Introduction
Myeloid sarcoma is a malignant neoplasm of myeloid
origin that locates outside the bone marrow. The tumor is also
known as a chloroma due to its green color when exposed to air.
Myeloid sarcoma can develop in any area of the body, including the
cervix, mediastinum, small intestines, lymph nodes and skin
(1). In the majority of cases,
myeloid sarcoma is an extramedullary presentation of leukemia, and
in a few cases, it can develop prior to onset or following the
remission of leukemia, presenting as a solitary extramedullary
neoplasm known as a solitary myeloid sarcoma or primary myeloid
sarcoma (2). The incidence of myeloid
sarcoma is only 1–2% of all acute myeloid leukemia cases, and only
6.5% of myeloid sarcomas derive from the gastrointestinal tract
(3). These patients usually present
with nonspecific symptoms, including abdominal pain, nausea,
vomiting, bowel obstruction or gastrointestinal bleeding without
bone marrow involvement, thus being easily misdiagnosed (4). The present study reports a case of
myeloid sarcoma derived from the gastrointestinal tract and reviews
the associated literature, in order to improve the understanding of
the disease and provide a reference for standardized and
individualized treatments.
Case report
A 31-year-old female was admitted to the Second
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, Zhejiang, China) on June 20, 2012, due to upper
abdominal pain and melena that had been apparent for 2 months and
vomiting that had persisted for 1 month. Physical examinations
showed jaundice of the skin and sclera, no compression pain of the
sternum and no enlarged superficial lymph nodes. The patient's
abdomen was soft, with moderate tenderness, but with no rebound
pain and no palpable mass. The liver and spleen were not palpable
beneath the ribs. A routine blood test showed a white blood cell
count of 9.5×109 cells/l (normal range,
4.0–10.0×109 cells/l), a hemoglobin level of 138 g/l
(normal range, 110–160 g/l) and a platelet level of
202×109 cells/l (normal range, 100–300×109
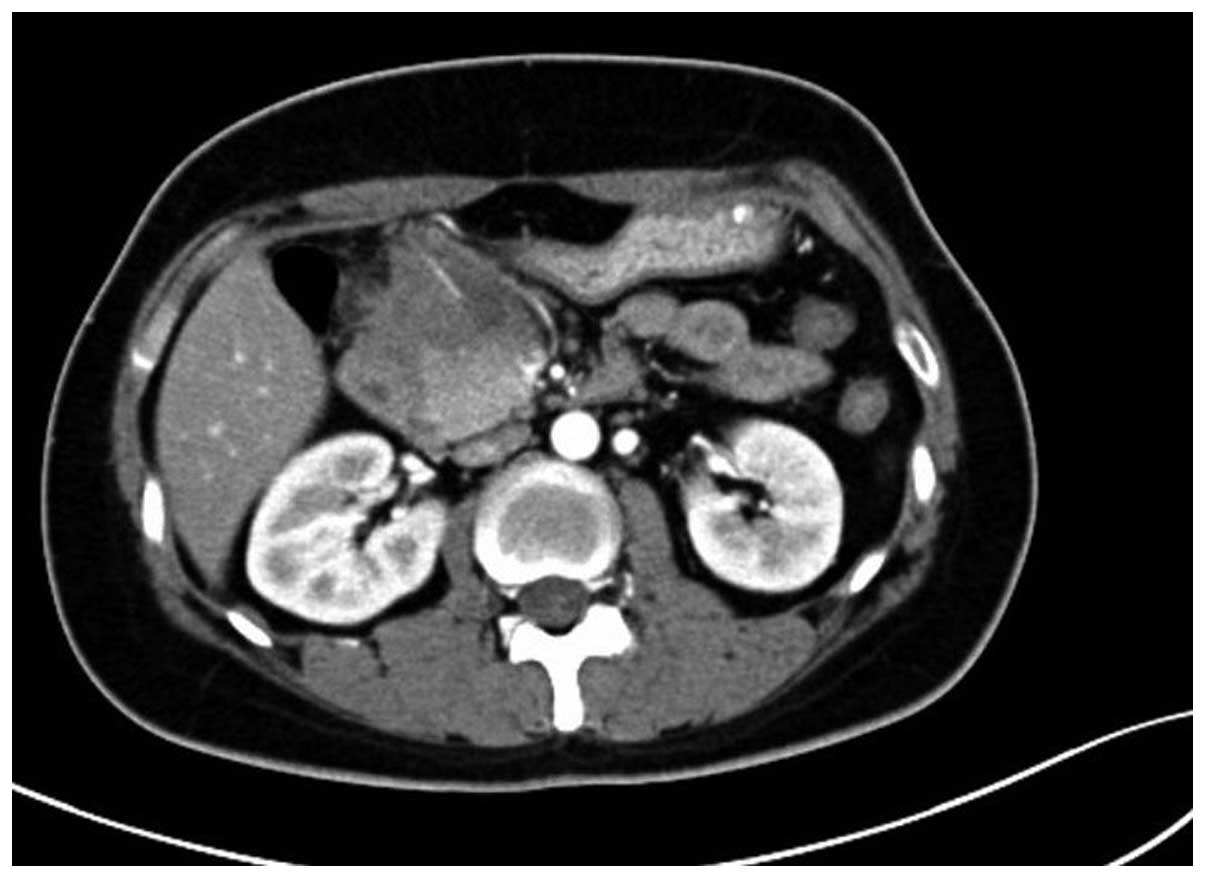
cells/l). The abdominal enhanced computed tomography (CT) scan
(Somatom Definition AS 64-row CT scanner; Siemens AG, Munich,
Germany) revealed a mass in the gastric antrum area, 5.2×6.2 cm in
size, with possible infiltration of the duodenum, gallbladder and
head of the pancreas, and possible retroperitoneal lymph node
metastasis (Fig. 1). Gastroscopy
revealed chronic gastritis and a mass in the cavity of the duodenal
bulb, from which a biopsy sample was taken. Positron emission
tomography-CT revealed a high metabolic tumor in the area of the
duodenal bulb. The tumor was 5.2×6.2 cm in size and its maximum
standard uptake value (SUVmax) was 10.39. The tumor was adhered to
the head of the pancreas, the gastric antrum and the liver.
