Introduction
Diagnosis of thyroid neoplasm mainly depends on
symptoms such as palpation, color Doppler ultrasound, endocrine
hormone, fine needle aspiration (FNA) and pathology. Susceptibility
of color Doppler ultrasound and computed tomography (CT) scan when
diagnosing thyroid neoplasm can be ≤75%, with a specificity of ≤73%
(1). Multiphase FNA can improve the
susceptibility of diagnosing thyroid neoplasm to 88% and
specificity to 79%, but it is difficult to trace (2) and requires high technology. Although
pathology is the ‘gold standard’ for diagnostics, surgery is
required to obtain specimens, delaying diagnosis and preventing
early screening of tumor. Recent findings have shown that as a type
of non-coding single-stranded RNA molecule coded by an endogenous
gene, microRNAs (miRNAs) are closely assoicated with the occurrence
and development of many diseases (3).
According to analysis of the miRNA chip, the expression of
miRNA146, miRNA155, miRNA187, miRNA221, miRNA222, and miRNA21 in
differentiated thyroid carcinoma (DTC) were ~5- to 10-fold higher
than that of normal thyroid tissues (4). miRNA224 and miRNA339 are highly
expressed in thyroid neoplasm, whereas miRNA141 was markedly
decreased in Hashimoto's thyroditis (5) and miRNA154, miRNA376b and miRNA431 were
markedly downregulated ~5-fold in Graves disease (6). Thus, miRNAs may be used as a reliable
marker and applied in the diagnosis and prognostic evaluation of
thyroid diseases (7). Thyroid
carcinoma papillary thyroid carcinoma (PTC) has the highest
incidence of thyroid carcinoma. Previous findings have identified
that miR146b, miR221 and miR222 is expressed in PTC (8). In the present study, the expression
conditions in various types of thyroid were examined using receiver
operating characteristic (ROC) curve to compare whether single
index or a combination of indices improved the susceptibility and
specificity of diagnosis.
Patients and methods
Patient information
A total of 120 patients admitted to Shaanxi
Provincial Tumor Hospital (Shaanxi, China) between August 2014 and
August 2015 were suspected of having thyroid carcinoma, and were
examined using clinical color Doppler ultrasound and CT. There were
75 males and 45 females, with an average age of 46.7±13.5 years.
Exclusion criteria for the study were hyperthyroidism,
hypothyroidism, thyroiditis, Graves disease, thyroid metastatic
carcinoma, women who were in treatment, gestation and lactation and
no access to obtain tissue specimens. Approval for the study was
obtained from the ethics committee of the Shaanxi Provincial Tumor
Hospital. Written informed informed was obtained from patients and
family members. To obtain tissue specimens, a FNA, multiphase
biopsy or surgical resection was used. The expression of miR146b,
miR221 and miR222 was detected by RT-quantitative polymerase chain
reaction (RT-qPCR) method.
Detection methods
The main reagents used in the present study were:
Absolute ethyl alcohol and isopropanol, chloroform (Tianjin Damao
Chemical Reagent Factory, Tianjin, China), DEPC and TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), reverse
transcription cDNA kit and PCR probe (Exiqon, Inc., Woburn, MA,
USA).
The main instruments used included, fluorescent
quantitative PCR (Rotor-Gene 6000; Qiagen, Hilden, Germany),
ultraviolet spectrophotometer (Smartspec Plus 300; Bio-Rad,
Berkeley, CA, USA), hypothermia high-speed centrifuge (centrifuge
2810R; Eppendorf AG, Hamburg, Germany), 96-well reverse
transcription plate (Axygen Scientific, Inc., Union City, CA, USA)
and −80°C deep freezer (Sanyo Electric Co., Ltd., Tokyo,
Japan).
