Introduction
Conjugation of small ubiquitin-related modifier
protein (SUMO) has been reported in organisms, including
Arabidopsis thaliana and mammals, indicating that SUMO is an
evolutionarily conserved protein that may have unique functions in
cellular metabolism (1). By
conjugating to protein substrates, SUMO regulates the localization
and activity of target proteins (1–5). SUMO
modification is involved in numerous metabolic processes in cells
and plays an important role in the balance and interaction between
proteins, transcriptional activity and cellular localization
(4,6–8).
De-SUMOylation, the reverse reaction of SUMOylation, is regulated
by the SENP family (9). Once the
balance between SUMOylation and de-SUMOylation is broken, there
will be an overexpression of SUMO or SENPs in cells, which may lead
to tumor development.
There are 6 SENPs in mammals (10). SENP1, a nuclear protease, promotes
de-SUMOylation processing of a variety of proteins that have been
SUMOylated. The majority of cases of prostate cancer demonstrate an
increased expression of SENP1 (11–13).
Subsequent to the comparison of SENP1 levels between normal
prostate and prostate cancer tissues, a previous study found that
increased expression of SENP1 was observed in 60% of patients with
prostate cancer (13). Androgens and
interleukin-6 easily increased SENP1 expression in prostate cancer
(14). SENP1 induction mediates cell
proliferation by increasing androgen receptor-dependent
transcription, c-Jun-dependent transcription and cyclin D1 levels
(14–16). However, the mechanism of SENP1 in
human glioma cells remains unclear. Since SUMO and proteins
modified by SUMO may be important in the occurrence of malignant
glioma (17), it is essential to
define the role of SENP1 in human glioma cells, which may aid in
the identification of potential therapeutic targets for malignant
glioma. Therefore, the present study aimed to define the role of
SENP1 in human glioma cells.
Materials and methods
Main reagents
Human glioma LN-299 cells were purchased from the
American Type Culture Collection (catalog no., CRL-2611; Manassas,
VA, USA). Dulbecco's modified Eagle's medium (DMEM) with high
glucose, fetal bovine serum (FBS) and phosphate buffer solution
(PBS) were obtained from HyClone (GE Healthcare, Logan, UT, USA).
Cell culture dishes were purchased from Corning Life Sciences
(Corning, NY, USA). TRIzol reagent and First Strand cDNA Synthesis
kit were purchased from Tiangen Biochemical Technology Co., Ltd.
(Beijing, China). Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection kit was purchased
from Nanjing KeyGen Biotech Co., Ltd. (catalog no., KGA106;
Nanjing, Jiangsu, China). Rabbit anti-SENP1 monoclonal (catalog
no., ab108981), rabbit anti-cyclin D1 monoclonal (catalog no.,
ab134175), rabbit anti-c-Jun monoclonal (catalog no., ab32137) and
rabbit anti-β-actin polyclonal (catalog no., ab8227) antibodies
were from Abcam (Cambridge, MA, USA).
shRNA design and expression plasmid
vector construction
Two shRNAs targeting the SENP1 gene were
synthesized: SENP1 shRNA-1, 5′-GCACCTCATCAGCCAAATAGC-3′; and SENP1
shRNA-2, 5′-GCATTCCGCTTGACCATTACA-3′. shRNA targeting scrambled
sequence (general sequence, 5′-TTCTCCGAACGTGTCACGT-3′) was designed
and acted as the negative control group. The DNA sequence targeting
the SENP1 gene was cloned into the pGenesil-1 vector (catalog no.,
VRG0358; Wuhan Genesil Biotechnology Co., Ltd., Wuhan, Hubei,
China), which expresses shRNA and enhanced green fluorescent
protein (EGFP) in mammalian cells. The recombinant plasmids
pGenesil/SENP1 and pGenesil/NC were constructed and verified by
Wuhan Genesil Biotechnology Co., Ltd.
Cell culture and transfection
Glioma cells were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS. All cells were cultured
in a humidified incubator, containing 5% CO2 at 37°C.
LN-299 cells were replated at a density of 5×106
cells/well in 6-well plates. When the cell density reached 40–50%,
cells were transfected with SENP1 shRNA using Lipofectamine 2000
(Invitrogen™; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Messenger RNA (mRNA) transcript expression was
quantified RT-qPCR and normalized against the expression of
β-actin. Using 1 ml TRIzol, cell lysis was performed at room
temperature for 5 min, followed by treatment with 0.2 ml chloroform
(Sigma-Aldrich, St. Louis, MO, USA) for 15 min at room temperature.