Multiple hypermetabolic lymph nodes were found in the
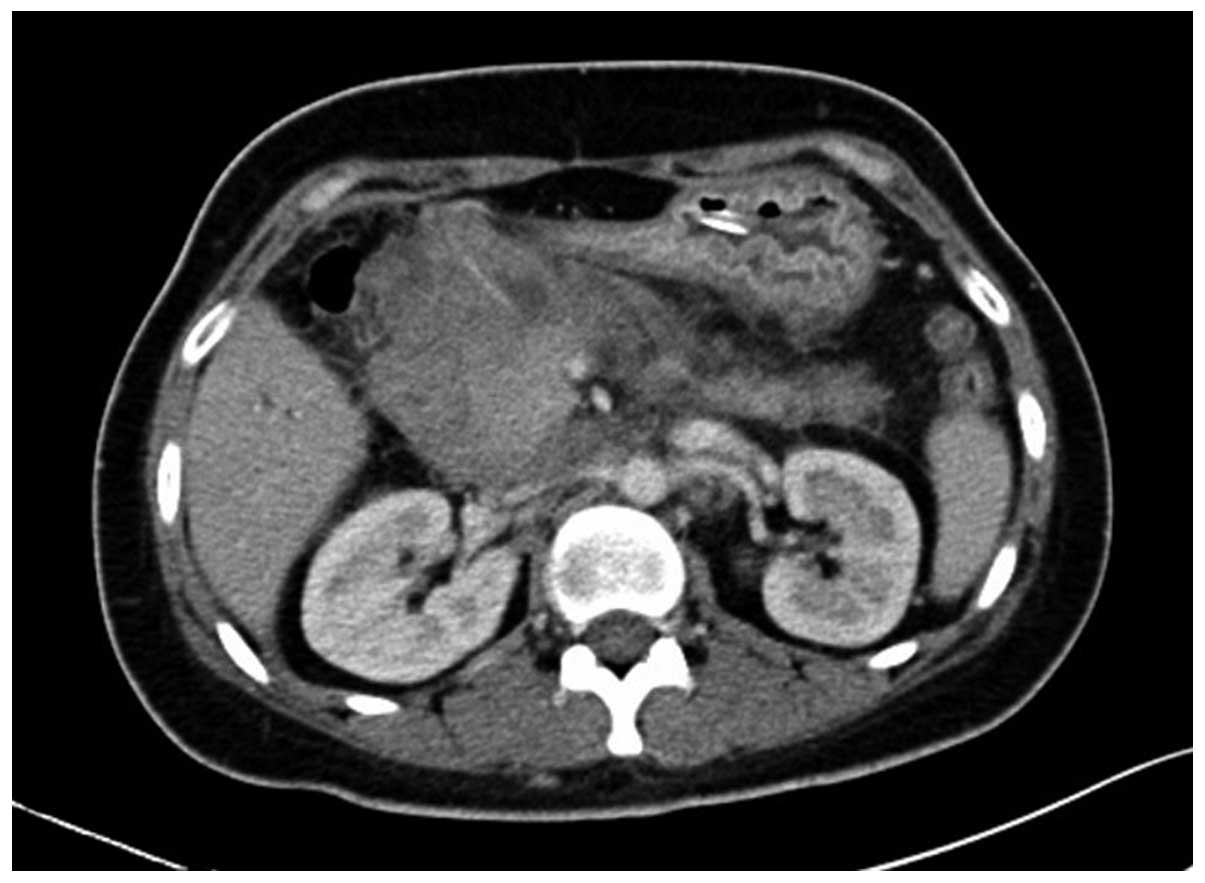
retroperitoneal area with an SUVmax of 5.03. Repeat abdominal CT
after 9 days showed marked enlargement of the tumor (12×9 cm), and
expansion of the superior segment of the common bile duct and the
intrahepatic bile ducts. This was considered to be due to
compression of the bile duct (Fig.
