Introduction
The overall incidence of cancer is continually
increasing, with an average annual incidence rate of 3–5% (1). Melanoma is a human malignancy
characterized by invasion and metastasis, in which the cells are
malignantly transformed from melanocytes. As the melanoma
demonstrates rapid progression, the prognosis is poor. There is no
effective treatment for melanoma at present, with no progress made
over the last 13 years (2,3). The angiopoietin
(Ang)-endothelial-specific receptor tyrosine kinase 2 (Tie2) system
is widely expressed in human tumor vasculature remodeling, but no
notable expression occurs in normal tissues, which results in the
Ang-Tie2 system being an attractive potential target for
anti-angiogenic cancer therapy (4).
Ang is expressed at a high level in malignant melanoma cells
compared with normal tissues (5).
Thus, it is possible to inhibit angiogenesis by blocking Ang
expression and to inhibit the growth of malignant melanoma,
therefore achieving a therapy for malignant melanoma (6).
Preliminary findings indicated that RNA interference
(RNAi) technology may silence Ang2 gene expression, thereby
inhibiting malignant melanoma xenograft angiogenesis and tumor
growth (7–11). However, in the preliminary studies
(8,10), a local intratumoral injection was
used, which is not suitable for routine clinical use, but the
intravenous injection of lentiviral vectors has safety and efficacy
issues that affected the feasibility and significance of its use in
additional studies. Based on the large specific surface area of
nanoscale genic carriers compared with vector, target cell surface
ligand or antibody can be coupled on the surface of nanomaterials.
Certain nanomaterials, including magnetic nanomaterials, such as
magnetic metal nanowire, magnetic nanofilms and nanocrystalline
soft magnetic materials, demonstrated magnetic compliance, and may
be directed to move in the magnetic field to obtain the
characteristics of active targeting. Active targeting vectors
demonstrate markedly improved specific gene delivery and enhance
the uptake of the target gene to the target cells.
The present study was based on previous studies
(7–11)
and combined nano-biotechnology with RNAi technology, with the
preparation of Ang2-small interfering (si)RNA magnetic chitosan
nanoparticles, which were used to transfect human malignant
melanoma cells. The measurement of the efficiency of the inhibition
of Ang2 gene expression provided a hypothetical basis and
experimental evidence for the safety of studying Ang2 in animals
in vivo, as well as inhibition experiments of tumor growth
in nude mice.
Materials and methods
Primer synthesis
Primers were synthesized by Takara (Dalian, China),
as follows: Ang2 upstream, 5′-GGGCATAATTGTGCTTGACTGG-3′ and
downstream, 5′-ATGGTCTTTAGAATTGGGTCACTGG-3′;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) upstream,
5′-GCACCGTCAAGGCTGAGAAC-3′ and downstream,
5′-TGGTGAAGACGCCAGTGGA-3′. The Ang2 fragment was 183 bp long and
the GAPDH fragment was 138 bp long.
Preparation of magnetic chitosan
nanoparticles
In total, 0.15 g Fe3O4
magnetic nanoparticles (Nanjing Emperor Nano Materials Co., Ltd.,
Nanjing, China) were dispersed in 20 ml 1.5% chitosan solution
(relative molecular weight, 1.38×106; degree of
deacetylation, 90%; Zhejiang Aoxing Biotechnology Co., Ltd.,
Kanmen, Zhejiang, China) subsequent to ultrasonic dispersal
(JY98-IIIDN; Shanghai Shengke Instrument & Equipment Co., Ltd.,
Shanghai, China) and stirring. Subsequently, 80 ml liquid paraffin
(Nujol; Hangzhou Yongxin Hardware Co., Ltd., Hangzhou, China) and
petroleum ether mixture (Jining Huakai Resin Co., Ltd, Jining,
China) containing 2 ml Span-80 (Jiangsu Haian Petrochemical Plant,
Nantong, China) was added to 20 ml 1.5% chitosan solution. The
volume ratio of Nujol (Hangzhou Yongxing Hardware & Electrical
Appliance Co. Ltd., Hangzhou, China) to petroleum ether was 7:5. An
emulsion was obtained by stirring at 40°C for 30 min using a
MYP2011-100 electric mixer (Shanghai Mei Yingpu Instrument
Manufacturing Co., Ltd., Shanghai, China). Then, l ml 25%
glutaraldehyde solution (Hubei Xinjing New Material Co., Ltd.,
Wuhan, China) was diluted to 10 ml, and the glutaraldehyde solution
was slowly added dropwise prior to incubation in a water bath at
40°C for 30 min. The pH of the glutaraldehyde solution was adjusted
with l mol/l NaOH solution (Fuzhou Xinrong Chemical Co., Ltd.,
Fuzhou, China) to pH 9.0. The solution was then heated to 60°C and
reacted at a constant temperature for 1 h. Finally, the solution
was thoroughly washed with ether (99.7%), acetone (99.7%),
anhydrous ethanol (99%) and distilled water (Fuzhou Xinrong
Chemical Co., Ltd.) in succession to obtain magnetic chitosan
nanoparticles.
Transmission electron microscopy (TEM)
of magnetic nanoparticles of chitosan
A 1 mg sample of magnetic chitosan nanoparticles
(Wujiang Hongli Chemical Co., Ltd., Suzhou, China) was added to a
10 ml glass bottle with 2 ml dispersant (0.5% sodium
hexametaphosphate; Wujiang Hongli Chemical Co., Ltd.). The solution
was ultrasonically dispersed using a JY98-IIIDN ultrasonic cell
disruptor (Shanghai Shengke Instrument & Equipment Co., Ltd.)
for 6 sec. The sample was placed on a copper grid within 2 min. The
size and morphology of the superparamagnetic particles chitosan
nanoparticles was directly observed by TEM using the JEM-2000EX
transmission electron microscope manufactured by JEOL USA, Inc.
(Peabody, MA, USA).
Sediment assays
For the sediment assays, 6 groups of 10 mg chitosan
magnetic nanoparticles were weighed, and each was dissolved in 20
ml phosphate-buffered saline (PBS; pH 6.5; Yocon Biotechnology Co.,
Ltd., Beijing, China). Ultrasonic oscillation (200 W; 3 min) was
then performed. Subsequently, 3 groups of chitosan magnetic
nanoparticles (10 mg) were treated by a magnetic field acting on
the base of the container for 5, 10 or 20 min. The remaining 3
groups were incubated at room temperature for 5, 10 or 20 min.
