Introduction
Renal cell carcinoma (RCC) accounts for 3% of all
solid tumors, with ~65,150 men and women diagnosed in United States
in 2013 (1). Clear cell RCC (ccRCC)
is the most common subtype of RCC (2). Von Hippel-Lindau (VHL) gene
mutation is a notable cause of ccRCC. The VHL gene is
located on chromosome 3p25-26, and the gene product regulates the
response to oxygen availability and functions as tumor suppressor
through degradation of hypoxia-induced factor (HIF) (3,4). HIF
consists of unstable α and stable β subunits. Three HIF-α genes,
HIF-1α, HIF-2α and HIF-3α, have been
identified in humans. HIF-1α and HIF-2α are the most important in
tumorigenesis; HIF-2α modulates the expression of genes involved in
energy metabolism, angiogenesis and apoptosis, while HIF-1α is the
major oxygen homeostasis regulator (5).
Concurrent hypoxia in the local microenvironment,
and mutation or hypermethylation of the VHL gene, result in
excessive HIF accumulation in cells. HIF is then transferred into
nucleus, where it binds to hypoxia response elements in the
promoters of hypoxia response genes, such as carbonic anhydrase IX
(CA9) and erythropoietin (EPO), resulting in the overexpression of
these genes (4).
Fructose-1,6-bisphosphatase (FBP1) is known as a
rate-limiting enzyme in gluconeogenesis, which is an important
process in cell energy metabolism. Recently, ubiquitous loss of
FBP1 expression and its catalytic activity-independent function in
ccRCC tissues has been identified, indicating that FBP1 may act as
a repressor of HIF in the nucleus by binding to the HIF inhibitory
domain (6). However, the association
between FBP1 expression status and hypoxia-related gene expression
has not been well investigated. Therefore, the present study aimed
to explore whether the expression of hypoxia-related genes is
correlated with FBP1 expression.
Patients and methods
Patients and samples
The study cohort consisted of 123 patients who
underwent radical nephrectomy or partial nephrectomy for ccRCC
between July and September 2012 at the Department of Urology,
Peking University First Hospital (Beijing, China). General patient
characteristics, including age, gender and tumor features, such as
location, T stage and Fuhrman grade (7,8), were
collected. The present study was approved by the institutional
review board of Peking University First Hospital.
Samples and tissue microarray (TMA)
construction
Following nephrectomy, tumor and adjacent control
surgical specimens (at least 1 cm from the tumor tissues) were
fixed in formaldehyde (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) and embedded in paraffin. Tissue sections (4 µm)
were stained with hematoxylin and eosin (OriGene Technologies,
Inc., Beijing, China), and reviewed by two professional urological
pathologists at the Department of Urology, Peking University First
Hospital. The TMA base mold and Quick Ray tip (cat. no. UB06-3;
Unitma Co., Ltd., Seoul, Korea) were used for TMA, according to the
manufacturer's instruction.
Immunochemical staining and expression
scoring of FBP1, HIF-1α, HIF-2α, CA9 and EPO in the 123 ccRCC
samples
Following deparaffinization with xylene and
rehydration in graded ethanol (Sinopharm Chemical Reagent Co.,
Ltd.), the TMA slides (4 µm) were heated in 10 mM sodium citrate
(pH 6.0; OriGene Technologies, Inc.) for antigen retrieval,
incubated in 0.3% H2O2 (OriGene Technologies,
Inc.) for 30 min and blocked in 10% goat serum (OriGene
Technologies, Inc.). The slides were then incubated with antibodies
against FBP1 (rabbit monoclonal; 1:50 dilution; cat no. ab109020;
Abcam, Cambridge, MA, USA), HIF-1α (rabbit polyclonal; 1:50
dilution; cat no. sc-10790; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), HIF-2α (rabbit polyclonal; 1:40 dilution; cat no. ab199;
Abcam), CA9 (rabbit polyclonal; 1:1,000 dilution; cat no. ab15086;
Abcam) and EPO (rabbit polyclonal; 1:100 dilution; cat no. ab30545;
Abcam) overnight at 4°C. After washing with 0.01 M
phosphate-buffered saline and incubating with the appropriate
secondary antibody (polyperoxidase-anti-rabbit immunoglobulin G;
catalog no., PV-9001; OriGene Technologies, Inc.), the slide was
developed by DAB staining (OriGene Technologies, Inc.).
