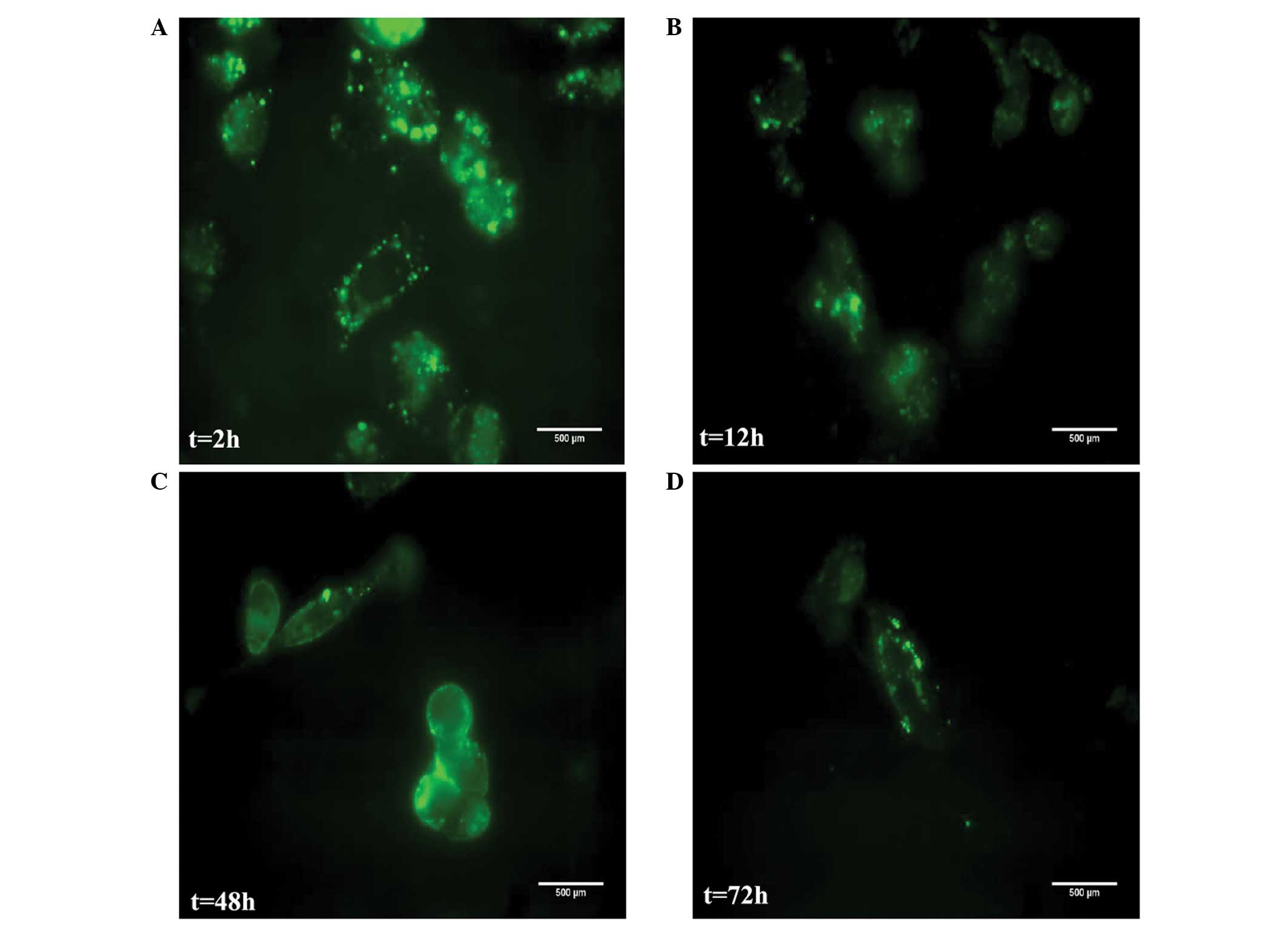
|
1
|
Cooke SP, Boxer GM, Lawrence L, Pedley RB,
Spencer DI, Begent RH and Chester KA: A strategy for antitumor
vascular therapy by targeting the vascular endothelial growth
factor: Receptor complex. Cancer Res. 61:3653–3659. 2001.PubMed/NCBI
|
|
2
|
Miller CR, Buchsbaum DJ, Reynolds PN,
Douglas JT, Gillespie GY, Mayo MS, Raben D and Curiel DT:
Differential susceptibility of primary and established human glioma
cells to adenovirus infection: Targeting via the epidermal growth
factor receptor achieves fiber receptor-independent gene transfer.