Images were captured, and the size and morphology of the chitosan
magnetic nanoparticles observed using a JEM-2000EX transmission
electron microscope manufactured by JEOL USA, Inc.
Ang2-siRNA vector combined with
chitosan magnetic nanoparticles
In total, 1 mg chitosan magnetic nanoparticles were
weighed and added to 1 ml PBS (pH 7.4). Ultrasonic oscillation (200
W; 3 min) was then performed. Subsequently, 2 ml polylysine
(Zhengzhou Bainafo Bioengineering Co., Ltd., Zhengzhou, China),
which was diluted with PBS to a concentration of 0.1 mg/ml, was
added to the nanoparticles and mixed thoroughly, prior to
incubation at room temperature for 10 min. The Ang2-siRNA plasmid
contained two RNAi sequences, as follows: S1,
5′-ACCCCACTGTTGCTAAAGATTCAAGAGATCTTTAGCAACAGTGGGGTTTTTT-3′; and S2,
5′-GCCACGGTGAATAATTCAGTTCTCGAGAACTGAATTATTCACCGTGGCTTTTT-3′ (Wuhan
Xima Biological Technology Co., Ltd., Wuhan, China). This plasmid
and the polylysine-modified magnetic nanoparticles of chitosan were
mixed at mass ratios of 1:1, 1:10, 1:100 and 1:1,000, and were then
incubated at room temperature for 1 h.
Transfection
The human malignant melanoma A-375 cells were
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). Cells (2–4×105)
were seeded with Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) in 6-well plates at 37°C and 5%
CO2 until the logarithmic growth phase was reached, and
2 h prior to transfection, serum-free medium was used instead. The
cells were transfected with 4 µg DNA per well, using serum-free
DMEM (HyClone; GE Healthcare Life Sciences) as the cell medium. The
Ang2-siRNA plasmid and magnetic chitosan nanoparticles were
combined into Ang2-siRNA magnetic chitosan nanoparticles at a mass
ratio of 1:1, 1:10, 1:100 or 1:1,000, and added to three 6-well
cell culture plates with diameter of 35 mm and cultured at 37°C in
a 5% CO2 incubator (YCP-200; Changsha Hua Xi Electronics
Technetronic Co., Ltd., Changsha, China) for 12 h. The cells were
aspirated and the original medium was then discarded. DMEM
containing 10% fetal bovine serum was added to the cells, and the
cells were cultured for 48 h. Subsequent to culture, red
fluorescent protein was observed under an Olympus CKX41
fluorescence microscope (On Haipu He Optoelectronics Technology
Co., Ltd., Shanghai, China) to assess protein
expression/transfection, and images were captured and recorded. The
Ang2-siRNA magnetic chitosan nanoparticle group with the highest
transfection efficiency was classified as the experimental group.
The group transfected with empty vector magnetic chitosan
nanoparticles with the same mass was classified as the control
group.
For the negative control, empty vector magnetic
chitosan nanoparticles with the same mass (mass ratio, 1:1, 1:10,
1:100 or 1:1,000) were added to three 6-well cell culture plates
with a diameter of 35 mm and cultured in a 5% CO2
incubator at 37°C for 12 h. Following incubation, the cells were
aspirated and the original culture medium was discarded. DMEM
containing 10% fetal bovine serum, and cultured in a 5%
CO2 incubator at 37°C for 48 h. Subsequent to
incubation, red fluorescent was observed under a fluorescence
microscope (Olympus CKX41; On Haipu He Optoelectronics Technology
Co., Ltd.) to assess protein expression/transfection, and images
were captured and recorded. The cells were digested with trypsin
(Shanghai Biological Technology Co., Ltd. Shanghai, China), and an
inverted fluorescence microscope was used to count the red
fluorescence cells under ordinary and mercury light sources. The
cell count results obtained using a hemocytometer (XB-K-25;
Shanghai Daming Laboratory Instrument Co., Ltd, Shanghai, China)
were used to calculate the transfection efficiency.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In total, 106 cells were extracted from
the experimental and control groups and total RNA was extracted
using the TRIzol method. Reverse transcription was performed to
obtain complementary (c)DNA using PrimeScript RT Reagent kit
(Takara), according to the manufacturer's protocol. Based on the
corresponding quantification cycle (Cq) value generated by the SYBR
Premix Ex Taq kit (Takara), the PCR system was performed as
follows: 45 cycles of 95°C for 5 sec, 60°C for 34 sec and 95°C for
15 sec. For the Cq value, the fluorescent signal in each reaction
tube should reach the set threshold number of cycles. The formula
∆∆Ct = ∆Ct1 - ∆Ct2 was used to calculate Ang2 inhibition, and the
formula N1 / N2 = 2−∆∆Ct was used to calculate the
expression efficiency of RNAi for inhibiting Ang2, where N1
indicates Ang2 expression of the experimental group and N2
indicates Ang2 expression of the control group.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Sciences, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). Data was compared using the independent
two-sample t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Visual observation of chitosan
magnetic nanoparticles
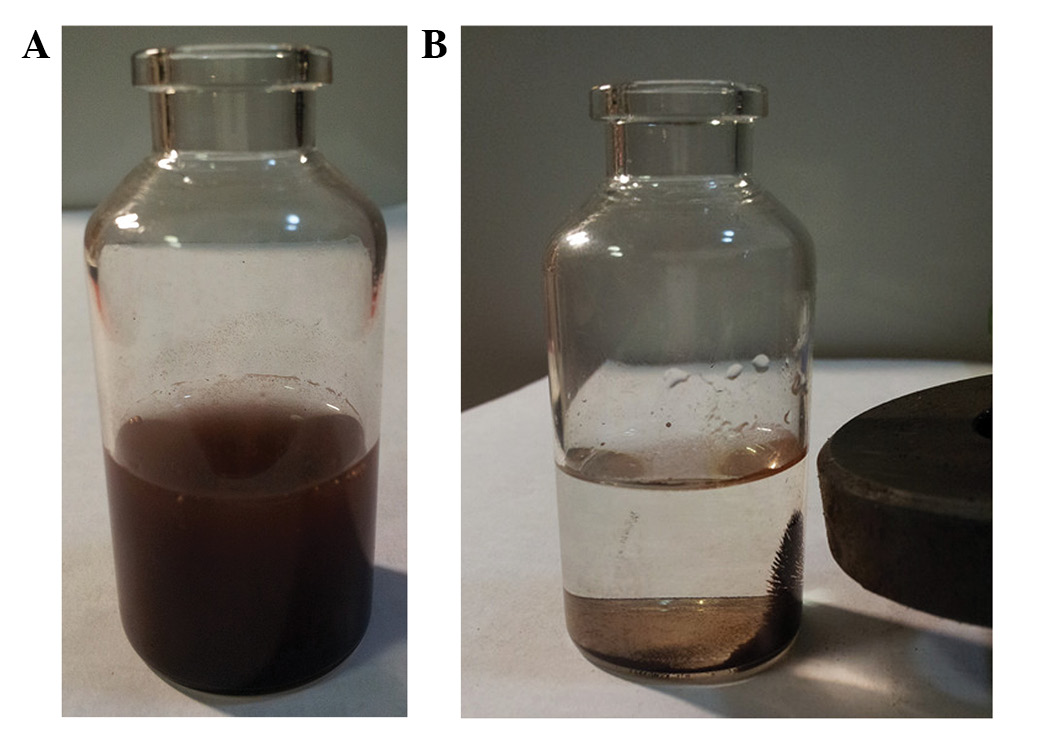
Chitosan magnetic nanoparticles may be well
dispersed in distilled water to yield a dark brown suspension
(Fig. 1A) with a good magnetic
response (Fig. 1B).