2). The jaundice and liver function of the patient worsened,
with test results as follows: Total bilirubin, 180.4 µmol/l (normal
range, 3.4–20.5 µmol/l); direct bilirubin, 147.0 µmol/l (normal
range, <6.8 µmol/l); alanine aminotransferase, 761 U/l (normal
range, <34 U/l); aspartate aminotransferase, 596 U/l (normal
range, <35 U/l); alkaline phosphatase, 1,092 U/l (normal range,
30–120 U/l); γ-glutamyl transpeptidase, 1451 U/l (normal range,
9–64 U/l); and lactate dehydrogenase, 489 U/l (normal range,
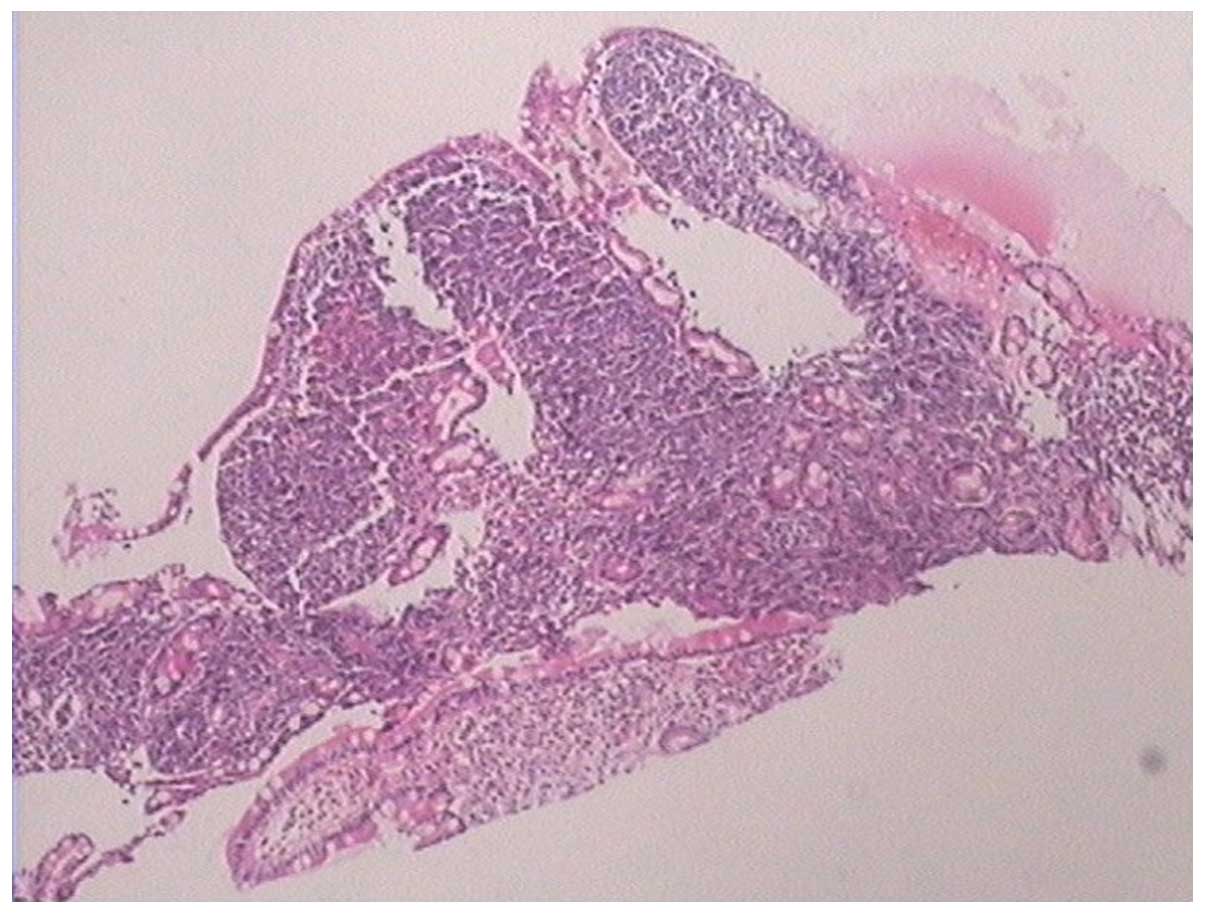
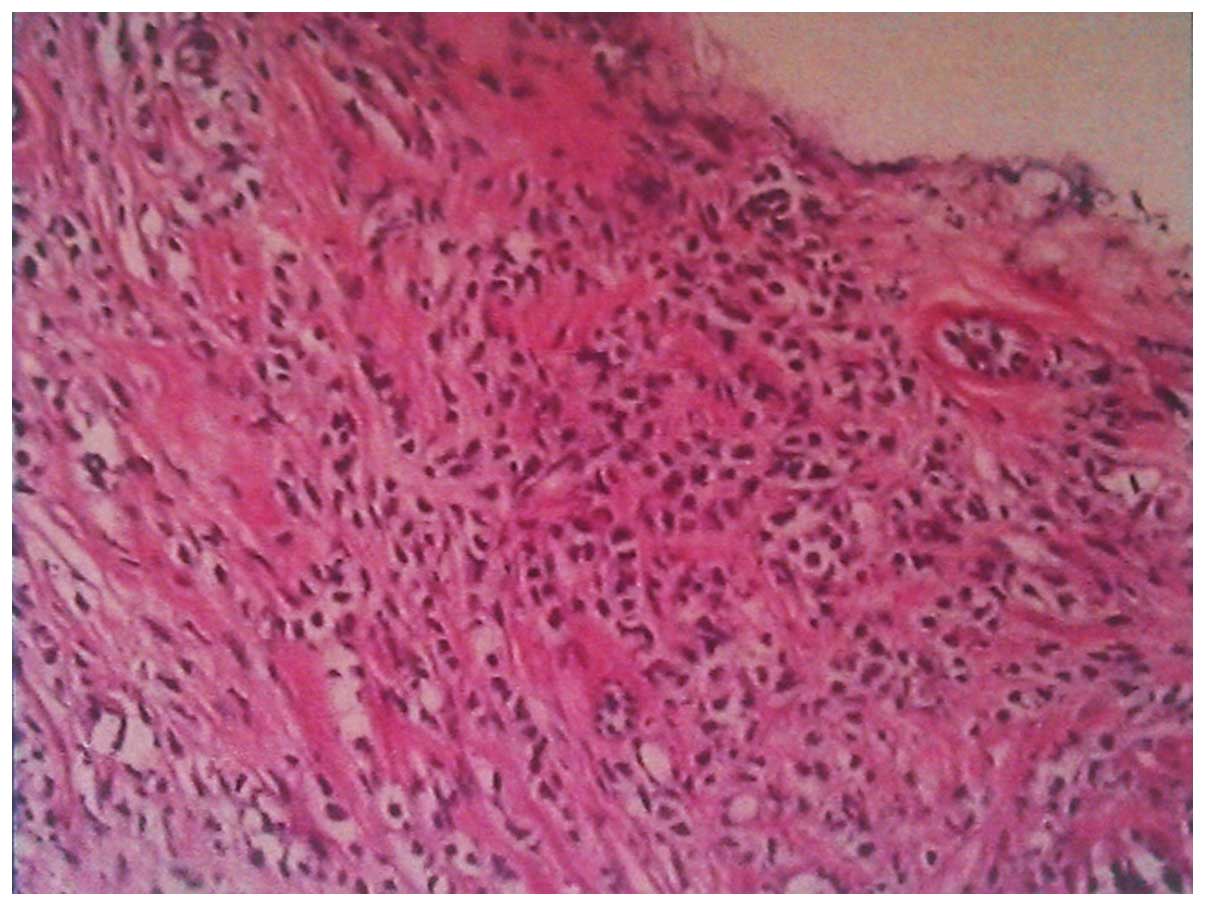
140–271 U/l). The pathological results of the gastroscopic biopsy
showed large abnormal cells with loose chromatin and nucleoli, and
immunohistochemical staining revealed that the tumor cells
expressed cluster of differentiation (CD)43 and myeloperoxidase
(MPO), with scattered CD123 and terminal deoxynucleotidyl
transferase expression (Fig. 3). The
diagnosis was of myeloid sarcoma. Bone marrow routine tests and
immunophenotyping were normal, however, gene screening of the bone
marrow cells showed increased MDS1 and EVI1 complex locus (EVI1)
gene expression. A percutaneous transhepatic cholangial drainage
(PTCD) procedure was performed on July 17, 2012, in order to
relieve the symptom of jaundice, and ~300 ml of yellow-green
colored bile was drained out everyday. Subsequent to PTCD, the
bilirubin level gradually decreased, liver function improved and
the jaundice gradually disappeared. Radiotherapy (6,000,000 eV
X-ray; total dose, 2,450 cGy; Clinac 21EX; Varian Medical Systems,