The aforementioned mixture was centrifuged at 12,000 × g for 15 min
at 4°C, and the supernatant was then mixed with 0.4 ml isopropyl
alcohol and placed for 10 min at room temperature. The centrifugal
sedimentation was obtained by centrifugation at 12,000 × g for 10
min at 4°C, was washed with diethylpyrocarbonate containing 75%
ethanol and placed at room temperature to dry. The quality of the
RNA was confirmed using an absorbance cut-off of A260/A280>1.8
(ND-2000; NanoDrop™, Thermo Fisher Scientific, Inc.). RT-qPCR was
performed using an ABI 7900HT Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the following
cycling conditions: 5°C for 1 min, followed by 40 cycles of 95°C
for 10 sec, 55°C for 20 sec and 72°C for 5 sec. The following
primers were used: SENP1 forward, 5′-CTACAAGAAGCCCAGCCTATCGTC−3′
and reverse, 5′-GTCACCTGAGCCAAGGAAACTG-3′; and β-actin forward,
5′-CTTTCTACAATGAGCTGCGTG-3′ and reverse,
5′-TCATGAGGTAGTCTGTCAGG-3′. Subsequent to confirmation of the
quality of RNA, 2 µg RNA was reverse transcribed into cDNA.
Western blot analysis
Total protein was extracted from cell lines using
the ReadyPrep Protein Extraction kit (catalog no., 163-2090;
Bio-Rad Laboratories, Hercules, CA, USA). The protein concentration
was determined using a Pierce BCA Protein Assay Kit (catalog no.,
23227; Thermo Fisher Scientific, Inc.). Protein lysates were loaded
on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Separated protein bands were electro-transferred onto
polyvinylidene fluoride (PVDF) membranes (catalog no., IPVH00010;
EMD Millipore, Billerica, MA, USA). The PVDF membranes were then
blocked in Tris-buffered saline (TBS; Neuromics, Inc., Minneapolis,
MN, USA) containing 10 mM Tris-HCl (pH 7.5) and 150 mM NaCl, and 5%
non-fat dry milk at room temperature for 1 h. The membranes were
then incubated at 4°C overnight with the rabbit anti-SENP1
monoclonal (catalog no., ab108981; dilution, 1:1,000; Abcam),
rabbit anti-c-Jun monoclonal (catalog no., ab32137; dilution,
1:1,000; Abcam), rabbit anti-cyclin D1 monoclonal (catalog no.,
ab314175; dilution, 1:1,000; Abcam) and rabbit anti-β-actin
polyclonal (catalog no., ab8227; dilution, 1:1,000; Abcam)
antibodies. Subsequent to incubation with the primary antibody, the
membranes were washed in TBS with 0.05% Tween-20 (Sigma-Aldrich),
and the secondary goat anti-rabbit immunoglobulin G heavy and light
chain horseradish peroxidase-conjugated antibody (catalog no.,
ab6721; dilution, 1:2,000; Abcam) was added. The membrane was then
incubated at 37°C for 2 h. Pierce ECL Plus Western Blotting
Substrate (catalog no., 32132; Thermo Fisher Scientific, Inc.) was
used to visualize the immunoreactive bands and Image J 1.42q
software (National Institutes of Health, Bethesda, MA, USA) was
used for quantification. Relative protein level was normalized
against the β-actin concentration. Three separate experiments were
performed in duplicate for each treatment.
Cell proliferation assay
Cell viability was assessed using a methyl thiazolyl
tetrazolium (MTT) assay (Thiazolyl Blue Tetrazolium Bromide;
catalog no., M2128; Sigma-Aldrich). Cells were cultured in 24-well
plates at a concentration of 5×106 cells per well and
allowed to adhere. Subsequent to treatment at various time
intervals (24, 48, 72 and 96 h), 100 µl MTT (0.5 mg/ml) was added
to the cells and the mixture was incubated for 4 h at 37°C.