TEM of magnetic chitosan
nanoparticles
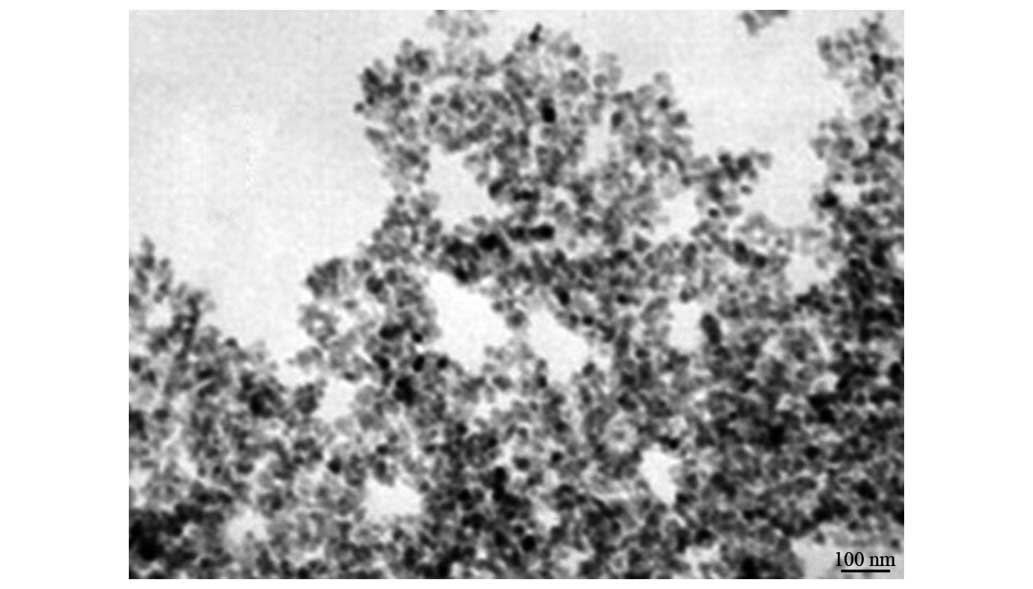
Under TEM, the majority of magnetic chitosan
nanoparticles were oval and round, with a small number
demonstrating an irregular shape. The boundaries of the
nanoparticles were clear. The central portion of the particles was
a darker color and the surroundings were shallow, suggesting the
existence of a shell core structure (Fig.
2).
Sediment assays
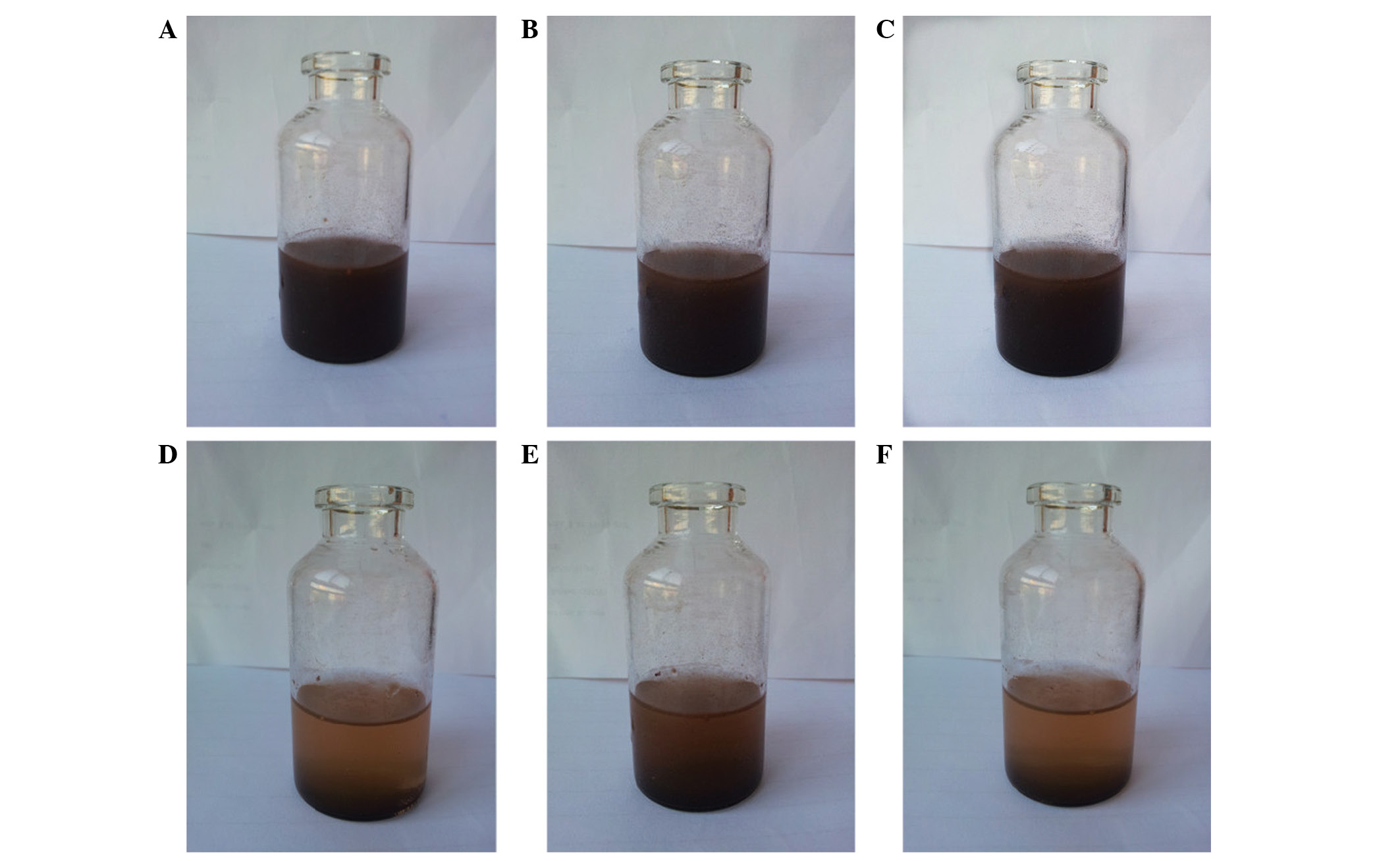
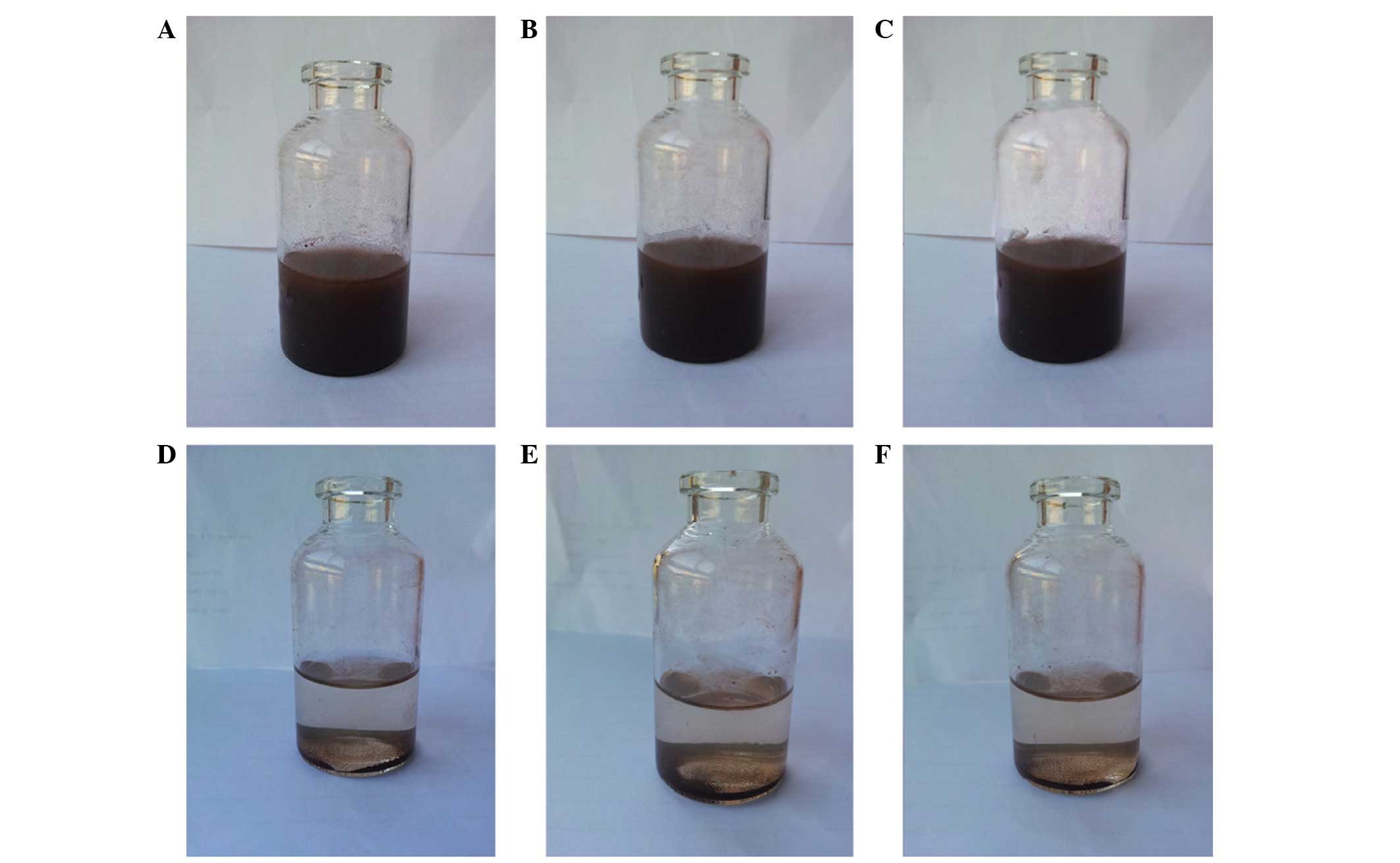
Subsequent to agitation, the magnetic chitosan
nanoparticles were well dispersed in distilled water (Figs. 3A-C and 4A-C), but the nanoparticles may precipitate
to the bottom of the container due to gravity. The supernatant
obtained by standing at room temperature for 10 min (Fig. 3E) was clearer compared with the
supernatant obtained by standing for 5 min (Fig. 3D), while the supernatant obtained
subsequent to 20 min (Fig. 3F) was
similar to that obtained at 10 min. The supernatant solutions
subsequent to 5 min (Fig. 4D), 10 min
(Fig. 4E) and 20 min (Fig. 4F) in the magnetic field were similar.