Inc., Palo Alto, CA, USA) was administered to the abdominal tumor 7
times (350 cGy each) after July 25, 2012, and the tumor gradually
became smaller. However, masses later appeared in the bilateral
orbits almost at the beginning of radiotherapy, and the mass in the
left orbit became progressively enlarged in the subsequent days.
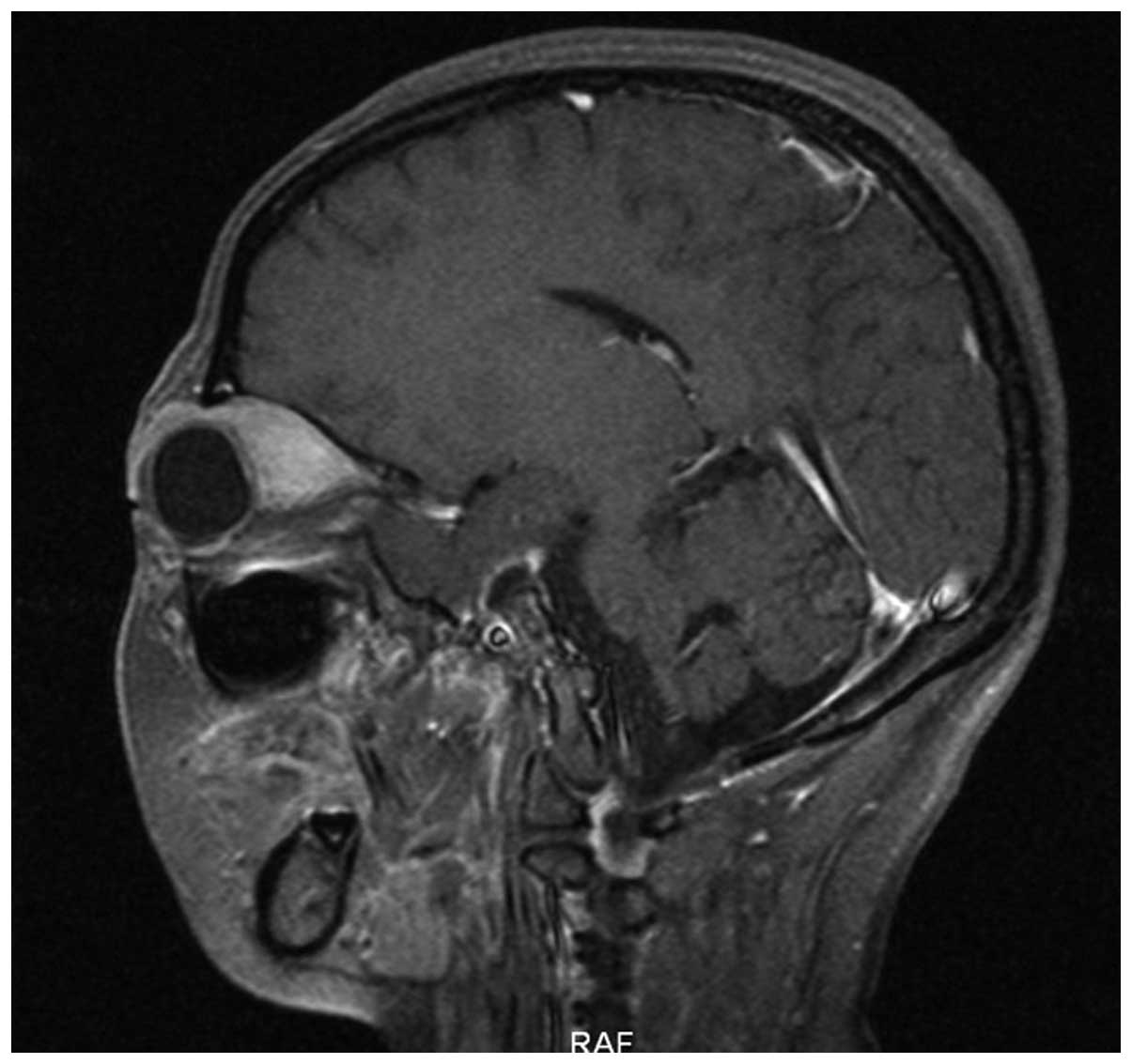
Simultaneously a mass was palpable in the left breast. Magnetic
resonance imaging (Magnetom Sonata 3.0T; Siemens AG) of the orbits
showed tumors in the bilateral superior rectus and eyelids
(Fig. 4). B-mode ultrasound showed a
hypoechoic area in the left breast. B-mode ultrasound-guided
fine-needle biopsy of the breast mass revealed fibrous tissues
infiltrated by abnormal cells and immunohistochemical staining
revealed a myeloid sarcoma.
Immunohistochemistry was performed with the
Histostain-Plus IHC kit (Dako, Glostrup, Denmark). Briefly, tumor
tissue samples, which were preserved in 2.5%
glutaraldehyde-polyoxymethylene solution, were dehydrated and
embedded in paraffin following routine methods. For
immunohistochemistry, paraffin was removed, and the sections were
immersed in distilled water, prior to be rinsed 3 times for 5 min
each in 0.01 M phosphate-buffered saline (PBS; pH 7.4) containing
0.05% Tween 20 (PBST; Fresenius Kabi, Bad Homburg vor der Höhe,
Germany), and then blocked with 3% peroxide-methanol at room
temperature for endogenous peroxidase ablation. All the subsequent
steps were conducted in a moist chamber: Sections were incubated