According to the TRIzol reagent instructions, the
one-step method was used to extract all RNAs in tissues. Agarose
gel electrophoresis was used to detect its integrity and measure
concentration and purity of the RNAs under an ultraviolet lamp and
ultraviolet spectrophotometer. Reverse transcription was then
performed. To determine the quantitative PCR reaction, samples were
preserved at −20°C by original LNA™ PCR primer set (miR-146b, −221
and −222) and internal reference U6 reference gene primer mix. cDNA
reaction product (10 µl) was diluted 80-fold, and 1.5 ml of diluted
cDNA (4 µl) was mixed with SYBR-Green master mix (5 µl) and primer
mix (1 µl) in a centrifuge tube at 11,000 × g for 3 min at room
temperature and subsequently transferred to nuclease-free PCR
tubes. The reaction conditions were: denaturation 95°C for 10 min
(polymerase activation), annealing 95°C for 10 sec, and extension
60°C for 1 min, with a decreasing velocity of 1.6°C/sec and 40
cycles. Rotor-Gene 6000 Series Software 1.7 (Qiagen, Valencia, CA,
USA) was used to process the data and obtain the Ct value. The
relative quantity of sample target genes were calculated by
2−ΔΔCt with U6 as an internal reference and
H2O as the negative control.
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for data input and analysis. Measurement data
were shown as the mean ± standard deviation and comparisons between
groups were analyzed by single-factor ANOVA. Independent samples
t-test was used to examine comparisons between two groups.
Diagnostic accuracy was analyzed by area under curve (AUC) of ROC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparisons of miRNA expression levels
between groups
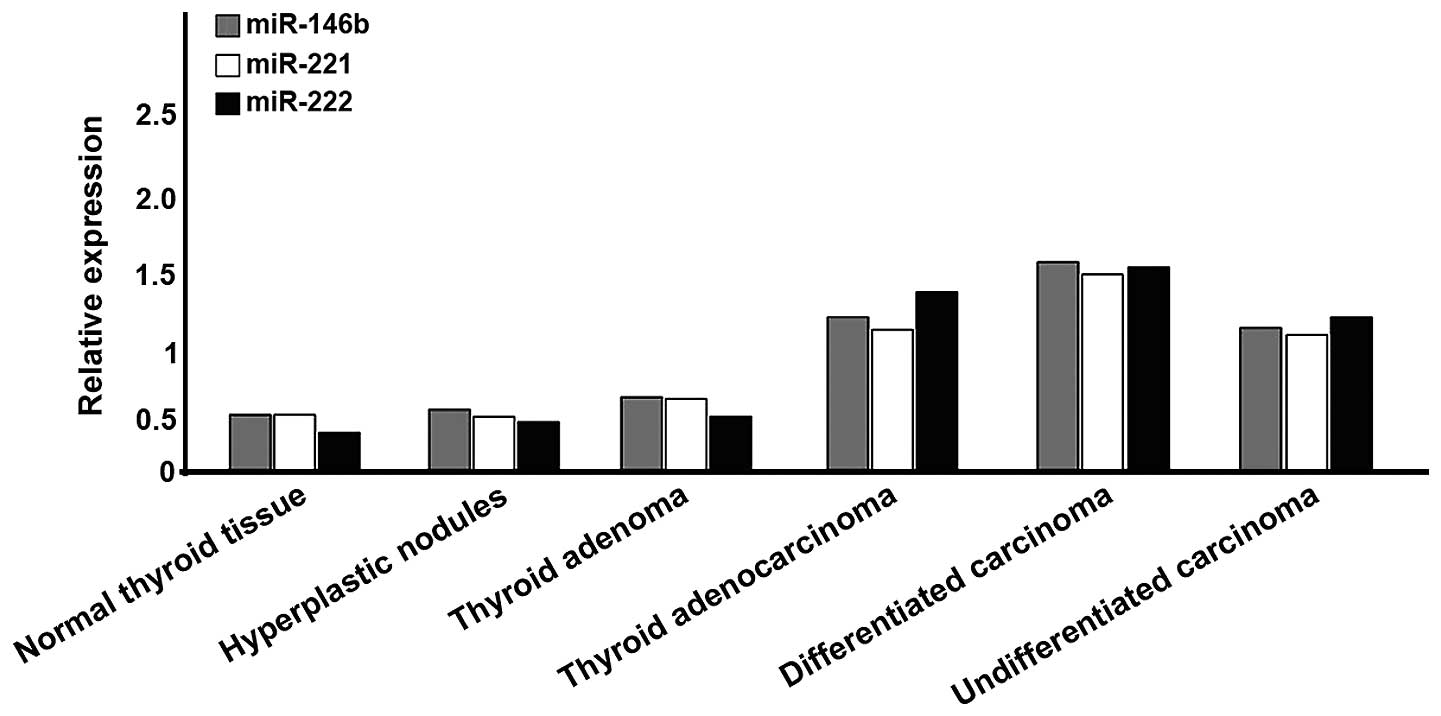
There were 8 cases of normal thyroid tissue; 9 cases
of hyperplastic nodules; 12 cases of thyroid adenoma; and 91 cases
of thyroid carcinoma, of which 59 cases were DTC, 15 cases were
follicular carcinoma and 17 cases were undifferentiated carcinoma.
