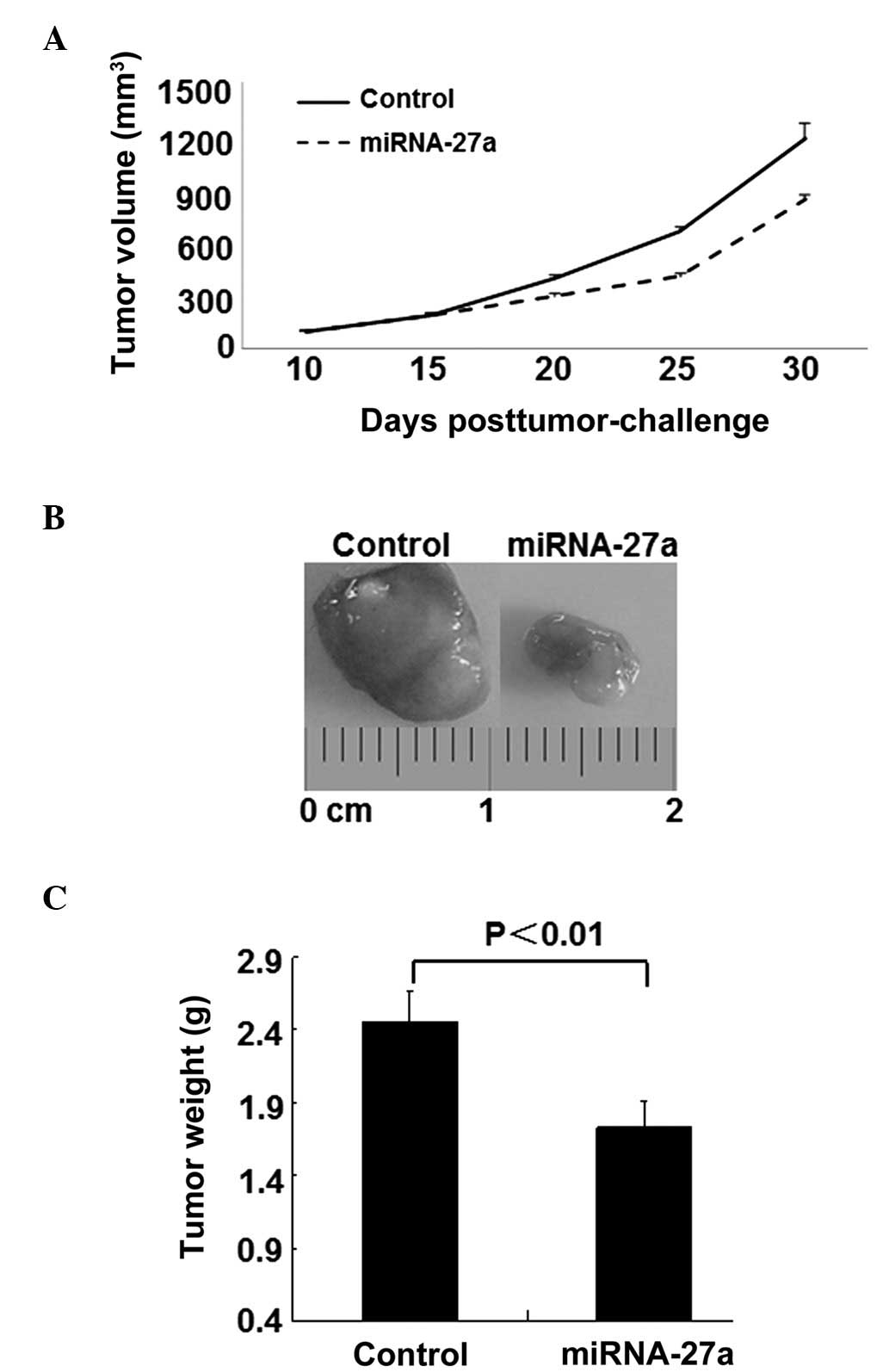
miR-27a attenuates RCC tumor growth in
mouse xenograft models
The present data demonstrated that miR-27a exhibited
antitumorigenic properties in RCC; therefore, the present study
subsequently established a 786-O xenograft model to investigate the
effect of miR-27a on tumorigenicity in vivo. As demonstrated
by Fig. 5A, 15 days subsequent to the
subcutaneous inoculation of 786-O cells, the mean tumor volume of
the mice in the control and miR-27a treated group was 200
mm3. However, by day 30, intratumoral delivery of
miR-27a induced a notable inhibitory response to tumor growth
compared with the control mice. In addition, the tumor weights were
markedly decreased in the miR-27a-treated mice (Fig. 5B).