Cancer Res. 58:5738–5748. 1998.PubMed/NCBI
|
|
3
|
Baselga J, Norton L, Albanell J, Kim YM
and Mendelsohn J: Recombinant humanized anti-HER2 antibody
(Herceptin) enhances the antitumor activity of paclitaxel and
doxorubicin against HER2 overexpressing human breast cancer
xenografts. Cancer Res. 58:2825–2831. 1998.PubMed/NCBI
|
|
4
|
Ruoslahti E and Pierschbacher MD: New
perspectives in cell adhesion: RGD and integrins. Science.
238:491–497. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elliott G and O'Hare P: Intercellular
trafficking and protein delivery by a herpesvirus structural
protein. Cell. 88:223–233. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muthumani K, Lambert VM, Shanmugam M,
Thieu KP, Choo AY, Chung JC, Satishchandran A, Kim JJ, Weiner DB
and Ugen KE: Anti-tumor activity mediated by protein and peptide
transduction of HIV viral protein R (Vpr). Cancer Biol Ther.
8:180–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CS, Kong B, Ma DX, Wang WX, Mq W and
Kao E: VP22 enhanced intercellular trafficking of HSV thymidine
kinase promoted an effective cell killing effect at lower
concentration of ganciclovir. Chin J Cancer Biother. 8:93–97.
2001.
|
|
10
|
Albarran B, To R and Stayton PS: A
TAT-streptavidin fusion protein directs uptake of biotinylated
cargo into mammalian cells. Protein Eng Des Sel. 18:147–152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong F, Xiao S, Peng F, Zheng H, Yu M,
Ruan Y, Li W, Shang Y, Zhao C, Zhou W, et al: Herpes simplex virus
VP22 enhances adenovirus-mediated microdystrophin gene transfer to
skeletal muscles in dystrophin-deficient (mdx) mice. Hum Gene Ther.
18:490–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ivanenkov VV, Felici F and Menon AG:
Targeted delivery of multivalent phage display vectors into
mammalian cells. Biochim Biophys Acta. 1448:463–472. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwarze SR, Hruska KA and Dowdy SF:
Protein transduction: Unrestricted delivery into all cells? Trends
Cell Biol. 10:290–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho A, Schwarze SR, Mermelstein SJ, Waksman
G and Dowdy SF: Synthetic protein transduction domains: Enhanced
transduction potential in vitro and in vivo. Cancer Res.
61:474–477. 2001.PubMed/NCBI
|
|
15
|
Rajotte D and Ruoslahti E: Membrane
dipetidase is the receptor for a lung-targeting peptide dentified
by in vivo phage display. J Biol Chem. 274:11593–11598. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dente L, Vetriani C, Zucconi A, Pelicci G,
Lanfrancone L, Pelicci PG and Cesareni G: Modified phage peptide
libraries as a tool to study specificity of phosphorylation and
recognition of tyrosine containing peptide. J Mol Biol.
269:694–703. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Guo XR, Gong HX, Ni YH, Fei L, Pan
XQ, Guo M and Chen RH: A resistin binding peptide selected by phage
display inhibits 3T3-L1 preadipocyte differentiation. Chin Med J
(Engl). 119:496–503. 2006.PubMed/NCBI
|
|
18
|
Pires D, Bemquerer M and Nascimento C:
Some mechanistic aspects on Fmoc solid phase peptide synthesis. Int
J Pept Res Ther. 20:53–69. 2014. View Article : Google Scholar
|
|
19
|
West MA, Bretscher MS and Watts C:
Distinct endocytotic pathways in epidermal growth factor-stimulated
human carcinoma A431 cells. J Cell Biol. 109:2731–2739. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furuchi T and Anderson RG: Cholesterol
depletion of caveolae causes hyperactivation of extracellular
signal-related kinase (ERK). J Biol Chem. 273:21099–21104. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subtil A, Hémar A and Dautry-Varsat A:
Rapid endocytosis of interleukin 2 receptors when clathrin-coated
pit endocytosis is inhibited. J Cell Sci. 107:3461–3468.