Due to the good response and superparamagnetic properties, the
magnetic nanoparticles may reach the location of the tumor in the
guidance of the magnetic field, which created the conditions for
magnetic targeting drug experiments.
Determination of the appropriate mass
ratio
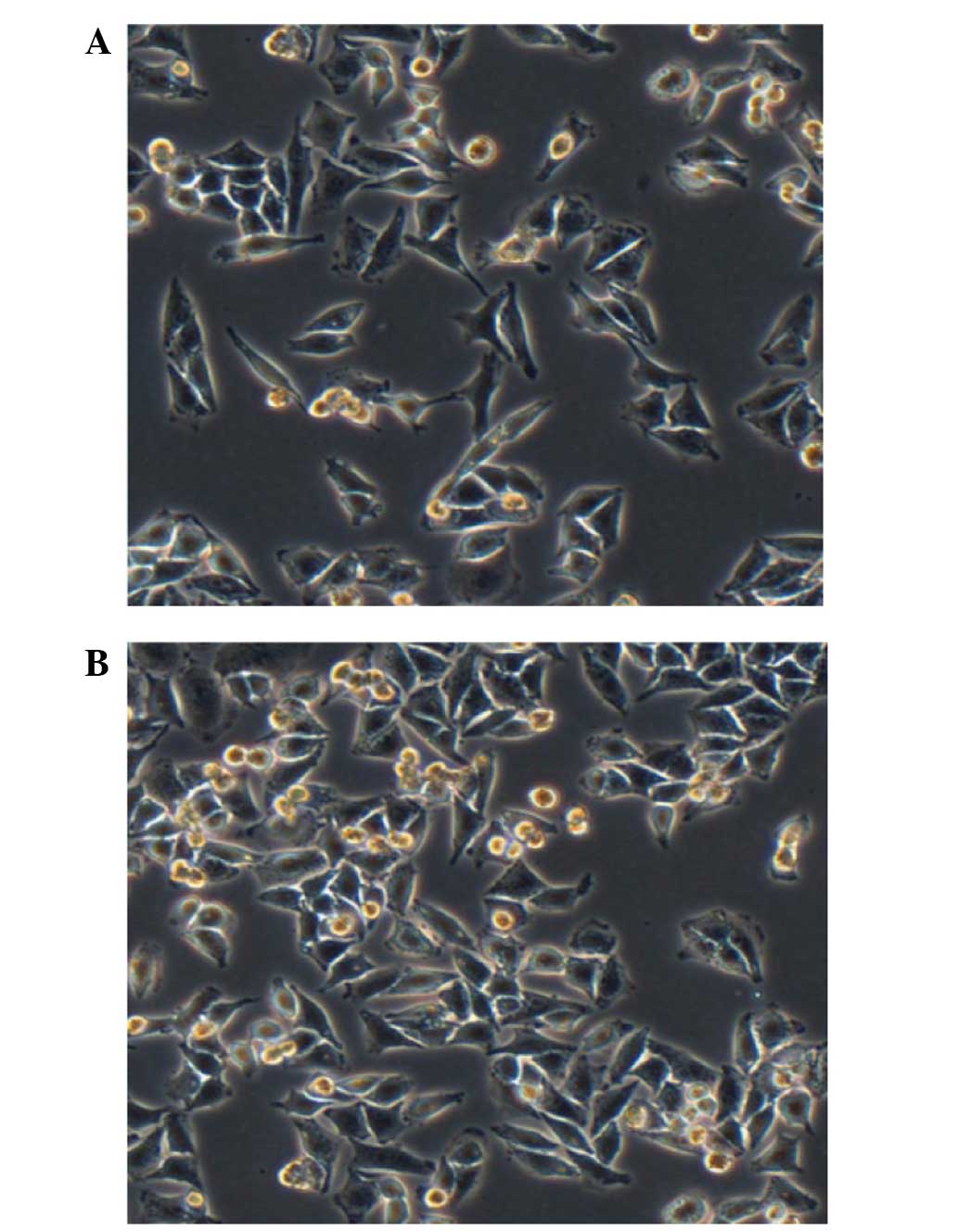
Malignant melanoma is a highly malignant tumor with
fast growth. The cells are loose, polygonal and adherent under
normal conditions (Fig. 5A). Prior to
the transfection assays, the malignant melanoma cells were
cultured, and transfection was conducted when the malignant
melanoma cells grew to ~75% confluency (Fig. 5B).