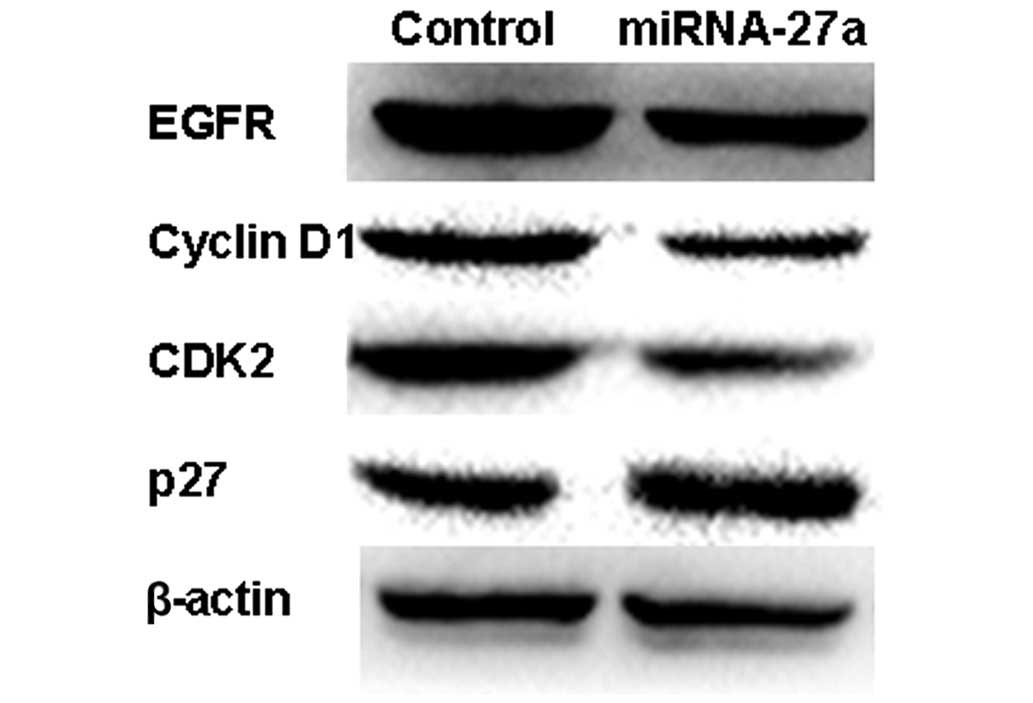
miR27a inhibits cell proliferation
through EGFR-dependent cell cycle regulation
To additionally elucidate the molecular mechanism of
the growth inhibition, the present study examined the expression of
EGFR and other cell cycle proteins using western blot analysis.
miR-27a reduced the expression of EGFR and its downstream gene
cyclin D1, CDK2, and upregulated the expression of the CDK
inhibitor p27 in 786-O cells (Figs.
6A). This suggests that miR-27a may suppress the growth of RCC
cells by targeting the EGFR-dependent cell cycle signaling
pathway.
Discussion
There are two crucial steps that enable cancer to
progress: Uncontrolled cell proliferation and aggressive tumor cell
metastasis (33). Numerous studies
have implicated miRNAs as regulatory molecules involved in cell
proliferation and metastasis of cancer in humans (34). Oncogenes and tumor suppressor genes
are two groups of miRNAs that possess tumorigenesis functions
(35). Numerous oncogenic miRNAs,
including miR-21, miRNA-17-92 and miRNA-23b-3p, are involved in
proliferative signaling, resisting growth suppression and
apoptosis, cell immortality and triggering angiogenesis, invasion
and metastasis of cells (36–38). Tumor suppressor miRNAs are involved in
the reverse processes, including miRNA-1285 and miR-138, which have
been demonstrated to be markedly decreased in RCC cells (20,39).