Immunohistochemical staining was evaluated by two independent
pathologists using an Olympus CK40 microscope (Olympus Corporation,
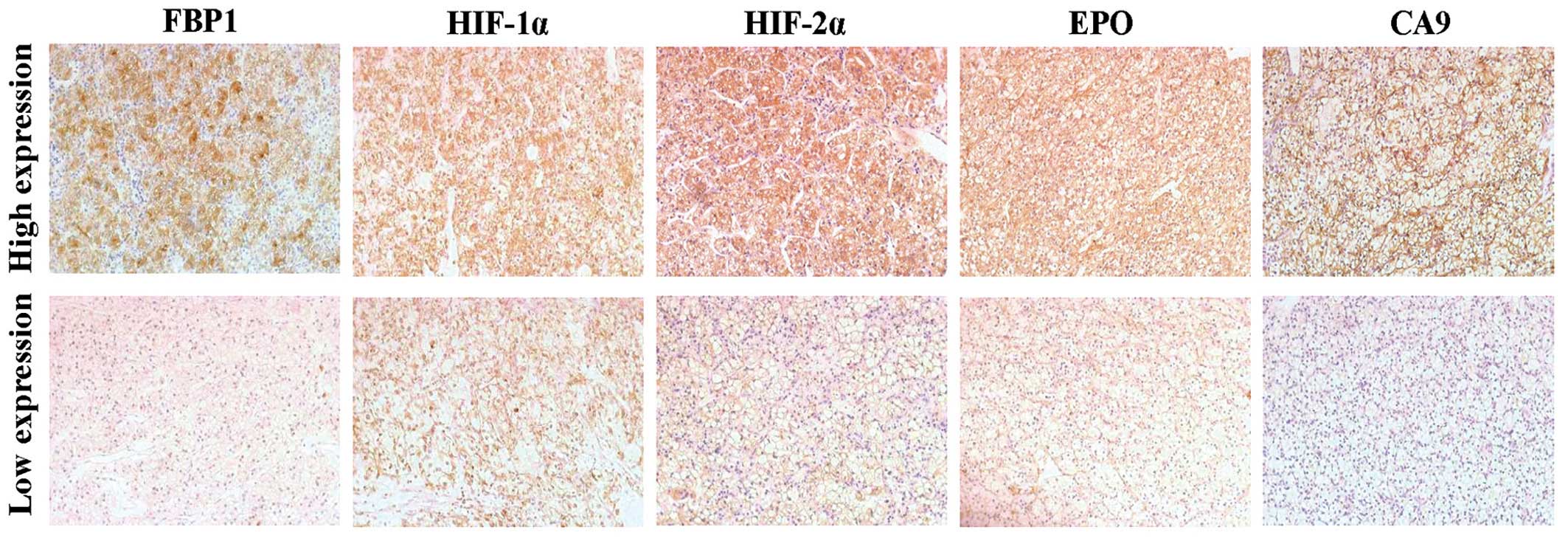
Tokyo, Japan). The staining intensity was represented by intensity
score, was which was follows: 0, no staining; 1, light yellow; 2,
brown-yellow; and 3, sepia. Tumor samples with an intensity score
of 0 or 1 were classified as low expression and samples with an
intensity score of 2 or 3 were classified as high expression
(Fig. 1).
Statistical analysis
Data is presented as the mean ± standard deviation
and the median value. Immunohistochemical assessment was repeated
three times for each sample. The correlation between FBP1
expression and clinicopathological factors or hypoxia-related
protein expression were analyzed by χ2 and Fisher's
exact tests. Differences in FBP1 expression between tumor tissues
and adjacent normal tissues was calculated by using the Wilcoxon
rank sum test. SPSS software version 19.0 (IBM SPSS, Armonk, NY,
USA) was used to perform statistical analysis, and P<0.05
indicated a statistically significant difference.