In thyroid carcinoma, the expression levels of miR-146b, −221 and
−222 were significantly higher than those of other tissues
(P<0.05). These indicators of differentiated type were also
significantly higher than those of undifferentiated type
(P<0.05). A comparison of the differentiated subunit, indicated
no statistically significant difference (P>0.05; Fig. 1).
Susceptibility and specificity of
miRNAs following diagnosis of DTC
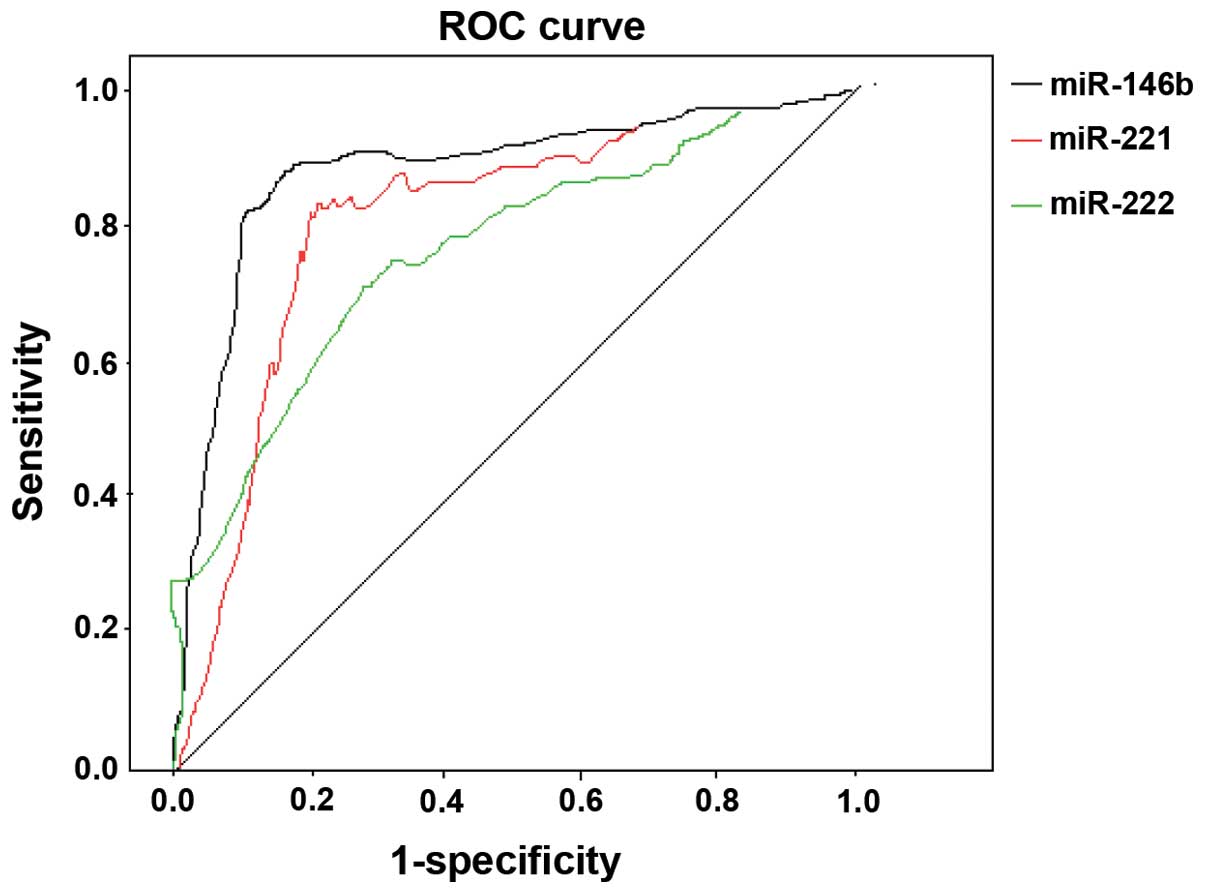
DTC was considered as a diagnosis and others
(diseases except DTC) as reference. The expression levels of
miR-146b, −221 and −222 were plotted into a ROC curve. The AUC of
miR-146 following diagnosis of DTC was 0.832, when
2−ΔΔCt =1.346, susceptibility was 83.2%, and specificity
was 79.6%. The AUC of miR-221 after diagnosis of DTC was 0.806,
when 2−ΔΔCt =1.213, susceptibility was 81.6%, and
specificity was 77.4%. The AUC of miR-222 following diagnosis of
DTC was 0.745, when 2−ΔΔCt =1.425, susceptibility was
78.4%, and specificity was 73.9% (Fig.
2).
Susceptibility and specificity of
single index and a combination of indices of miRNAs following
diagnosis of PTC