However, the effects of miR-27a on RCC have remain to be fully
elucidated.
The present study demonstrated that miR-27a
transfection suppressed cell growth, migration and invasion and
induced cell apoptosis in human RCC 786-O cells in vitro.
Furthermore, intratumoral delivery of miR-27a triggered regression
of tumor growth in an RCC xenograft model in vivo. The
present results suggest that miR-27a is a tumor suppressor and may
be of therapeutic use in RCC. In addition, miR-27a was observed to
become downregulated in several other tumor types, including
colorectal cancer (40,41), malignant melanoma (42), prostate cancer (43), oral squamous cell carcinoma (44) and acute promyelocytic leukemia
(45), indicating that miR-27a is a
possible tumor suppressor, which is consistent with the present
results in RCC. However, miR-27a is regarded as an oncogene in
several types of tumors. It has been reported that suppression of
miR-27a inhibits gastric cancer cell growth by targeting prohibitin
(29), and an additional study
confirmed that the overexpression of miR-27 promotes metastasis of
the human gastric cancer AGS cell line, and its depletion decreases
metastasis (46). This inconsistency
may be due to the various tumor types and cellular context. In the
present study, restoration of miR-27a significantly inhibited cell
migration and invasion, as demonstrated by a predication formed by
bioinformatics and subsequent experimental demonstration.
The cell cycle is essential for maintaining balance
between cell proliferation and cell death. Uncontrolled cell
proliferation is a hallmark of cancer. Growth factors are critical
in triggering signaling pathways, and stimulating cell cycle
progression, which is critical for tumorigenesis (47). The EGFR family of tyrosine kinases is
important in the etiology and progression of various carcinomas,
including RCC (48,49). EGFR demonstrates numerous oncogenic
effects, including the initiation of DNA synthesis, regulation of
the cell cycle, enhancement of cell growth and cell invasion and
metastasis (50,51). In the present study, overexpression of
EGFR was observed in the RCC cells. The increased expression of
EGFR was associated with the deficiency of EGFR-targeting miRNA. A
negative association was identified between the expression of EGFR
and miR-27a in RCC tissues, confirming that EGFR may be a novel
target of miR-27a. Furthermore, several cell cycle-associated
proteins, including cyclin D1 and CDK 2, were observed to be
downregulated following miR-27a overexpression, which resulted in
the progression of the cell cycle. This may be attributed to the
inhibition of the EGFR/protein kinase B/nuclear factor-κB/cyclin D1
survival signaling pathway (52).
Consequently, the inhibition of 786-O cell proliferation by miR-27a
may be partly due to EGFR-dependent cell cycle regulation.
In summary, the present study demonstrated that
miR-27a altered the cell proliferation, percentage of apoptotic
cells and the migration and invasion of human RCC 786-O cells.
Overexpression of miR-27a inhibited the EGFR signaling pathway in
786-O cells, which in turn regulated the cell cycle. The present
results may aid in the understanding of the molecular function of
miR-27a in RCC tumorigenesis and may provide novel diagnostic and
therapeutic options for RCC.
Acknowledgements
The present study was supported by the Science and
Technology Planned Projects of Xuzhou City (grant no.
KC14SH099).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pascual D and Borque A: Epidemiology of
kidney cancer. Adv Urol. 2008:7823812008. View Article : Google Scholar
|
|
3
|
Koul H, Huh JS, Rove KO, Crompton L, Koul
S, Meacham RB and Kim FJ: Molecular aspects of renal cell
carcinoma: A review. Am J Cancer Res. 1:240–254. 2011.PubMed/NCBI
|
|
4
|
Reeves DJ and Liu CY: Treatment of
metastatic renal cell carcinoma. Cancer Chemother Pharmacol.