Subsequently, the supernatant was removed, and dimethyl sulfoxide
(catalog no., D2650; Sigma-Aldrich) was used to dissolve the
resultant formazan crystals. The absorbance value was read at 570
nm using a microplate reader (Automated Microplate Reader EL309;
Bio-Tek Instruments, Inc., Winooski, VT, USA). Six wells were
measured for each group, and the experiment was repeated three
times.
Flow cytometry analysis of cell
apoptosis
To detect cell apoptosis, the proliferating phase
LN-299 was trypsinized, washed with cold PBS and resuspended in
binding buffer using the Annexin V-FITC/PI Apoptosis Detection kit
(catalog no., KGA106; Nanjing KeyGen Biotech Co., Ltd.) according
to the manufacturer's protocol. Annexin V-FITC and PI were added to
the fixed cells for 20 min in darkness at room temperature. Annexin
V binding buffer was then added to the mixture prior to the
fluorescence being measured on FACSort flow cytometer (BD
Biosciences, San Jose, CA, USA). Cell apoptosis was analyzed using
the Cell Quest 3.0 software (BD Biosciences).
Transwell migration assay
Cell culture inserts and Transwell chamber (catalog
no., 3464; Corning Life Sciences, Tewksbury, MA, USA) were
pre-warmed up at 37°C. Cells in the logarithmic phase of growth
were rinsed with PBS and adjusted to an appropriate concentration.
Resuspended cells were cultured in the upper chamber of the
Transwell inserts. The lower chambers contained ~600 µl culture
medium containing 10% FBS. Following 24 h of incubation,
transmigrated cells were fixed with 800 µl methyl alcohol and
stained with Giemsa (Sigma-Aldrich) at room temperature for 30 min.
The number of migratory cells were counted by capturing images of
the membrane under a microscope (BX51-P; Olympus Corporation,
Tokyo, Japan).
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for the data analysis. All experiments were repeated three times
and data are presented as the mean ± standard deviation (SD). The
data were compared between two groups using the two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
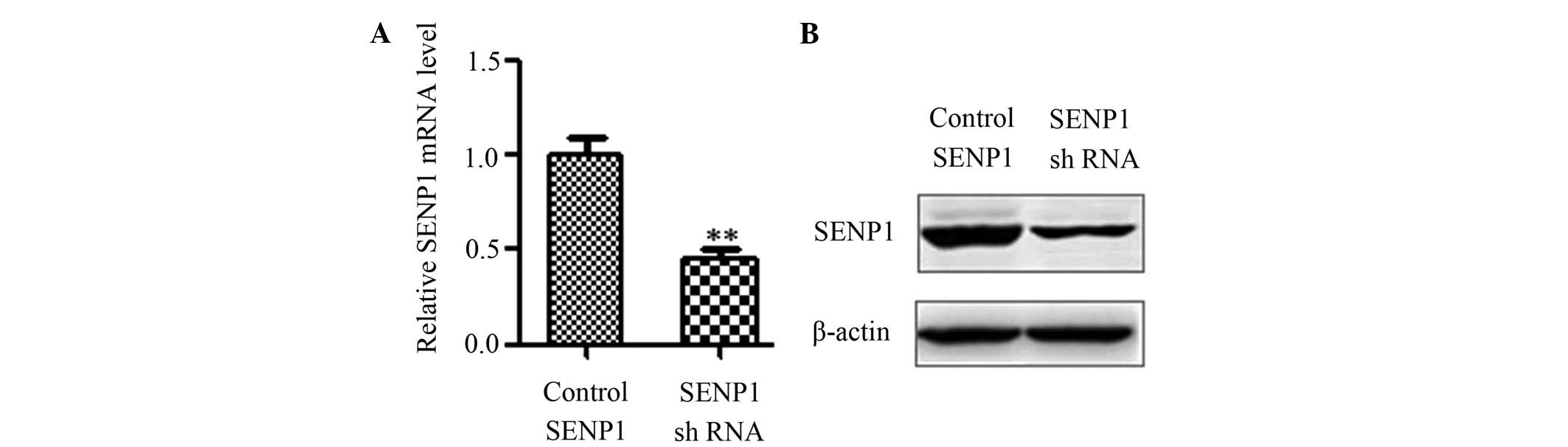
SENP1 expression was downregulated by
SENP1 shRNA
To knockdown SENP1, SENP1 shRNA and control shRNA
were transfected into glioma cells. Subsequent to 48 h of
culturing, RNA was extracted, and reverse transcription reactions
were performed to illustrate various mRNA expression levels of
SENP1. It was found that SENP1 shRNA effectively downregulated the
mRNA levels of SENP1 (Fig. 1A;
0.45±0.04 vs. 1.00±0.14; P=0.02). Accordingly, the downregulation
of the SENP1 protein was detectable by western blot analysis
(Fig. 1B).