Results
Clinicopathological characteristics
and FBP1 expression status in ccRCC tumor tissues
Surgical ccRCC samples were obtained from 123
patients (83 males and 40 females). The mean age was 55.1±12.3
years old, 62 patients (50.4%) were aged ≤55 years old and 61
patients (49.6%) were aged >55 years old. Using the TNM staging
system, tumors were classified as T1a in 59 cases (48.0%), T1b in
39 cases (31.7%), T2 in 8 cases (6.5%) and T3 in 17 cases (13.8%).
According to the Fuhrman grading system, tumors were graded as G1
in 42 cases (34.1%), G2 in 73 cases (59.4%) and G3 in 8 cases
(6.5%) (Table I).
 | Table I.Correlation between FBP1 expression
and clinicopathological factors in clear cell renal cell carcinoma
tissues. |
Table I.
Correlation between FBP1 expression
and clinicopathological factors in clear cell renal cell carcinoma
tissues.
|
|
| FBP1 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Patients (n=123) | Low (n=95) | High (n=28) | χ2 | P-value |
|---|
| Age (years), mean ±
SD | 55.1±12.3 | 55.3±11.9 | 54.0±13.4 | 0.827 | 0.363 |
| Gender, n (%) |
|
|
| 1.765 | 0.184 |
| Male | 83 (67.5) | 67
(54.5) | 16
(13.0) |
|
|
|
Female | 40 (32.5) | 28
(22.8) | 12 (9.7) |
|
|
| T stage, n (%) |
|
|
| 1.203 | 0.752 |
| T1a | 59 (48.0) | 48
(39.0) | 11 (8.9) |
|
|
| T1b | 39 (31.7) | 29
(23.6) | 10 (8.1) |
|
|
| T2 | 8 (6.5) | 6
(4.9) | 2
(1.6) |
|
|
| T3 | 17 (13.8) | 12 (9.8) | 5
(4.1) |
|
|
| Fuhrman grade, n
(%) |
|
|
| 2.025 | 0.363 |
| G1 | 42 (34.1) | 35
(28.5) | 7
(5.7) |
|
|
| G2 | 73 (59.4) | 55
(44.7) | 18
(14.6) |
|
|
| G3 | 8 (6.5) | 5
(4.1) | 3
(2.4) |
|
|
Immunohistochemistry staining revealed significantly
higher rates of low FBP1 expression in tumor tissues compared with
adjacent healthy tissues (P<0.001). The rate of low FBP1
expression in ccRCC tissues was 77.2% (95/123 cases) while the rate
in adjacent normal tissues was 8.1% (10/123 cases; data not shown).
In the ccRCC tumor tissues, 28 cases (22.8%) were identified as
exhibiting high FBP1 expression and 95 cases (77.2%) exhibited low
expression. No correlations were identified between FBP1 expression
level status, and age, gender, T stage or Fuhrman grade in the
ccRCC patients (Table I).
Correlation of FBP1 expression with
HIF-1α, HIF-2α, EPO and CA9 expression in ccRCC tumor tissues
HIF-1α, HIF-2α, EPO and CA9 were highly expressed in
the majority of samples (HIF-1α in 101/123 cases, 82.1%; HIF-2α in
77/123 cases, 62.6%; CA9 in 77/123 cases, 62.6%; and EPO in 66/123
cases, 53.7%; Table II). The
expression of HIF-1α and EPO were significantly correlated with
FBP1 (P=0.005 and P=0.010, respectively; Table II). However, this significant
correlation was not observed between HIF2α (P=0.123) or CA9
(P=0.513) expression, and FBP1 expression.
 | Table II.Correlation between expression of
FBP1, and HIF1α, HIF2α, CA9 and EPO in clear cell renal cell
carcinoma. |
Table II.
