Introduction
Pituitary adenoma is the third most frequently
diagnosed of all intracranial tumors, comprising ~10–25% of all
intracranial tumors (1). Human
clinically nonfunctioning pituitary adenomas (NFPAs) are defined as
adenomas lacking symptoms or signs secondary to oversecretion of
pituitary hormones by the tumor (2).
NFPAs constitute 9–50% of all pituitary tumors and ~80% of all
pituitary macroadenomas (3).
Effective medical therapy has been demonstrated in FPAs (4). By contrast, for NFPAs, surgery is the
first-line treatment (2). However,
aggressive NFPAs are not always amenable to complete resection, and
radiotherapy should be considered for residual tumors (5). Current detection strategies for
pituitary adenoma do not reliably detect the disease at an early
stage, and are unable to distinguish between aggressive and
non-aggressive pituitary adenoma; thus, effective prognosis and
treatment guidance are missing (6,7). At
present, the extent of adenoma aggressiveness is mainly determined
by computed tomography (CT) and magnetic resonance imaging (MRI)
(8). However, the current standards
to determine tumor aggressiveness are questioned by numerous
scholars, and the effectiveness of the above techniques remains to
be determined (6,7,9).
Therefore, specific molecular markers for aggressiveness of NFPAs
will be more helpful to guide the clinical diagnosis and treatment
of aggressive NFPAs.
Wnt signaling is involved in cell fate,
proliferation, polarity and death (10,11).
Aberrant regulation of the Wnt signaling pathway has been suggested
to be involved in tumorigenesis (12). Three intracellular pathways are known
to induce Wnt signaling: i) The Wnt-β-catenin signaling pathway, in
which Wnt signaling alters transcription, and it is referred to as
‘canonical’ Wnt signaling (13); ii)
the Wnt-Ca2+ signaling pathway; and iii) the Wnt-c-Jun N-terminal
kinase signaling pathway, which do not involve the nucleus or
transcription, but rather drive cytoplasmic changes involving the
actin cytoskeleton and intracellular calcium stores. Activation of
the Wnt-β-catenin signaling pathway results in the accumulation of
free β-catenin in the nucleus (14).
This in turn activates various transcription factors such as T-cell
factor to induce the expression of target genes that participate in
cell proliferation, including c-Myc and cyclin D1 (14). The Wnt canonical signaling pathway has
been studied most extensively in cancer, but it has also been
implicated as an important contributor to the pathogenesis of other
human diseases (15).
Recent evidence suggests that soluble extracellular
Wnt antagonists regulate Wnt signaling (16). One class of such antagonists is the
secreted frizzled-related protein (sFRP) family (16). The expression levels of sFRPs are
inversely correlated with the grade and invasive ability of
different tumors, including prostate, ovarian and cervical cancer,
which suggests that sFRPs activities are fundamental for tissue
homeostasis (17–22).
In the present study, it was hypothesized that sFRP2
was downregulated and functioned as a tumor suppressor gene in
NFPAs. To address this issue, the messenger (m)RNA and protein
expression levels of sFRP2 were compared in non-aggressive NFPAs,
aggressive NFPAs and normal pituitary tissues. In addition, the
present study investigated whether the Wnt canonical signaling
pathway was activated in NFPAs, and examined the association
between sFRP2 and the Wnt canonical signaling pathway.
Materials and methods
Sample collection
Informed consent was obtained from all patients
participating in the present study, which was approved by the
Ethics Committee of Beijing Tiantan Hospital (Beijing, China).
Between January 2010 and December 2013, 50 NFPA
cases (age range, 15–68 years) were obtained from the Beijing
Tiantan Hospital. The samples were classified into three groups:
Normal pituitary control group, non-aggressive NFPA group and
aggressive NFPA group. The diagnosis was based on MRI/CT.
Aggressive adenomas were defined as fulfilling one of the following
conditions: i) Hardy classification grades III and IV (23); and/or ii) Knosp classification grades
III and IV (24). There were 30
non-aggressive NFPAs and 20 aggressive NFPAs. In the non-aggressive
group, there were 12 female cases and 18 male cases, whose median
age was 42 years. In the aggressive group, there were 7 female
cases and 13 male cases, whose median age was 45 years. Tumors were
removed by transsphenoidal surgery and immediately ‘flash-frozen’
in liquid nitrogen until used. Suitable parts of each sample were
embedded in paraffin.