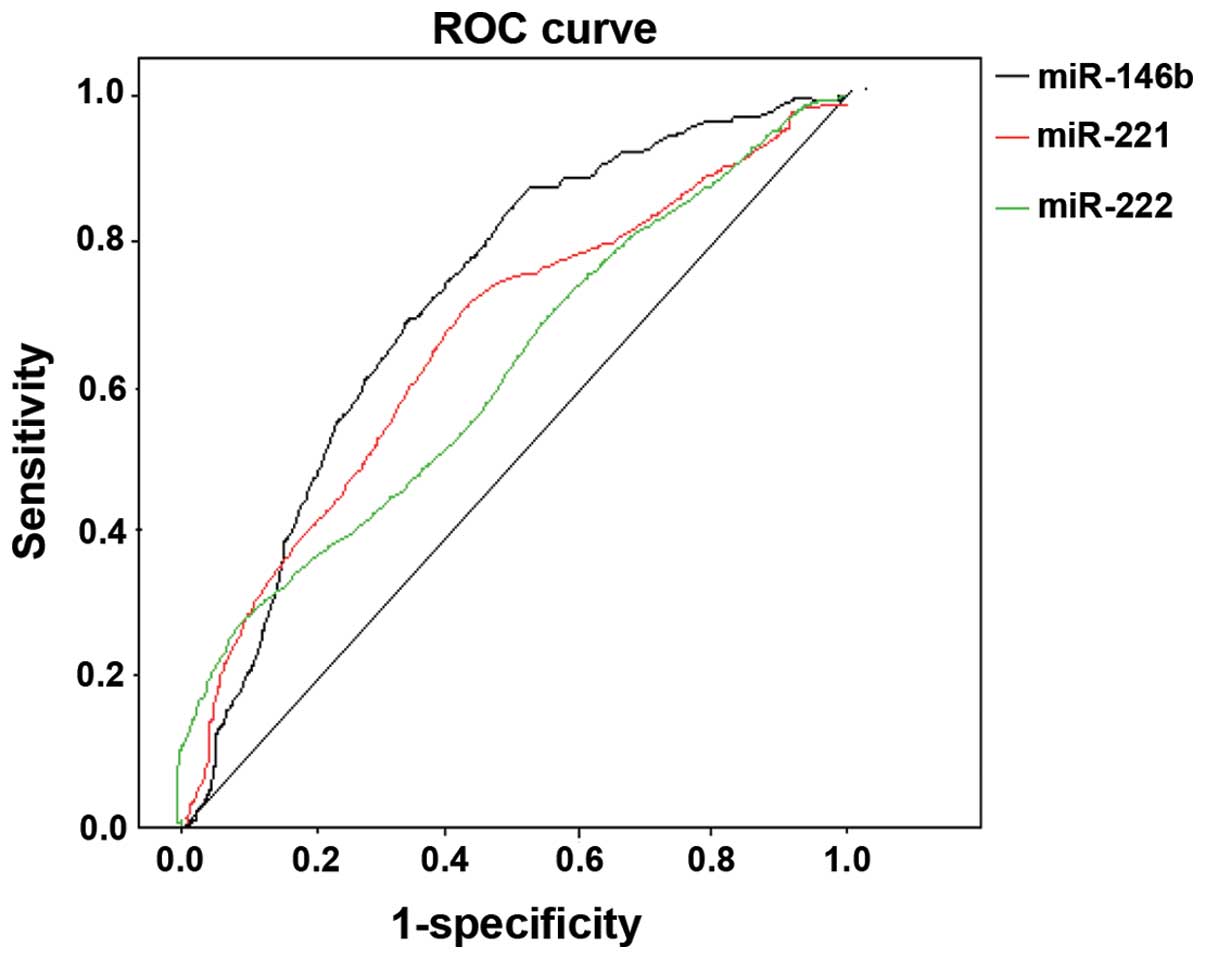
PTC was considered as the diagnosis and follicular
carcinoma as the reference. The expression levels of miR-146b,
−221, −222 and combinations of two types were plotted into a ROC
curve. The AUC of miR-146 following diagnosis of PTC was 0.644,
when 2−ΔΔCt =1.572, susceptibility was 72.8%, and
specificity was 62.3%. The AUC of miR-221 following diagnosis of
PTC was 0.633, when 2−ΔΔCt =1.492, susceptibility was
71.5%, and specificity was 60.9%; the AUC of miR-222 following
diagnosis of PTC was 0.615, when2−ΔΔCt =1.448,
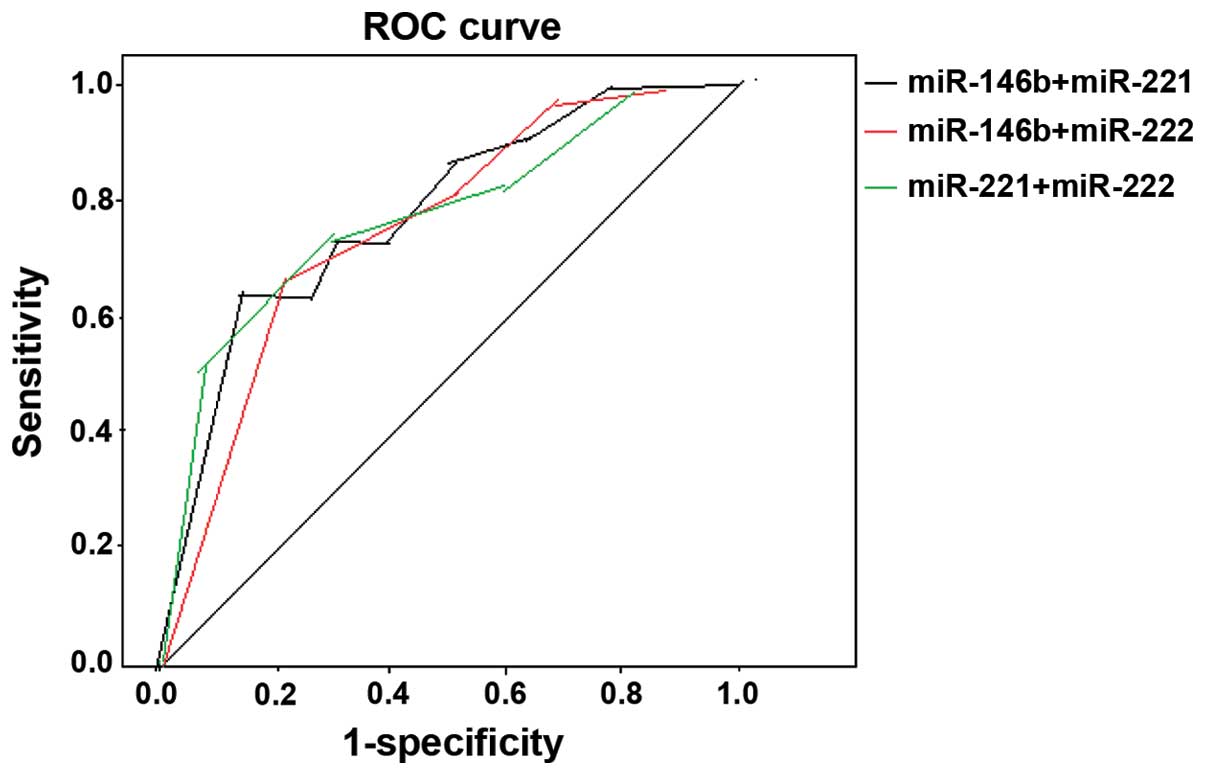
susceptibility was 68.7%, and specificity was 59.3% (Fig. 3). The AUC of combined miR-146b and
−221 following diagnosis of PTC is 0.695, when 2−ΔΔCt
was 1.506 and 1.462, respectively, susceptibility was 78.9%, and
specificity was 68.5%. The AUC of combined miR-146b and −222
following diagnosis of PTC is 0.677, when 2−ΔΔCt was
1.523 and 1.443, respectively, susceptibility was 76.3%, and
specificity was 66.4%. The AUC of combined miR-221 and −222
following diagnosis of PTC is 0.662, when 2−ΔΔCt was