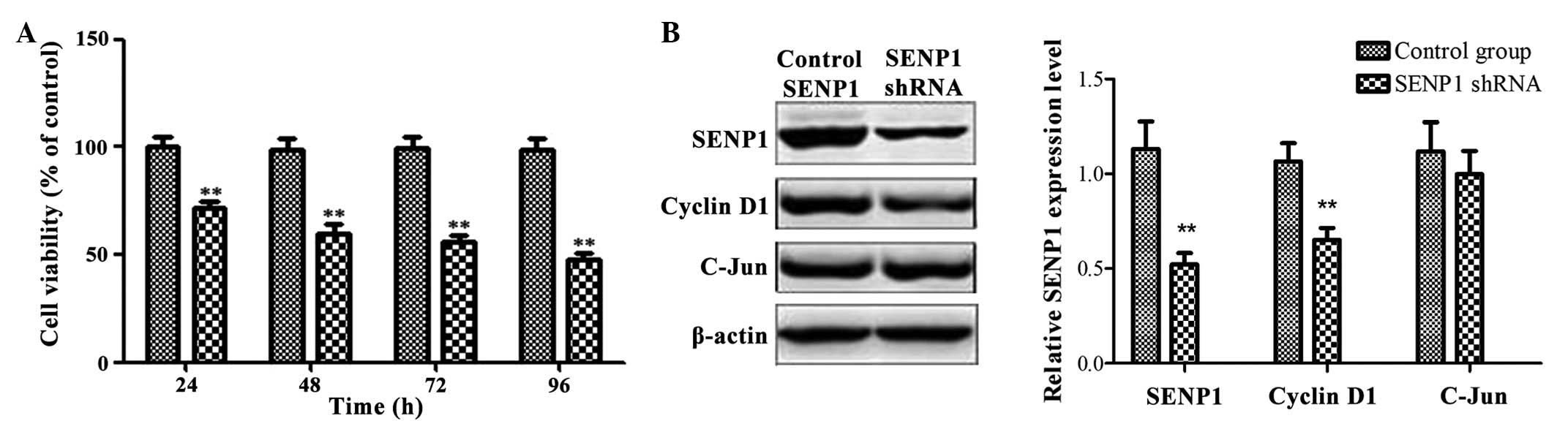
Downregulation of SENP1 inhibited cell
proliferation and viability
The effect of the downregulation of SENP1 on the
proliferation and viability of glioma cells was detected. The
viable cells were counted using a cell counting chamber every 24 h
for 4 days subsequent to transfection. The viable cells in the
control shRNA group were defined as 1 × 100%, and data were
expressed as the percentage of growth inhibition as follows:
Relative cell viability = A595 (SENP1 shRNA group) / A595 (control
shRNA group) × 100%. Following knockdown of SENP1, the number of
viable human glioma cells decreased in a time-dependent manner
(Fig. 2A) (SENP1 shRNA group vs.
control group: 24 h, 71.01±3.43 vs. 100±3.95%, P=0.0024; 48 h,
58.47±4.69 vs. 100±2.66%, P=0.0002; 72 h, 54.99±2.50 vs. 100±3.25%,
P<0.0001; 96 h 46.21±2.81 vs. 100±2.59%, P<0.0001). Compared
to the control group, the viable cells in the SENP1 shRNA group
decreased to ~50% at 96 h subsequent to transfection (SENP1 shRNA
group vs. control group: 100±2.59 vs. 46.21±2,81%, P<0.0001).
These results indicated that downregulation of SENP1 may
effectively kill tumor cells. To define the present conclusion,
levels of cyclin D1 protein was determined by semi-quantified
western blot analysis. As shown in Fig.
2B, SENP1 knockdown inhibited the expression of cyclin D1 to a
certain degree, suggesting that SENP1 knockdown may inhibit tumor
cell proliferation and viability by mediating the expression of
cyclin D1.