64:11–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow TF, Youssef YM, Lianidou E, Romaschin
AD, Honey RJ, Stewart R, Pace KT and Yousef GM: Differential
expression profiling of microRNAs and their potential involvement
in renal cell carcinoma pathogenesis. Clin Biochem. 43:150–158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hadoux J, Vignot S and De La Motte Rouge
T: Renal cell carcinoma: Focus on safety and efficacy of
temsirolimus. Clin Med Insights Oncol. 4:143–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Y, Dai Y, Yang J, Chen T, Yin Y,
Tang M, Hu C and Zhang L: Microarray analysis of microRNA
expression in renal clear cell carcinoma. Eur J Surg Oncol.
35:1119–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naito S, Tomita Y, Rha SY, Uemura H, Oya
M, Song HZ, Zhong LH and Wahid MI: Kidney Cancer Working Group
report. Jpn J Clin Oncol. 40(Suppl 1): i51–i56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Agarwal N, Beard C, Bhayani S,
Bolger GB, Carducci MA, Chang SS, Choueiri TK, Hancock SL, Hudes
GR, et al: National Comprehensive Cancer Network: Kidney cancer. J
Natl Compr Canc Netw. 9:960–977. 2011.PubMed/NCBI
|
|
12
|
Agarwala SS and Case S: Everolimus
(RAD001) in the treatment of advanced renal cell carcinoma: A
review. Oncologist. 15:236–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White NM and Yousef GM: MicroRNAs:
Exploring a new dimension in the pathogenesis of kidney cancer. BMC
Med. 8:652010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiemer EA: The role of microRNAs in
cancer: No small matter. Eur J Cancer. 43:1529–1544. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamasaki T, Seki N, Yamada Y, Yoshino H,
Hidaka H, Chiyomaru T, Nohata N, Kinoshita T, Nakagawa M and
Enokida H: Tumor suppressive microRNA 138 contributes to cell
migration and invasion through its targeting of vimentin in renal
cell carcinoma. Int J Oncol. 41:805–817. 2012.PubMed/NCBI
|
|
21
|
Yamasaki T, Seki N, Yoshino H, Itesako T,
Hidaka H, Yamada Y, Tatarano S, Yonezawa T, Kinoshita T, Nakagawa M
and Enokida H: MicroRNA-218 inhibits cell migration and invasion in
renal cell carcinoma through targeting caveolin-2 involved in focal
adhesion pathway. J Urol. 190:1059–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F,
Guo Z, Zhang X, Liu J, Shen Q, et al: Let-7d suppresses growth,
metastasis, and tumor macrophage infiltration in renal cell
carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 13:2062014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Ruan A, Wang X, Han W, Wang R, Lou
N, Ruan H, Qiu B, Yang H and Zhang X: miR-129-3p, as a diagnostic
and prognostic biomarker for renal cell carcinoma, attenuates cell
migration and invasion via downregulating multiple
metastasis-related genes. J Cancer Res Clin Oncol. 140:1295–1304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu D, Pan H, Zhou Y, Zhou J, Fan Y and Qu
P: microRNA-133b downregulation and inhibition of cell
proliferation, migration and invasion by targeting matrix
metallopeptidase-9 in renal cell carcinoma. Mol Med Rep.
9:2491–2498. 2014.PubMed/NCBI
|
|
25
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chintharlapalli S, Papineni S, Abdelrahim
M, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott S,
Vanderlaag K, Cho SD, et al: Oncogenic microRNA-27a is a target for
anticancer agent methyl
2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer
cells. Int J Cancer. 125:1965–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vimalraj S, Miranda PJ, Ramyakrishna B and
Selvamurugan N: Regulation of breast cancer and bone metastasis by
microRNAs. Dis Markers. 35:369–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi D, Li P, Ma L, Zhong D, Chu H, Yan F,
Lv Q, Qin C, Wang W, Wang M, et al: A genetic variant in
pre-miR-27a is associated with a reduced renal cell cancer risk in
a Chinese population. PLoS One. 7:e465662012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reutelingsperger CP: Annexins: Key
regulators of haemostasis, thrombosis, and apoptosis. Thromb
Haemost. 86:413–419. 2001.PubMed/NCBI
|
|
32
|
National Research Council: Guide for the
Care and Use of Laboratory Animals (8th). National Academies Press.