1994.PubMed/NCBI
|
|
22
|
Garcia-Lora A, Algarra I and Garrido F:
MHC class I antigens, immune surveillance and tumor immune escape.
J Cell Physiol. 195:346–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adessi C, Miege C, Albrieux C and
Rabilloud T: Two-dimensional electrophoresis of membrane proteins:
A current challenge for immobilized pH gradients. Electrophoresis.
18:127–135. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huebener N, Lange B, Lemmel C, Rammensee
HG, Strandsby A, Wenkel J, Jikai J, Zeng Y, Gaedicke G and Lode HN:
Vaccination with minigenes encoding for novel ‘self’ antigens are
effective in DNA-vaccination against neuroblastoma. Cancer Lett.
197:211–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dean-Colomb W and Esteva FJ: Her2-positive
breast cancer: Herceptin and beyond. Eur J Cancer. 44:2806–2812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davoli A, Hocevar BA and Brown TL:
Progression and treatment of HER2-positive breast cancer. Cancer
Chemother Pharmacol. 65:611–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fogelman DR, Kopetz S and Eng C: Emerging
drugs for colorectal cancer. Expert Opin Emerg Drugs. 13:629–642.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim R and Toge T: Changes in therapy for
solid tumors: Potential for overcoming drug resistance in vivo with
molecular targeting agents. Surg Today. 34:293–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ran JT, Zhou YN, Tang CW, Lu JR, Wu J, Lu
H and Yang GD: Celecoxib induces apoptosis and inhibites
angiogenesis in gastric cancer. Zhonghua Zhong Liu Za Zhi.
30:448–451. 2008.(In Chinese). PubMed/NCBI
|
|
32
|
Ortega J, Vigil CE and Chodkiewicz C:
Current progress in targeted therapy for colorectal cancer. Cancer
Control. 17:7–15. 2010.PubMed/NCBI
|
|
33
|
Mailliez A, Baldini C, Van JT, Servent V,
Mallet Y and Bonneterre J: Nasal septum perforation: A side effect
of bevacizumab chemotherapy in breast cancer patients. Br J Cancer.
103:772–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeda A, Loveman E, Harris P, Hartwell D
and Welch K: Time to full publication of studies of anti-cancer
medicines for breast cancer and the potential for publication bias:
A short systematic review. Health Technol Assess. 12:1–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown KC: Peptidic tumor targeting agents:
The road from phage display peptide selections to clinical
applications. Curr Pharm Des. 16:1040–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laakkonen P and Vuorinen K: Homing
peptides as targeted delivery vehicles. Integr Biol (Camb).
2:326–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller WH, Alberts DP, Bhatnagar PK,
Bondinell WE, Callahan JF, Calvo RR, Cousins RD, Erhard KF,
Heerding DA, Keenan RM, et al: Discovery of orally active
nonpeptide vitronectin receptor antagonists based on a
2-benzazepine gly-Asp mimetic. J Med Chem. 43:22–26. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Wang JC, Sun QS, Luo CL and Zhang
Q: RGD-based strategies for improving antitumor activity of
paclitaxel-loaded liposomes in nude mice xenografted with human
ovarian cancer. J Drug Target. 17:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong J, Liu WQ, Jiang AM, Zhang KJ and
Chen MQ: A novel peptide, selected from phage display library of
random peptides, can efficiently target into human breast cancer
cell. Chinese Science Bulletin. 53:860–867. 2008.
|
|
40
|
Zitzmann S, Krämer S, Mier W, Hebling U,
Altmann A, Rother A, Berndorff D, Eisenhut M and Haberkorn U:
Identification and evaluation of a new tumor cell-binding peptide,
FROP-1. J Nucl Med. 48:965–972. 2007. View Article : Google Scholar : PubMed/NCBI
|