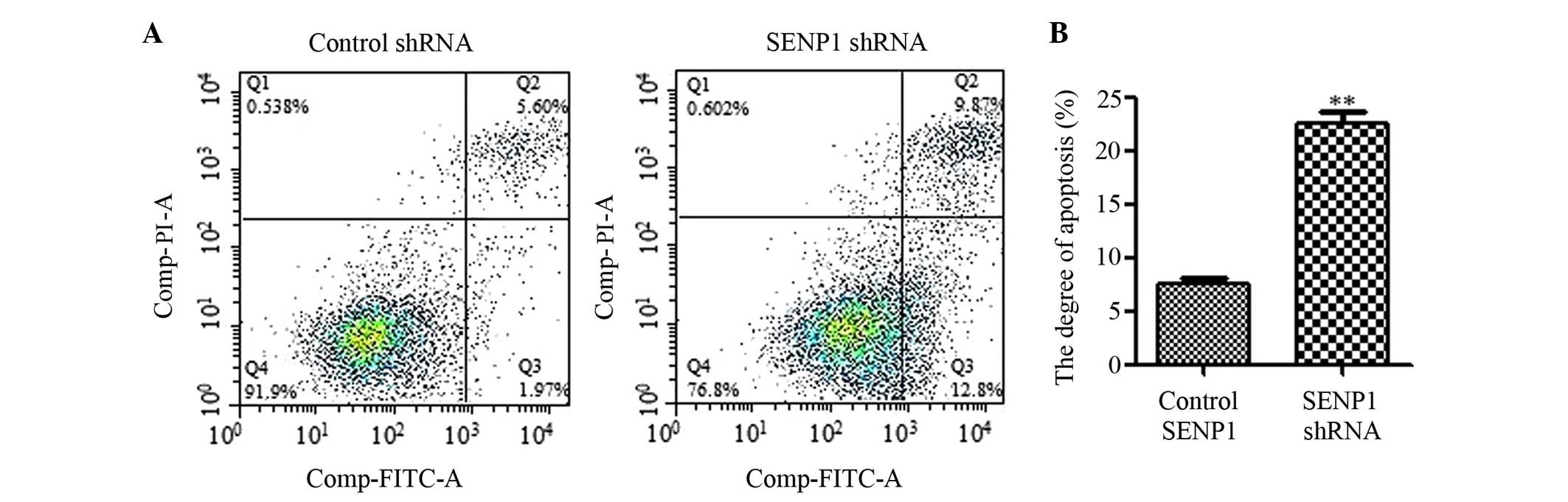
Downregulation of SENP1 induced
apoptosis
To explore the role of SENP1 in glioma cells,
Annexin V-FITC/PI Apoptosis Detection kit (catalog no., KGA106;
Nanjing KeyGen Biotech Co., Ltd.) was used for apoptosis analysis.
SENP1 knockdown was found to significantly induce apoptosis in
human glioma cells following 72 h of transfection (SENP1 shRNA
group vs. control group, 22.46±0.91 vs. 7.43±0.72%; P<0.0001),
in accordance with the inhibitory effect on cell proliferation and
viability (Fig. 3).