with blocking buffer (normal goat serum) at room temperature for 20
min, followed by incubation for 2 h at 37°C with mouse anti-MPO,
anti-cytokeratin AE1/AE3, anti-epithelial membrane antigen (EMA),
anti-vimentin, anti-CD45, anti-Ki-67, anti-CD20, anti-CD79a,
anti-CD10, anti-CD3, anti-CD45RO, anti-CD117, anti-CD15 and
anti-CD43 antibodies. Upon rinsing in PBST (3×5 min), sections were
incubated for 30 min at 37°C with goat anti-mouse immunoglobulin G.
Next, sections were rinsed in PBST (3×5 min) and incubated with
streptavidin-horseradish peroxidase conjugate at 37°C for 30 min.
Following rinsing in PBST (3×5 min), sections were stained with
3,3′-diaminobenzidine at room temperature for 10 min. Subsequently,
sections were washed with distilled water and stained with
hematoxylin, prior to dehydration, clearing and mounting with
neutral gums. The negative control was analyzed as above, with the
exception of the replacement of the aforementioned antibodies by
PBS. The immunohistochemical results were as follows: Cytokeratin
AE1/AE3(−), EMA(−), vimentin(−), CD45 (leukocyte common
antigen)(++), MPO(+++), Ki-67 of 70%(+), CD20(−), CD79a(−),
CD10(−), CD3(−), CD45RO(−), CD117(++), CD15(+) and CD43(++)
(Fig. 5).
Myeloid sarcoma involving the breast and orbits was
considered. An idarubicin and cytarabine regimen (10 mg idarubicin
on days 1–3 and 150 mg cytarabine on days 1–7) was administered
between August 3 and August 9, 2012, after which, the tumors in the
gastrointestinal tract, orbits and breast decreased in size.