1.564 and 1.437, respectively, susceptibility was 74.9%, and
specificity was 68.2% (Fig. 4).
Discussion
Mature miRNA engages in the formation of RNA-induced
silencing complex, known as the miRNA ribonucleoprotein complex
(7). In the majority of cases,
single-stranded miRNA of compounds and 3′UTR of its homologous mRNA
have incomplete complementary pairing, which interrupts translation
of this gene and regulates the protein expression. In addition,
miRNA and its mRNA have complete complementary pairing, which leads
to a specific fracture of target mRNA in the complementary region
and gene silencing (9). This feature
of miRNA can be used as a sensitive indication during the diagnosis
of diseases in the early stage (10).
Chen et al (11) identified that compared with follicular
adenoma, PTC markedly upregulates miR-146b, −221 and −222, while no
difference was identified for the expression of −146a, −155 and
−187. Compared with non-PTC, including poorly differentiated
adenocarcinoma, hyperplastic nodules and normal thyroid tissue,
miR-146b was highly expressed in PTC while there was no distinctive
difference in the subtypes of DTC. Nikiforova et al
(12) also compared BRAF, RAS and
RET, three types of different mutations of PTC, and identified that
miR-187 and −146b expression was downregulated in the RAS and BRAF
mutational group, while this expression was upregulated in the RET
mutational group, with the expression levels of the BRAF and RAS
mutational groups being the highest (12). Through flat plate clone test, it was
found that compared with the control group, clone numbers of PTC
cells of transfected miR-221 were more than double (13). Conversely, by calculating cells at 72
and 96 h after knocking out miR-221, it was found that cell counts
decreased ~0.3-fold compared with the control group. Thus, the
expression of miR-221 may be significantly associated with the cell
proliferation of PTC. Follicular adenocarcinoma (FTC) is only 10%
of DTC. Colamaio et al (14)
found that compared with normal thyroid tissues, the expression of
miR-191 significantly decreased in FTC, and that upregulated
miR-191 in the WRO cell line of FTC, not only changes cell
morphology, but also inhibits cyclin CDK6, and blocks cells in the
G1 period, which inhibits cell proliferation and migration.