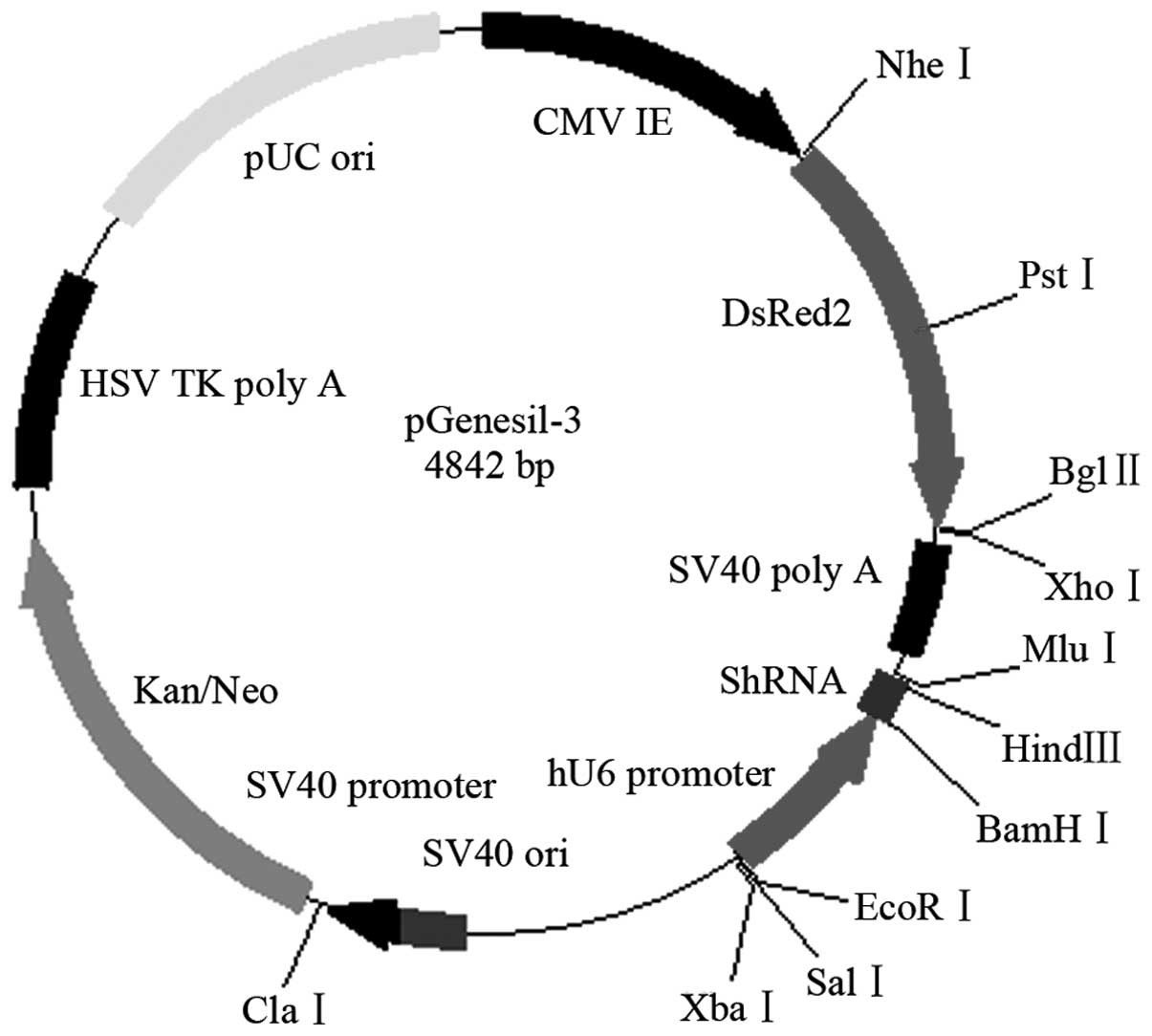
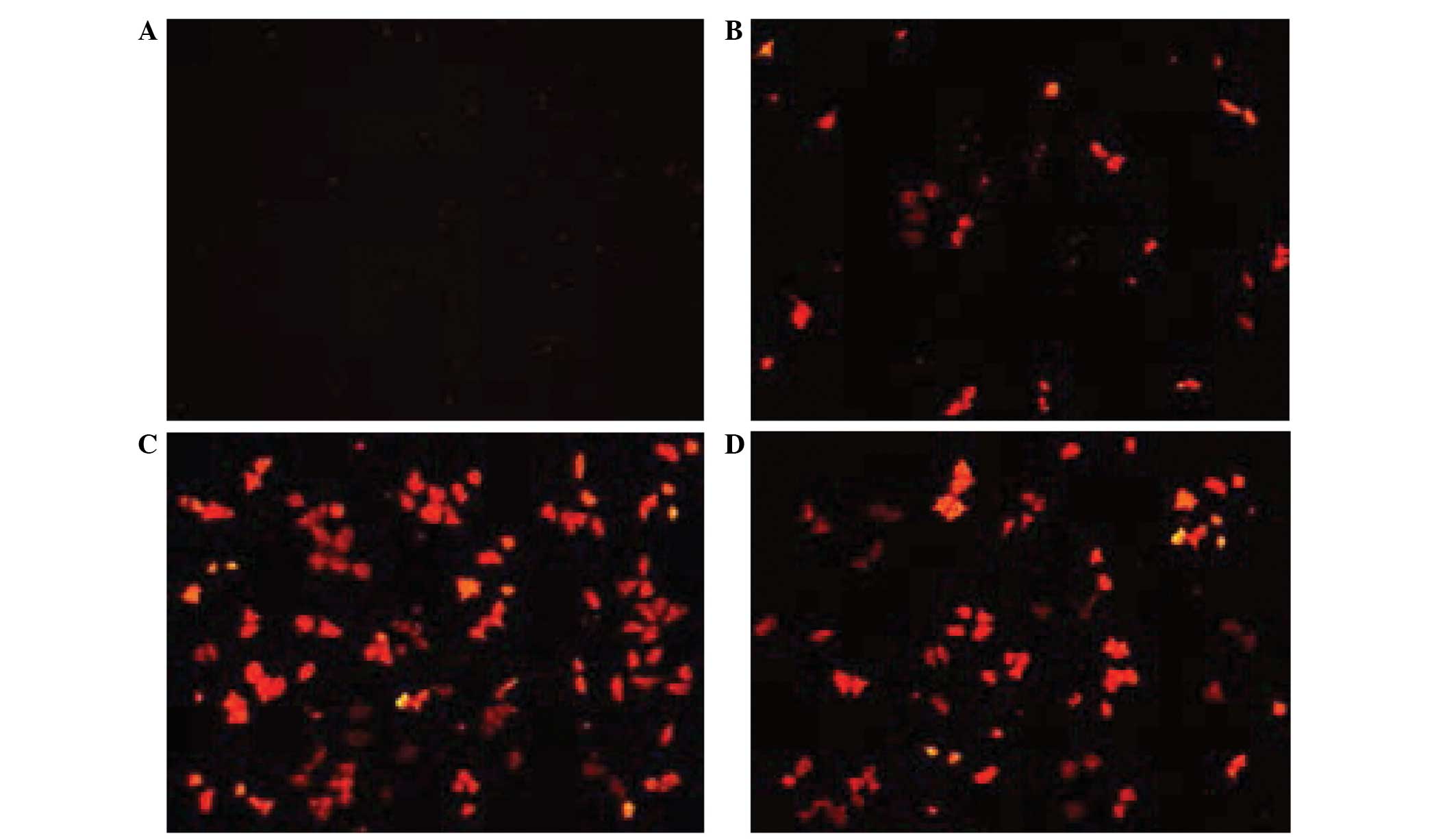
The Ang2-siRNA vector (Fig. 6) and chitosan magnetic nanoparticles
at a mass ratio of 1:1, 1:10, 1:100 or 1:1,000 were transfected
into human malignant melanoma cells, and the cells were then
observed under a fluorescent microscope (Olympus CKX41; On Haipu He
Optoelectronics Technology Co., Ltd.) and images were captured
(Fig. 7A–D). When the mass ratio was
1:100, the emitted red fluorescence was the strongest (Fig. 7C). The cells in each group were
digested into single cell suspensions and counted using a
hemocytometer (Table I), and the
appropriate mass ratio was determined to be 1:100 for subsequent
experiments.
 | Table I.Efficiency of angiopoietin-2-small
interfering RNA plasmid chitosan magnetic nanoparticle transfection
into human malignant melanoma cells. |
Table I.