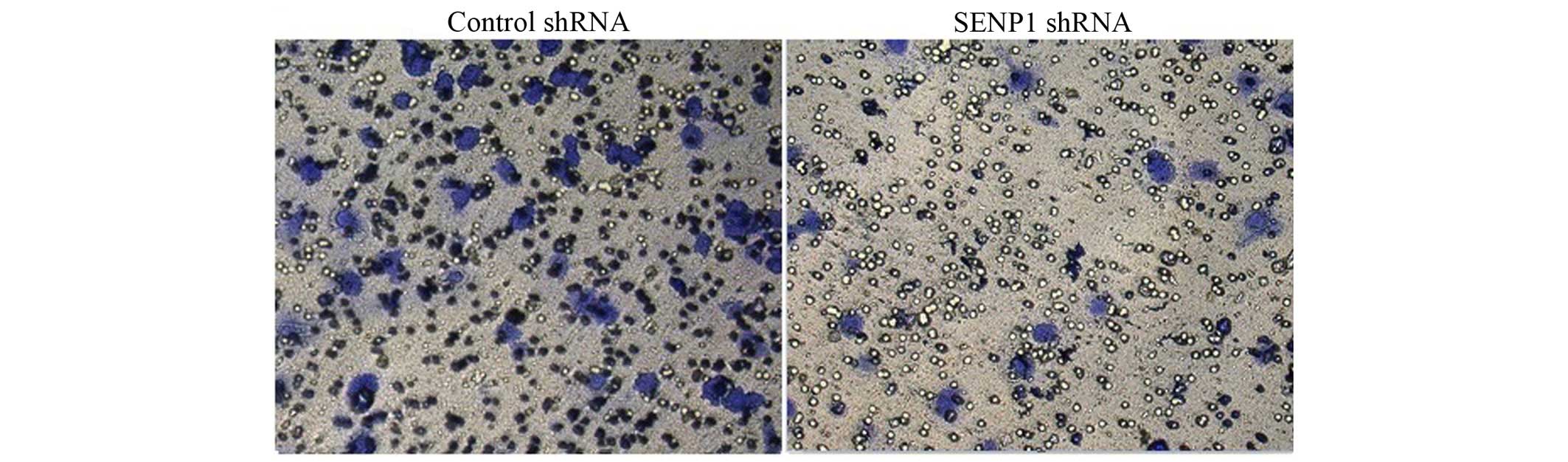
Downregulation of SENP1 inhibited cell
migration
Migration is one of the features of tumor cells. The
effect of the downregulation of SENP1 on cell migration was
investigated using a Transwell migration system. As the
representative images show in Fig. 4,
SENP1 knockdown was found to evidently suppress tumor cell
migration (SENP1 shRNA group vs. control group, 37.93±8.29 vs.
100±5.78%; P<0.0001).
Discussion
Recently, SENP1 has been less frequently reported in
studies. It has previously been reported that SENP1 was
overexpressed in prostate cancer tissue samples from patients, and
the overexpression of SENP1 contributes to the progression of
prostate cancer (11). High
expression of SENP1 is also associated with the occurrence and
development of gastric cancer (18).
However, the effect of SENP1 on human glioma cells remains
unclear.
In the present study, SENP1 was found to perform a
vital function in human glioma cells. The results in this study
indicate that SENP1 acts as a positive regulator in human glioma
cell viability, migration and proliferation (Fig. 2A). This is consistent with the results
of previous study (11), in which
SENP1 was reported to affect the tumorigenesis of prostate cancer
cells in vivo and in vitro. Decreased levels of the
cyclin D1 protein were also detected in glioma cells to a certain
degree following downregulation of SENP1, which suggested that
SENP1 knockdown may suppress cell proliferation and viability by
mediating the cell cycle. In addition, the present study showed
that SENP1 knockdown significantly induced apoptosis in human
glioma cells (Fig. 3) and suppressed
tumor cell migration (Fig. 4), which
was similar to the effects of SENP1 on prostate cancer and
hepatocellular carcinoma cells as reported by previous studies
(11,19). Two reasons may exist for cell
migration suppression. One reason is that the migration-associated
genes expression may be inhibited by the downregulation of SENP1
expression, and the other reason is that migrated cells became
decreased in number due to the decrease in viable cells and
increase in apoptotic cells. These results indirectly demonstrate
that SENP1 is likely to play a critical role in human glioma cells,
as shown by the results from additional studies (19,20).
However, the definite mechanism of SENP1 in cell proliferation and
apoptosis requires investigation in additional studies.
In conclusion, the present experimental results show
the anti-tumor effects of SENP1 on the proliferation and apoptosis
of human glioma LN-299 cells. SENP1 may be a potential therapeutic
target for the inhibition of growth and progression of glioma
cells. The present study may provide insight into the tumorigenesis
and development of glioma cells and provide novel strategies and
targets for glioma treatment. However, additional studies are
required to clarify the associated molecular mechanisms and signal
transduction of SENP1.
Acknowledgements
The present study was supported by the Basic
Research Project from Science and Technology Innovation Commission
of Shenzhen Municipality of Guangdong Province (ågrant no.
jcyj20130401112059058) and the Project from Health Department of
Guangdong Province (grant no. A2013349).
References
|
1
|
Geiss-Friedlander R and Melchior F:
Concepts in sumoylation: A decade on. Nat Rev Mol Cell Biol.