Correlation between expression of
FBP1, and HIF1α, HIF2α, CA9 and EPO in clear cell renal cell
carcinoma.
|
|
| FBP1 expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Expression | Patients, n (%) | Low (n=95) | High (n=28) | χ2 | P-value |
|---|
| HIF-1α |
|
|
| 7.897 | 0.005 |
| Low | 22
(17.9) | 22 (23.2) | 0 (0.0) |
|
|
| High | 101 (82.1) | 73 (76.8) | 28
(100.0) |
|
|
| HIF-2α |
|
|
| 2.380 | 0.123 |
|
Low | 46 (37.4) | 39 (41.1) | 7
(25.0) |
|
|
|
High | 77 (62.6) | 56 (58.9) | 21 (75.0) |
|
|
| CA9 |
|
|
| 0.428 | 0.513 |
|
Low | 46 (37.4) | 37 (38.9) | 9
(32.1) |
|
|
|
High | 77 (62.6) | 58 (61.1) | 19 (67.9) |
|
|
| EPO |
|
|
| 6.640 | 0.010 |
|
Low | 57 (46.3) | 50 (52.6) | 7
(25.0) |
|
|
|
High | 66 (53.7) | 45 (47.4) | 21 (75.0) |
|
|
Discussion
The present study analyzed the association between
FBP1 expression and the expression of various hypoxia-related
proteins in ccRCC samples. The majority of ccRCC samples exhibited
low expression of FBP1, accompanied by high expression of HIF-1α
and EPO. However, there was not a significant association between
low FBP1 expression, and HIF-2α and CA9 expression.
Low expression of FBP1 has previously been observed
in human hepatocellular carcinoma (HC), colon cancer (CC), breast
cancer (BC), gastric cancer (GC) and ccRCC tissues (6,9–11). The current data is consistent with the
latter study. The reason for low FBP1 expression in HC, CC, BC and
GC has been identified as hypermethylation of the promoter region
of the FBP1 gene (9–11). However, the mechanism of FBP1
inhibition in ccRCC tissues has not been clearly identified and
requires further investigation (12,13).
Recently, an association between FBP1
expression and various pathological variables in patients with
ccRCC was demonstrated at the transcription level (6). By contrast, the current results did not
identify a significant association between FBP1 protein expression
level and T stage (P=0.75) or Fuhrman grade (P=0.36) in ccRCC. This
conflicting association between FBP1 mRNA and FBP1 protein
level may be partially due to the high proportion of patients with
low T stage (T1 + T2, 86.2%) and low Fuhrman grade (G1 + G2, 93.5%)
in the current cohort. Analysis of a greater number of ccRCC cases
with various T stages and Fuhrman grades are required to understand
the clinical significance of FBP1 expression levels in ccRCC.
HIF-1α and HIF-2α are the two most
important hypoxia-related genes, and are regulated by VHL protein
and oxygen in the local environment. HIF-1α and HIF-2α are
overexpressed in various types of cancer, including melanoma and
breast cancer (14–16), and have a role of tumorigenesis and
tumor progression. However, these two subunits occasionally have
different effects on cell biological behavior (17). HIF1α dominantly regulates processing
mechanisms, particularly the glucose processing mechanism, while
HIF2α influences various processes, including tumor cell growth
(18). The present study observed
that FBP1 low expression is associated with high expression of
HIF1α but not of HIF2α. In agreement with this finding, FBP1 is a
critical enzyme in glucose metabolism, with certain research
indicating that FBP1 may inhibit the function of HIF located in the
nucleus (6); whether HIF1α can
regulate FBP1 expression has not been well discussed. Identifying
the role of HIF1α in the regulation of FBP1 expression may propose
a novel function of HIF1α and may be useful for clarifying ccRCC
tumorigenesis.
EPO is widely used to treat tumor-related anemia,
however, the effect of EPO on RCC has not been well delineated
(19). Several studies observed that
exogenous recombinant human EPO inhibits cancer progression,
however, other studies indicated that it promotes cancer
progression (20–22). EPO expression under a hypoxic
environment is predominantly regulated by HIF2α (23). In the present study, it was identified
that FBP1 expression is significantly associated with EPO, but is
not significantly associated with HIF2α. These findings indicate
that FBP1 may directly interact with EPO expression. Understanding
the interaction between FBP1 and EPO may aid in comprehending the
role of FBP1 and EPO in ccRCC.
In conclusion, FBP1 expression was positively
correlated with the expression levels of HIF-1α and EPO in ccRCC
tissues. The current findings confirm the association between FBP1
and hypoxia-related gene expression, and may facilitate
understanding of the mechanisms of ccRCC tumorigenesis.