Radiotherapy and consolidation chemotherapy were administered at
regular intervals, as follows: Two additional cycles of idarubicin
and cytarabine regimens (10 mg idarubicin on days 1–3, and 150 mg
cytarabine on days 1–7) were administered on September 13 and
October 30, 2012; a medium dose of cytarabine (2 g cytarabine every
12 h on days 1–3) was administered on December 14, 2012; and FLAG
regimen [40 mg fludarabine on days 1–5, 2.8 g cytarabine on days
1–5 and 200 µg granulocyte-colony stimulating factor (G-CSF) on
days 0–5] was administered on March 1, 2013. However, in April
2013, the tumor in the breast progressed and became resistant to
chemotherapy, therefore, salvage allogeneic peripheral blood stem
cell transplantation was performed on May 6, 2013. The peripheral
hematopoietic stem cells were obtained from an unrelated donor of
the China Marrow Donor Program (http://www.cmdp.org.cn/show/1022765.html), and 9 of 10
human leukocyte antigens were matched. The volume of the graft was
191 ml, in which the number of mononuclear cells was
5.42×108 cells/kg, and the number of CD34-positive cells
was 4.06×106 cells/kg. Consolidation radiotherapy
(6,000,000 eV X-ray; total dose, 2,400 cGy) was administered to the
orbit tumor 12 times (200 cGy each) after June 11, 2013.. At 5
months post-transplantation, a routine B-mode ultrasound
examination found a hypoechoic, 7.1×5.1-cm mass on the top left
side of the uterus, which suggested disease relapse. In
consequence, the patient received HAG regimen chemotherapy (1 mg
homoharringtonine on days 1–14, 50 mg cytarabine on days 1–14 and
300 µg G-CSF on days 0–14) on November 13, 2013. Donor lymphocytes
infusions were performed on December 9, 2013 (total mononuclear
cells, 3.42×107 cells/kg) and March 9, 2014 (total
mononuclear cells 3.87×107 cells/kg). The patient
declined further treatment and succumbed to disease at home on May
19, 2015.
Discussion
The primary symptoms of the patient in the present
study were bleeding and obstruction of the gastrointestinal tract
caused by the tumor. After initial relief from the symptoms, tumors
in the orbits and left breast were found. The pathological results
of the tumors in the abdomen and left breast confirmed a diagnosis
of myeloid sarcoma. Similar cases have seldom been reported
globally.
Myeloid sarcoma can be found in patients with a wide
spectrum of ages, with cases reported in individuals between 5
months and 89 years old. Despite this, the tumor mainly affects
children and the younger population, with no clear gender
preference (2). Myeloid sarcoma turns
green when exposed to air, so it is also known as chloroma. This
change occurs due to the presence of peroxidase, however, not all
myeloid sarcomas are green and certain tumors are white in color.
The nomenclature of such tumors at present is myeloid sarcoma or
granulocytic sarcoma (1). There are
four clinical presentations of myeloid sarcoma (5): i) Accompanied with acute myeloid
leukemia (AML); ii) could be the portent of AML; iii) could be
associated with myelodysplastic disease transforming to leukemia;
and iv) solitary tumors. However, such localized primitive
granulocytic sarcomas are extremely rare and they could develop to
AML if no treatment is provided (5).
The case in the current study presented as multiple solitary tumors
and the routine bone marrow examination was normal. However, EVI1
gene overexpression occurred in the bone marrow cells, which
suggested a poor prognosis (6).
Therefore, the standard chemotherapy and radiotherapy were followed
by an allogenic peripheral blood stem cell transplantation.
Myeloid sarcoma can be divided into three
pathological categories according to the proportion of immature
granulocytes in different stages of differentiation: The blast cell
type, the partially-differentiated type and the differentiated type
(7). Blast cell-type myeloid sarcoma
mainly consists of myeloblasts, and there are few differentiated
promyelocytes. The partially-differentiated type mainly consists of
myeloblasts and promyelocytes. The differentiated type mainly
consists of promyelocytes and granulocytes of late mature stages,
which mostly include eosinophilic granulocytes. The diagnosis is
often difficult according to routine histology slices, as the tumor
consists of relatively consistent immature cells, particularly in
the following situations: i) When the tumor is solitary and no
leukemia is evident in peripheral blood smears or bone marrow
smears; ii) when the tumor is located in less common areas, such as
the small intestines; iii) when the tumor is not green; and iv)
when the tumor cells are poorly differentiated (4).
MPO has a high sensitivity and specificity for
myeloid cells, and its expression rate in myeloid tumors is as high
as 93%, so it is the most important marker for myeloid sarcomas.
However, Mourad et al (8)
reported that only 66% cases of myeloid sarcomas express MPO.
Traweek et al (9) reported
that MPO is the most useful marker for distinguishing myeloid
sarcomas, but that the expression of MPO varies with degrees of
differentiation. Using MPO accompanied with CD68, CD43 and CD20,
>96% of myeloid sarcomas could be distinguished. Furthermore,
the lysozyme expression rate is 60–93% in myeloid sarcomas, which
could be due to expression in granulocytes and monocytes. However,
the specificity of lysozyme is less than that of MPO, as a lot of
tissues contain lysozyme. Other studies (10) have suggested that lysozyme and its
relative antigen, CD68, are the most sensitive markers for myeloid
cells, which makes them of great significance for the diagnosis of
myeloid sarcomas. A study by Amador-Ortiz et al (11) analyzed 82 cases of myeloid sarcoma and
found that the expression rate of MPO and lysozyme were 95.9 and
95.5%, respectively. CD43 is expressed in almost all myeloid
sarcomas, but it is often used to mark T cells, so it has a high
sensitivity but poor specificity. When tumor cells of unknown
origin express CD43 but are negative for CD3, the possibility of
myeloid sarcoma should be considered. CD68 and CD117 are sensitive
markers of myeloid tumors that are mainly expressed in immature
myeloid tumors and are not expressed in lymphomas. The use of two
such markers is extremely useful in distinguishing between myeloid
sarcoma and lymphomas. CD45 exhibits moderate positive expression
in myeloid sarcomas. CD20 is a characteristic differentiation
antigen of B cells, and it is expressed by B cells from the pre-B
cell period until their differentiation into plasma cells (10). The majority of studies consider that
myeloid sarcomas are CD20-negative, however, Mourad et al
(8) reported that the CD20 expression
rate is 13% in myeloid sarcomas.