Based on the above results, the present study
identified that miR-146b,-221 and −222 are highly expressed in DTC,
although there was no difference in the subtypes of DTC. According
to the ROC curve, single miR-146b, −221 or −222 can be used as
higher indices of susceptibility and specificity while diagnosing
DTC, but single index does not perform well while distinguishing
PTC, and combinations of the two types improved diagnostic
accuracy, and there was no distinctive difference. However,
compared with the single index while diagnosing DTC, susceptibility
and specificity were not optimal; thus, there was no distinctive
difference between miR-146b, −221 or −222 in the different subtypes
of DTC. The significance of the different expressions of miRNA in
various diseases remains to be determined. In addition, how it
regulates and controls cell proliferation, migration and
differentiation in various types of tumor and specific cell
signaling pathways has yet to be determined. Thus, whether
different subtypes of DTC have miRNAs of obvious differential
expression remains to be elucidated.
References
|
1
|
Wu Q, Li Y and Wang Y: Diagnostic value of
‘absent’ pattern in contrast-enhanced ultrasound for the
differentiation of thyroid nodules. Clin Hemorheol Microcirc. Dec
15–2015.(Epub ahead of print).
|
|
2
|
Fang Q, Cai C and Chen H: Application
value of fine needle aspiration and cell block in preoperative
diagnosis of thyroid cancer and discrimination of follicular tumor.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 50:668–672. 2015.(In
Chinese). PubMed/NCBI
|
|
3
|
Kouniavsky G and Zeiger MA: The quest for
diagnostic molecular markers for thyroid nodules with indeterminate
or suspicious cytology. J Surg Oncol. 105:438–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dorris ER, Smyth P, O'Leary JJ and Sheils
O: MIR141 expression differentiates Hashimoto thyroiditis
from PTC and benign thyroctytes in Irish archival thyroid tissues.
Front Endocrinol (Lausanne). 3:1022012.PubMed/NCBI
|
|
6
|
Liu R, Ma X, Xu L, Wang D, Jiang X, Zhu W,
Cui B, Ning G, Lin D and Wang S: Differential microRNA expression
in peripheral blood mononuclear cells from Graves' disease
patients. J Clin Endocrinol Metab. 97:E968–E972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graham ME, Hart RD, Douglas S, Makki FM,
Pinto D, Butler AL, Bullock M, Rigby MH, Trites JR, Taylor SM, et
al: Serum microRNA profiling to distinguish papillary thyroid
cancer from benign thyroid masses. J Otolaryngol Head Neck Surg.
44:332015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou CK, Chen RF, Chou FF, Chang HW, Chen
YJ, Lee YF, Yang KD, Cheng JT, Huang CC and Liu RT: miR-146b is
highly expressed in adult papillary thyroid carcinomas with high
risk features including extrathyroidal invasion and the BRAF(V600E)
mutation. Thyroid. 20:489–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Connerty P, Ahadi A and Hutvagner G: RNA
Binding Proteins in the miRNA Pathway. Int J Mol Sci. 17:172015.
View Article : Google Scholar
|
|
10
|
Lee JC, Zhao JT, Gundara J, Serpell J,
Bach LA and Sidhu S: Papillary thyroid cancer-derived exosomes
contain miRNA-146b and miRNA-222. J Surg Res. 196:39–48. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YT, Kitabayashi N, Zhou XK, Fahey TJ
III and Scognamiglio T: MicroRNA analysis as a potential diagnostic
tool for papillary thyroid carcinoma. Mod Pathol. 21:1139–1146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikiforova MN, Tseng GC, Steward D, Diorio
D and Nikiforov YE: MicroRNA expression profiling of thyroid
tumors: Biological significance and diagnostic utility. J Clin
Endocrinol Metab. 93:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pallante P, Visone R, Ferracin M, Ferraro
A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M,
Negrini M, et al: MicroRNA deregulation in human thyroid papillary
carcinomas. Endocr Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colamaio M, Borbone E, Russo L, Bianco M,
Federico A, Califano D, Chiappetta G, Pallante P, Troncone G,
Battista S, et al: miR-191 down-regulation plays a role in thyroid
follicular tumors through CDK6 targeting. J Clin Endocrinol Metab.
96:E1915–E1924. 2011. View Article : Google Scholar : PubMed/NCBI
|