8:947–956. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hay RT: SUMO: A history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh ET, Gong L and Kamitani T:
Ubiquitin-like proteins: New wines in new bottles. Gene. 248:1–14.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gill G: SUMO and ubiquitin in the nucleus:
Different functions, similar mechanisms? Genes Dev. 18:2046–2059.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saitoh H: The role of ubiquitin-like
protein SUMO-1 in the nucleus. Tanpakushitsu Kakusan Koso. 44(12
Suppl): 1852–1859. 1999.(In Japanese). PubMed/NCBI
|
|
6
|
Dünnebier T, Bermejo JL, Haas S, Fischer
HP, Pierl CB, Justenhoven C, Brauch H, Baisch C, Gilbert M, Harth
V, et al: Common variants in the UBC9 gene encoding the
SUMO-conjugating enzyme are associated with breast tumor grade. Int
J Cancer. 125:596–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hay RT: SUMO-specific proteases: A twist
in the tail. Trends Cell Biol. 17:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu G, Xu C and Staudinger JL: Pregnane X
receptor is SUMOylated to repress the inflammatory response. J
Pharmacol Exp Ther. 335:342–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pourcet B, Pineda-Torra I, Derudas B,
Staels B and Glineur C: SUMOylation of human peroxisome
proliferator-activated receptor alpha inhibits its trans-activity
through the recruitment of the nuclear corepressor NCoR. J Biol
Chem. 285:5983–5992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chow KH, Elgort S, Dasso M, Powers MA and
Ullman KS: The SUMO proteases SENP1 and SENP2 play a critical role
in nucleoporin homeostasis and nuclear pore complex function. Mol
Biol Cell. 25:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease 1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanna K, Tiina J, Ulla K, Rytinki MM,
Makkonen H, Gioeli D, Paschal BM and Palvimo JJ: SUMO-specific
protease 1 (SENP1) reverses the hormone-augmented SUMOylation of
androgen receptor and modulates gene responses in prostate cancer
cells. Mol Endocrinol. 23:292–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng J, Bawa T, Peng L, Lee P, Gong L and
Yeh ET: Role of desumoylation in the development of prostate
cancer. Neoplasia. 8:667–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM
and Yeh ET: SENP1 induces prostatic intraepithelial neoplasia
through multiple mechanisms. J Biol Chem. 285:25859–25866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dammer EB, Leon A and Sewer MB:
Coregulator exchange and sphingosine-sensitive cooperativity of
steroidogenic factor-1, general control nonderepressed 5, p54 and
p160 coactivators regulate cyclic adenosine
3′,5′-monophosphate-dependent cytochrome P450c17 transcription
rate. Mol Endocrinol. 21:415–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee WP, Jena S, Doherty D, Ventakesh J,
Schimdt J, Furmick J, Widener T, Lemau J, Jurutka PW and Thompson
PD: SUMO specific proteases as novel tissue-selective modulators of
vitamin D receptor-mediated signaling. PLoS One. 9:e895062014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi L, Wang WY, Zheng ZH, Zhang YH, Zhao L,
Zhong XH, Zhao DH, Yang S, Yang NJ and Ren K: Effects of SUMO and
proteins modified by SUMO on occurrence of malignant gliomas. Ji
Lin Da Xue Xue Bao. 42:7–10. 2016.(In Chinese).
|
|
18
|
Chen J, Gao Q, Wang XY, et al: Protein
expression and significance of SENP1 and HER-2 in the gastric
cancer. Chinese Journal of Current Advances in General Surgery.
16:102–106. 2013.
|
|
19
|
Zhang W, Sun H, Shi X, Wang H, Cui C, Xiao
F, Wu C, Guo X and Wang L: SENP1 regulates hepatocyte growth
factor-induced migration and epithelial-mesenchymal transition of
hepatocellular carcinoma. Tumor Biol. Dec 22–2015.(Epub ahead of
print).
|
|
20
|
Ma C, Wu B, Huang X, Yuan Z, Nong K, Dong
B, Bai Y, Zhu H, Wang W and Ai K: SUMO-specific protease 1
regulates pancreatic cancer cell proliferation and invasion by
targeting MMP-9. Tumour Biol. 35:12729–12735. 2014. View Article : Google Scholar : PubMed/NCBI
|