Efficiency of angiopoietin-2-small
interfering RNA plasmid chitosan magnetic nanoparticle transfection
into human malignant melanoma cells.
|
| Total number of red
fluorescent cells, n |
|
|---|
|
|
|
|
|---|
| Mass ratio | Under mercury
light | Under ordinary
light | Transfection
efficiency, % |
|---|
| 1:1 | 0 | 118 | 0.00 |
| 1:10 | 10 | 107 | 9.35 |
| 1:100 | 63 | 103 | 61.17 |
| 1:1,000 | 35 | 84 | 41.67 |
Efficiency of RNAi inhibition of Ang2
expression
Total RNA was extracted from malignant melanoma
cells in the experimental group and the control group. The RNA was
reverse transcribed into cDNA, and RT-qPCR was performed using an
equal amount of GAPDH cDNA to obtain the Cq value. The differences
in the Cq value between the groups ranged between 0.01 and 0.1
(data not shown). First, RNA purity of the two samples was
determined to be good. Second, the cDNA template of the two samples
participating the reactions was extremely similar. An equal amount
of the cDNA template was used for the two samples in the Ang2 gene
RT-qPCR reactions to obtain the corresponding Cq values, which were
calculated as follows: ∆Ct = Ang2 Ct value - GAPDH Ct value
(Table II). According to the formula
N1 / N2 = 2−∆∆Ct, in which ∆∆Ct = ∆Ct experimental group
- ∆Ct control group, the efficiency of RNAi inhibition of the
expression of the Ang2 gene was calculated, and the inhibition rate
ratio between the experimental group and the control group was
found to be 59.56% (P<0.05). The Ang2 plasmid magnetic chitosan
nanoparticles should be selected for animal tumor suppression
experiments in future studies.
 | Table II.Efficiency of the inhibition of
angiopoietin-2 expression by RNA interference. |
Table II.
Efficiency of the inhibition of
angiopoietin-2 expression by RNA interference.
| Group (n=3) | Average ∆Cq
values | ∆∆Cq | Expression efficiency
(%) |
|---|
| Control (Cq) | 9.389+0.223 |
|
|
| Experimental
(Cq) |
10.695+0.329a | 1.306 | 59.56 |
Discussion
With the increased complexity of basic medical
research, gene therapy has become an important component of
biological tumor therapy, which is the current focus of biomedical
research (12,13). One challenge of gene therapy studies
is the choice of a vector system for gene therapy (14).
Gene vectors are usually divided into viral vectors
and non-viral vectors. Viral gene vectors include retroviral and
adenoviral vectors. Although these vectors demonstrate high
transfection efficiency, there is the fundamental issue of toxicity
and immune response; thus, non-viral gene vectors have become an
extremely important aspect of the pharmaceutical carrier gene
(15,16). Chitosan has biodegradable and
biocompatible features (17), in
addition to the characteristics of low toxicity and cationic
polymers; these natural properties of chitosan have caused chitosan
to become one of the most promising non-viral gene vectors. In
non-viral gene vectors, the intake of gene carrier complexes and
gene delivery are also affected by particle sizes (18). Chitosan, as a gene carrier, has
previously been a focus of studies (19). Studies have shown that the chitosan
molecular weight (20) and the degree
of deacetylation (21) have a notable
impact on gene transfection. Chitosan nanoparticles are favored for
cellular uptake, primarily by endocytosis into cells, and the
nanoparticles gradually release nucleic acids by degrading polymer
materials. Chitosan nanoparticle vectors are susceptible to
covalent and targeted cell ligand modification on the surface,
which can result in active targeting of gene transfer. PEG also may
be used for surface modification and extending the half-life of the
carrier in vivo (22).
Therefore, chitosan nanoparticle vectors developed to be gene
carriers demonstrate good prospects in gene therapy.
In the present study, the chitosan magnetic
nanoparticles were used as a transfection vector for Ang2-siRNA
plasmids to induce RNA interference and silence the expression of
the Ang2 gene in human malignant melanoma cells. When the mass
ratio of chitosan magnetic nanoparticles to Ang2-siRNA plasmids
increased between 0.05 and 1.00, the combination rate of the two
demonstrated a rapid increase, while the increase in the binding
rate slowed when the amount of chitosan magnetic nanoparticles was
increased. At a 1:1 mass ratio of chitosan magnetic nanoparticles
and Ang2-siRNA plasmids, the chitosan magnetic nanoparticles bind
the majority of the Ang2-siRNA plasmids. Therefore, 4 mass ratios
of the Ang2-siRNA plasmid to chitosan magnetic nanoparticles were
used in the present study, consisting of 1:1, 1:10, 1:100 and
1:1,000. Following transfection, fluorescence expression of
melanoma cells was counted. When the mass ratio of plasmid and
chitosan magnetic nanoparticles was 1:1, the human malignant
melanoma cells showed no red fluorescence protein expression. At a
mass ratio of plasmids to magnetic nanoparticles of 1:10, 1:100 or
1:1,000, red fluorescent protein was visible. At a mass ratio of
1:100, the emitted red fluorescence was the strongest, as the
largest number of human malignant melanoma cells expressed red
fluorescent protein, therefore, 1:100 was identified as the best
mass ratio and was set as the proportion of plasmids to chitosan
magnetic nanoparticles in the experimental and control groups.