Acknowledgements
The present study was supported by grants from the
Program for New Century Excellent Talents in Universities (grant
no. NCET-10-0190) and the National Natural Science Foundation of
China (grant nos. 81172418 and 81172419). The abstract was
published as AB180 in Transl Androl Urol 4 (Suppl 1), 2015.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kim WY and Kaelin WG: Role of VHL gene
mutation in human cancer. J Clin Oncol. 22:4991–5004. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Latif F, Tory K, Gnarra J, Yao M, Duh FM,
Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al:
Identification of the von Hippel-Lindau disease tumor suppressor
gene. Science. 260:1317–1320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Clark PE: The role of VHL in clear-cell
renal cell carcinoma and its relation to targeted therapy. Kidney
Int. 76:939–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Arjumand W and Sultana S: Role of VHL gene
mutation in human renal cell carcinoma. Tumour Biol. 33:9–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Li B, Qiu B, Lee DS, Walton ZE, Ochocki
JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I and Simon MC:
Fructose-1,6-bisphosphatase opposes renal carcinoma progression.
Nature. 513:251–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Elmore JM, Kadesky KT, Koeneman KS and
Sagalowsky AI: Reassessment of the 1997 TNM classification system
for renal cell carcinoma. Cancer. 98:2329–2334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hong SK, Jeong CW, Park JH, Kim HS, Kwak
C, Choe G, Kim HH and Lee SE: Application of simplified Fuhrman
grading system in clear-cell renal cell carcinoma. BJU Int.
107:409–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen M, Zhang J, Li N, Qian Z, Zhu M, Li
Q, Zheng J, Wang X and Shi G: Promoter hypermethylation mediated
downregulation of FBP1 in human hepatocellular carcinoma and colon
cancer. PloS One. 6:e255642011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Alderton GK: Tumorigenesis: FBP1 is
suppressed in kidney tumours. Nat Rev Cancer. 14:5752014.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Phillips R: Kidney cancer: FBP1 depletion
feeds ccRCC. Nat Rev Urol. 11:4822014. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Giatromanolaki A, Sivridis E, Kouskoukis
C, Gatter KC, Harris AL and Koukourakis MI: Hypoxia-inducible
factors 1alpha and 2alpha are related to vascular endothelial
growth factor expression and a poorer prognosis in nodular
malignant melanomas of the skin. Melanoma Res. 13:493–501. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Theodoropoulos VE, Lazaris A, Sofras F,
Gerzelis I, Tsoukala V, Ghikonti I, Manikas K and Kastriotis I:
Hypoxia-inducible factor 1 alpha expression correlates with
angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol.
46:200–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Helczynska K, Larsson AM, Mengelbier
Holmquist L, Bridges E, Fredlund E, Borgquist S, Landberg G,
Påhlman S and Jirström K: Hypoxia-inducible factor-2alpha
correlates to distant recurrence and poor outcome in invasive
breast cancer. Cancer Res. 68:9212–9220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011.PubMed/NCBI
|
|
18.
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Morais C, Johnson DW, Vesey DA and Gobe
GC: Functional significance of erythropoietin in renal cell
carcinoma. BMC Cancer. 13:142013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Abhold E, Rahimy E, Wang-Rodriguez J,
Blair KJ, Yu MA, Brumund KT, Weisman RA and Ongkeko WM: Recombinant
human erythropoietin promotes the acquisition of a malignant
phenotype in head and neck squamous cell carcinoma cell lines in
vitro. BMC Res Notes. 4:5532011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Phillips TM, Kim K, Vlashi E, McBride WH
and Pajonk F: Effects of recombinant erythropoietin on breast
cancer-initiating cells. Neoplasia. 9:1122–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhou B, Damrauer JS, Bailey ST, Hadzic T,
Jeong Y, Clark K, Fan C, Murphy L, Lee CY, Troester MA, et al:
Erythropoietin promotes breast tumorigenesis through
tumor-initiating cell self-renewal. The Journal of clinical
investigation. 124:553–563. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rankin EB, Biju MP, Liu Q, Unger TL, Rha
J, Johnson RS, Simon MC, Keith B and Haase VH: Hypoxia-inducible
factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin
Invest. 117:1068–1077. 2007. View
Article : Google Scholar : PubMed/NCBI
|