Washington, DC: 2011.
|
|
33
|
Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q
and Ma B: MicroRNA-34a inhibits the proliferation and metastasis of
osteosarcoma cells both in vitro and in vivo. PLoS
One. 7(3): e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng S, Calin GA, Croce CM, Coukos G and
Zhang L: Mechanisms of microRNA deregulation in human cancer. Cell
Cycle. 7:2643–2646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Xin S, He Z, Che X, Wang J, Xiao X,
Chen J and Song X: MicroRNA-21 (miR-21) post-transcriptionally
downregulates tumor suppressor PDCD4 and promotes cell
transformation, proliferation, and metastasis in renal cell
carcinoma. Cell Physiol Biochem. 33:1631–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chow TF, Mankaruos M, Scorilas A, Youssef
Y, Girgis A, Mossad S, Metias S, Rofael Y, Honey RJ, Stewart R, et
al: The miR-17-92 cluster is over expressed in and has an oncogenic
effect on renal cell carcinoma. J Urol. 183:743–751. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hidaka H, Seki N, Yoshino H, Yamasaki T,
Yamada Y, Nohata N, Fuse M, Nakagawa M and Enokida H: Tumor
suppressive microRNA-1285 regulates novel molecular targets:
Aberrant expression and functional significance in renal cell
carcinoma. Oncotarget. 3:44–57. 2012.PubMed/NCBI
|
|
40
|
Xi Y, Shalgi R, Fodstad O, Pilpel Y and Ju
J: Differentially regulated micro-RNAs and actively translated
messenger RNA transcripts by tumor suppressor p53 in colon cancer.
Clin Cancer Res. 12:2014–2024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dai Y, Sui W, Lan H, Yan Q, Huang H and
Huang Y: Comprehensive analysis of microRNA expression patterns in
renal biopsies of lupus nephritis patients. Rheumatol Int.
29:749–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fletcher CE, Dart DA, Sita-Lumsden A,
Cheng H, Rennie PS and Bevan CL: Androgen-regulated processing of
the oncomir miR-27a, which targets Prohibitin in prostate cancer.
Hum Mol Genet. 21:3112–3127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saumet A, Vetter G, Bouttier M,
Portales-Casamar E, Wasserman WW, Maurin T, Mari B, Barbry P,
Vallar L, Friederich E, et al: Transcriptional repression of
microRNA genes by PML-RARA increases expression of key cancer
proteins in acute promyelocytic leukemia. Blood. 113:412–421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stumm G, Eberwein S, Rostock-Wolf S, Stein
H, Pomer S, Schlegel J and Waldherr R: Concomitant overexpression
of the EGFR and erbB-2 genes in renal cell carcinoma (RCC) is
correlated with dedifferentiation and metastasis. Int J Cancer.
69:17–22. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dias F, Teixeira AL, Santos JI, Gomes M,
Nogueira A, Assis J and Medeiros R: Renal cell carcinoma
development and miRNAs: A possible link to the EGFR pathway.
Pharmacogenomics. 14:1793–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shelton JG, Steelman LS, Abrams SL, White
ER, Akula SM, Franklin RA, Bertrand FE, McMahon M and McCubrey JA:
Conditional EGFR promotes cell cycle progression and prevention of
apoptosis in the absence of autocrine cytokines. Cell Cycle.
4:822–830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lui VW and Grandis JR: EGFR-mediated cell
cycle regulation. Anticancer Res. 22(1A): 1–11. 2002.PubMed/NCBI
|
|
52
|
Liu W, Yin T, Ren J, Li L, Xiao Z, Chen X
and Xie D: Activation of the EGFR/Akt/NF-κB/cyclinD1 survival
signaling pathway in human cholesteatoma epithelium. Eur Arch
Otorhinolaryngol. 271:265–273. 2014. View Article : Google Scholar : PubMed/NCBI
|