RT-qPCR was used to determine the expression of the Ang2 gene
between the experimental group and the control group, and then the
efficiency of the plasmid interference with Ang2 gene expression
was analyzed by Ang2-siRNA chitosan magnetic nanoparticles. The
results showed that the Ang2-siRNA plasmids and chitosan magnetic
nanoparticles may interfere in human malignant melanoma cells in
vitro with a higher transfection efficiency and inhibit the
expression of the Ang2 gene in malignant melanoma cells.
In summary, the present results revealed that
Ang2-siRNA chitosan magnetic nanoparticles could intervene in human
malignant melanoma cells in vitro at a high transfection
efficiency, and inhibit the expression of the Ang2 gene in
malignant melanoma cells, which may lay the foundation and provide
experimental evidence for additional in vivo targeting of
the angiogenesis and tumor growth of malignant melanoma xenografts
in nude mice.
Acknowledgements
The present study was supported by the Foundation of
National Key Clinical Specialty Discipline Construction Program,
Scientific Research Foundation of National Health and Family
Planning Commission-Joint Research Projects of Fujian Provincial
Health and Education (grant no. WKJ-FJ-03) and Projects of Fujian
Provincial Natural Science Foundation (grant no. 2012J01125).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yakovleva ME, Welinder C, Sugiharaa Y,
Pawlowskid K, Rezelib M, Wieslandera E, Malme J and Marko-Varga G:
Workflow for large-scale analysis of melanoma tissue samples. EuPA
Open Proteomics. 8:78–84. 2015. View Article : Google Scholar
|
|
3
|
Essner R: Surgical treatment of malignant
melanoma. Surg Clin North Am. 83:109–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliner J, Min H, Leal J, Yu D, Rao S, You
E, Tang X, Kim H, Meyer S, Han SJ, et al: Suppression of
angiogenesis and tumor growth by selective inhibition of
angiopoietin-2. Cancer Cell. 6:507–516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Helfrich I, Edler L, Sucker A, Thomas M,
Christian S, Schadendorf D and Augustin HG: Angiopoietin-2 levels
are associated with disease progression in metastatic malignant
melanoma. Clin Cancer Res. 15:1384–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schlaak M, Kreuzberg N, Mauch C and
Kurschat P: Personalized therapy concepts for malignant melanoma.
Internist (Berl). 54:188–193. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boguslawska J and Małecki M: siRNA
preparations in gene therapy of melanoma. Med Wieku Rozwoj.
17:196–201. 2013.PubMed/NCBI
|
|
8
|
Dolinsek T, Markelc B, Sersa G, Coer A,
Stimac M, Lavrencak J, Brozic A, Kranjc S and Cemazar M: Multiple
delivery of siRNA against endoglin into murine mammary
adenocarcinoma prevents angiogenesis and delays tumor growth. PLoS
One. 8:e587232013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu ZL, Wang B, Guo GX, Shan XY, Wang MS,
Zhuang FL, Cai CS, Zhang MF and Zhang YD: Construction of
recombinant lentiviral vector of siRNA for Ang2 and the effect of
the expression of Ang2 gene in malignant melanoma cells. J Med Mol
Biol. 8:494–500. 2011.
|
|
10
|
Wang B, Lin JH, Zhang WQ, Zhang MF, Liu
ZL, Shan XY, Wang MS and Zhuang FL: Ang2-siRNA lentivirus
interfernce on melanoma xenograft in nude mice. J Med Mol Biol.
9:79–83. 2012.
|
|
11
|
Wang B, Liu Z, Zhang M, San X, Zhang Y,
Zhang W and Wang M: Interfering growth of malignant melanoma with
Ang2-siRNA. Mol Biol Rep. 40:1463–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kenjo E, Asai T, Yonenaga N, Ando H, Ishii
T, Hatanaka K, Shimizu K, Urita Y, Dewa T, Nango M, et al: Systemic
delivery of small interfering RNA by use of targeted polycation
liposomes for cancer therapy. Biol Pharm Bull. 36:287–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Todorovic V, Sersa G and Cemazar M: Gene
electrotransfer of siRNAs against CD146 inhibits migration and
invasion of human malignant melanoma cells SK-MEL28. Cancer Gene
Ther. 20:208–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stein S, Scholz S, Schwäble J, Sadat MA,
Modlich U, Schultze-Strasser S, Diaz M, Chen-Wichmann L,
Müller-Kuller U, Brendel C, et al: From bench to bedside:
Preclinical evaluation of a self-inactivating gammaretroviral
vector for the gene therapy of X-linked chronic granulomatous
disease. Hum Gene Ther Clin Dev. 24:86–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonamassa B and Liu D: Nonviral gene
transfer as a tool for studying transcription regulation of
xenobiotic metabolizing enzymes. Adv Drug Deliv Rev. 62:1250–1256.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Rorke S, Keeney M and Pandit A:
Non-viral polyplexes: Scaffold mediated delivery for gene therapy.
Prog Polym Sci. 35:441–458. 2010. View Article : Google Scholar
|
|
17
|
Sionkowska A, Wisniewski M, Skopinska J,
Kennedy CJ and Wess TJ: Molecular interactions incollagen and
chitosan blends. Biomaterials. 25:795–801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pang SW, Park HY, Jang YS, Kim WS and Kim
JH: Effects of charge density and particle size of poly
(styrene/(dimethylamino)ethyl methacrylate) nanoparticle for gene
delivery in 293 cells. Colloids Surf B Biointerfaces. 26:213–222.
2002. View Article : Google Scholar
|
|
19
|
Mansouri S, Lavigne P, Corsi K, Benderdour
M, Beaumont E and Fernandes JC: Chitosan-DNA nanopartieles as
non-viral vectors in gene therapy: Strategies to improve
transfection efficacy. Eur J Pharm Biopharm. 57:1–8. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato T, Ishii T and Okahata Y: In vitro
gene delivery mediated by chitosan. Effect of pH, serum, and
molecular mass of chitosan on the transfection efficiency.
Biomaterials. 22:2075–2080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiang T, Wen J, Lim HW and Leong KW: The
effect of the degree of chitosan deacetylation on the efficiency of
gene transfection. Biomaterials. 25:5293–5301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin
KY, Wang Y, August JT and Leong KW: Chitosan-DNA nanoparticles as
gene carriers: Synthesis, characterization and transfection
efficiency. J Control Release. 70:399–421. 2001. View Article : Google Scholar : PubMed/NCBI
|