The literature has reported varied cytogenetic
abnormities in myeloid sarcomas (12), such as the inversion of chromosome 16
and its associated molecular genetic change resulting in the core
binding factor β/myosin, heavy chain 11, smooth muscle (CBFβ/MYH11)
fusion gene (13). In the present
case, the patient exhibited a normal karyotype of 46,XX and was
negative for the CBFβ/MYH11 fusion gene; however, EVI1 gene
overexpression was recorded.
The differential diagnosis of myeloid sarcoma should
include several diseases (14): i)
Non-Hodgkin's lymphoma (NHL). No eosinophilic granulocytes are
present in NHL tissues, and NHL can express differentiation
antigens of B or T lymphocytes instead of MPO or lysozyme. ii)
Ewing's sarcoma or primitive neuroectodermal tumors. These diseases
are mostly observed in younger individuals. Real or false rosettes
can be found without expression of hematological markers such as
CD45 or MPO. iii) High risk small cells type stromal tumors. The
mesenchymomas usually have clear boundaries to the naked eye and
certain tumors exhibit pseudocapsules. Under the microscope,
fusiform cells and epithelioid differentiated areas can be found,
as well as tumor cells without acidophile cell plasma. The
immunophenotyping is positive for CD117 and discovered on GIST-1,
but negative for MPO and CD45. Myeloid sarcomas have the appearance
of lymphomas and exhibit infiltrative growth without clear
boundaries. Under the microscope, similar diffuse small cells
without fusiform areas are present, with almost no interstitial
elements. Occasionally areas of fibrosis can be found. Tumor cells
with acidophile cell plasma may be found, and immunophenotyping
reveals positive expression for CD117, MPO, B-cell lymphoma 2 and
CD43. iv) Undifferentiated small cell lung cancer. The cancer cells
are diffusely distribute, but with a tendency for nest forming.
Mitotic figures can be easily observed. EMA and PCK are positively
expressed, but the markers for myeloid tissues are negative. In
conclusion, the correct diagnosis of myeloid sarcoma could be made
with carefully observation of hematoxylin-eosin staining slides,
observation of tumor cells differentiating to granulocytes in
mature stages, and following integration of these findings with
immunophenotypical staining (15).
The prognosis of patients with myeloid sarcoma is
extremely poor and the majority succumb to the disease within a
short time. Few patients experience long complete remission periods
after effective treatments. Untreated primary myeloid sarcoma
ultimately transforms to AML usually within 10 months of the
diagnosis of myeloid sarcoma. However, in rare instances, cases
have been reported in which transformation to leukemia has not
occurred in a follow-up time of >16 years (16). Yamauchi and Yasuda (17) reported that the median time for
myeloid sarcoma transformation to AML is 10–12 months.
Previously, treatments for primary myeloid sarcoma
have included surgical resection, local radiotherapy and systemic
chemotherapy. However, surgical resection and local radiotherapy
cannot delay the transformation from myeloid sarcoma to AML or
improve the prognosis (18). This
indicates that primary myeloid sarcoma is a type of systemic
disease that consequently requires systemic treatment. Systemic
chemotherapy should perform for all solitary myeloid sarcomas, even
if surgical resections have been performed. The present hypothesis
is that the application of anti-leukemia chemotherapy soon after
surgery is useful for controlling the develop of the disease and
improving the prognosis (19). The
preferred regimen uses anthracyclines combined with cytarabine
(20). Allogeneic hematopoietic stem
cell transplantation could also be an effective treatment for
myeloid sarcoma (21,22). Yagi et al (23) reported the successful treatment of a
case of myeloid sarcoma using allogeneic hematopoietic stem cell
transplantation following systemic chemotherapy and additional
radiotherapy.
In conclusion, myeloid sarcoma is a malignant
neoplasm of myeloid origin that could develop in any area of the
body. Myeloid sarcoma derived from the gastrointestinal tract is
relatively rare and tends to be misdiagnosed. Intensive systemic
chemotherapy and allogeneic hematopoietic stem cell transplantation
should be performed in addition to surgical resection and
radiotherapy. The prognosis of myeloid sarcoma remains poor, and
further randomized controlled trials are required to improve
clinical practice
References
|
1.
|
Vardiman JW: The World Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pantanowitz L and Thompson L: Myeloid
sarcoma. Ear Nose Throat J. 84:470–471. 2005.PubMed/NCBI
|
|
3.
|
Pileri SA, Ascani S, Cox MC, Campidelli C,
Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero
D, et al: Myeloid sarcoma: Clinico-pathologic, phenotypic and
cytogenetic analysis of 92 adult patients. Leukemia. 21:340–350.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Narayan P, Murthy V, Su M, Woel R,
Grossman IR and Chamberlain RS: Primary myeloid sarcoma
masquerading as an obstructing duodenal carcinoma. Case Rep
Hematol. 2012:4904382012.PubMed/NCBI
|
|
5.
|
Shimizu H, Saitoh T, Hatsumi N, Takada S,
Yokohama A, Handa H, Jimbo T, Sakura T, Tsukamoto N, Murakami H, et
al: Clinical significance of granulocytic sarcoma in adult patients
with acute myeloid leukemia. Cancer Sci. 103:1513–1517. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Haferlach C, Bacher U, Grossmann V,
Schindela S, Zenger M, Kohlmann A, Kern W, Haferlach T and
Schnittger S: Three novel cytogenetically cryptic EVI1
rearrangements associated with increased EVI1 expression and poor
prognosis identified in 27 acute myeloid leukemia cases. Genes
Chromosomes Cancer. 51:1079–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li JM, Liu WP, Zhang MH, Wei X, Gu JM, Han
AJ, Wu WQ and Chen XY: Clinicopathologic and immunophenotypic
analysis of myeloid sarcoma. Zhonghua Bing Li Xue Za Zhi.
35:606–611. 2006.(In Chinese). PubMed/NCBI
|
|
8.
|
Mourad W, Kfoury H and Al Husseini H: The
value of CD34, myeloperoxidase and chloroacetate esterase (Leder)
stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med.
21:287–291. 2001.PubMed/NCBI
|
|
9.
|
Traweek ST, Arber DA, Rappaport H and
Brynes RK: Extramedullary myeloid cell tumors. An
immunohistochemical and morphologic study of 28 cases. Am J Surg
Pathol. 17:1011–1019. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Audouin J, Comperat E, Le Tourneau A,
Camilleri-Broët S, Adida C, Molina T and Diebold J: Myeloid
sarcoma: Clinical and morphologic criteria useful for diagnosis.
Int J Surg Pathol. 11:271–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Amador-Ortiz C, Hurley MY, Ghahramani GK,
Frisch S, Klco JM, Lind AC, Nguyen TT, Hassan A, Kreisel FH and
Frater JL: Use of classic and novel immunohistochemical markers in
the diagnosis of cutaneous myeloid sarcoma. J Cutan Pathol.
38:945–953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Xavier SG, Fagundes EM, Hassan R, Bacchi
C, Conchon M, Tabak DG, Spector N and Zalcberg IR: Granulocytic
sarcoma of the small intestine with CBFbeta/MYH11 fusion gene:
Report of an aleukaemic case and review of the literature. Leuk
Res. 27:1063–1066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Skalska-Sadowska J,
Januszkiewicz-Lewandowska D, Derwich K, Pieczonka A, Samborska M
and Wachowiak J: Ph-negative isolated myeloid sarcoma with NPM1
gene mutation in adolescent with Ph-positive chronic myeloid
leukemia in remission after treatment with allogeneic bone marrow
transplantation and imatinib mesylate. Pediatr Blood Cancer.
62:1070–1071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Deeb G, Baer MR, Gaile DP, Sait SN, Barcos
M, Wetzler M, Conroy JM, Nowak NJ, Cowell JK and Cheney RT: Genomic
profiling of myeloid sarcoma by array comparative genomic
hybridization. Genes Chromosomes Cancer. 44:373–383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Menasce LP, Banerjee SS, Beckett E and
Harris M: Extra-medullary myeloid tumour (granulocytic sarcoma) is
often misdiagnosed: A study of 26 cases. Histopathology.
34:391–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Movassaghian M, Brunner AM, Blonquist TM,
Sadrzadeh H, Bhatia A, Perry AM, Attar EC, Amrein PC, Ballen KK,
Neuberg DS and Fathi AT: Presentation and outcomes among patients
with isolated myeloid sarcoma: A surveillance, epidemiology and end
results database analysis. Leuk Lymphoma. 56:1698–1703. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yamauchi K and Yasuda M: Comparison in
treatments of nonleukemic granulocytic sarcoma: Report of two cases
and a review of 72 cases in the literature. Cancer. 94:1739–1746.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
He J, Zhu L, Ye X, Li L, Zhu J, Zhang J,
Xie W, Shi J, Zheng W, Wei G, et al: Clinical characteristics and
prognosis of nonleukemic myeloid sarcoma. Am J Med Sci.
347:434–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chelly I, Mekni A, Kchir N, Karim BH,
Khadija B, Selma B, Slim H, Khaldi M and Zitouna M: Intracerebellar
granulocytic sarcoma. A case report. Pathologica. 97:335–337.
2005.PubMed/NCBI
|
|
20.
|
Tsimberidou AM, Kantarjian HM, Wen S,
Keating MJ, O'Brien S, Brandt M, Pierce S, Freireich EJ, Medeiros
LJ and Estey E: Myeloid sarcoma is associated with superior
event-free survival and overall survival compared with acute
myeloid leukemia. Cancer. 113:1370–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Widhalm G, Dietrich W, Müllauer L,
Streubel B, Rabitsch W, Kotter MR, Knosp E and Roessler K: Myeloid
sarcoma with multiple lesions of the central nervous system in a
patient without leukemia. Case report. J Neurosurg. 105:916–919.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chevallier P, Mohty M, Lioure B, Michel G,
Contentin N, Deconinck E, Bordigoni P, Vernant JP, Hunault M,
Vigouroux S, et al: Allogeneic hematopoietic stem-cell
transplantation for myeloid sarcoma: A retrospective study from the
SFGM-TC. J Clin Oncol. 26:4940–4943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yagi T, Ishikawa J, Takahashi M, Yamashita
Y, Kusakabe S, Yoshinami T, Masaie H, Sugimoto N, Yoshida H and
Imamura F: Successful treatment of duodenal myeloid sarcoma with
allogeneic bone marrow transplantation and additional radiotherapy.
Intern Med. 51:769–772. 2012. View Article : Google Scholar : PubMed/NCBI
|