The 10 normal pituitary glands were obtained from a
donation program between February 2010 and October 2013 in the
Beijing Tiantan Hospital (patient age range, 21–45 years). All
donors had succumbed to non-neurological diseases. The 10 samples
were rinsed in sterile saline and snap-frozen in liquid nitrogen.
Suitable parts of each normal pituitary tissue were embedded in
paraffin.
All samples were analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). A
total of 15 aggressive and 20 non-aggressive NFPA specimens were
randomly selected for western blot analysis, while 10 aggressive
and 10 non-aggressive NFPA paraffin specimens were randomly
selected for immunohistochemical (IHC) analysis.
IHC analysis
Sections (5-µm-thick) were prepared from the
formalin-fixed paraffin-embedded blocks. Briefly, slides were
deparaffinized, rehydrated in xylene and subjected to an alcohol
gradient, and subsequently incubated with anti-sFRP2 (rabbit
polyclonal; 1:100 dilution; ab86379; Abcam, Cambridge, UK) and
anti-β-catenin (rabbit monoclonal; 1:100 dilution; ab32572; Abcam)
antibodies overnight at 4°C. The slides were then incubated for 45
min at room temperature with anti-rabbit immunoglobulin G antibody
conjugated with horseradish peroxidase-labeled polymer (EnVision™+
System; Dako, Glostrup, Denmark). Slides were counterstained with
hematoxylin and then examined under a light microscope.
Staining for sFRP2 and β-catenin was quantified
according to the percentage of positive cells (25). Slides were scored by two pathologists
(Department of Pathology, Beijing Neurosurgical Institute, Beijing,
China) who were blinded to the patients' characteristics. In case
of disagreement, the status was determined by consensus following
simultaneous dual reexamination.
RT-qPCR
Total RNA was isolated from frozen pituitary
adenomas and normal pituitaries (100–150 mg) using TRIzol
(15596-026; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). RT-qPCR analysis was performed on an ABI 7500 Real-Time
system (Applied Biosystems, Foster City, CA, USA), with the
following conditions: 50°C for 2 min, 95°C for 10 min, and 40
cycles each at 95°C for 15 sec and 60°C for 1 min. The housekeeping
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control. Relative quantification of gene expression was
determined using the 2-ΔΔCT method, as described by Livak and
Schmittgen (26). The primers used in
the RT-qPCR assay were as follows: sFRP2, forward
5′-AGGACAACGACCTTTGCATC-3′ and reverse 5′-TTTGCAGGCTTCACATACCTT-3′;
β-catenin, forward 5′-GAGAATTCATGTCTGAGGACAAGCCA-3′ and reverse
5′-GACTCGAGGGATCCTTACAGGTCAGTATCAAACCAGGC-3′; dishevelled segment
polarity protein 3 (DVL-3) forward 5′-AACCAGGGGGTTATGATAGCTC-3′ and
reverse 5′-TATCTCCTGGCTCGATGCGTCC-3′; c-Myc forward
5′-TTCGGGTAGTGGAAAACCAG-3′ and reverse 5′-CAGCAGCTCGAATTTCTTCC-3′;
cyclin D1 forward 5′-AACTACCTGGACCGCTTCCT-3′ and reverse
5′-CCACTTGAGCTTGTTCACCA-3′; and GAPDH forward
5′-ACCACAGTCCATGCCATCACT-3′ and reverse
5′-GTCCACCACCCTGTTGCTGTA-3′. The specificity of the PCR products
was verified by performing dissociation reaction plots.
Protein extraction and western blot
analysis
Western blotting was performed according to
published methods (27). Blots were
incubated with the primary antibody overnight at 4°C. The primary
antibodies used were as follows: Rabbit polyclonal anti-GAPDH
(1:1,000; G9545; Sigma-Aldrich, St. Louis, MO, USA), rabbit
monoclonal anti-β-catenin (1:5,000; ab32572; Abcam), rabbit
polyclonal anti-sFRP2 (1:5,000; ab86379; Abcam), rabbit monoclonal
anti-cyclin D1 (1:1,000; ab40754; Abcam), rabbit monoclonal
anti-DVL-3 (1:1,000; ab76081; Abcam) and rabbit monoclonal
anti-c-Myc (1:1,000; ab32072; Abcam). Horseradish
peroxidase-conjugated secondary antibody was next added (1:5,000;
SI-B5645; Sigma-Aldrich) for 1 h at room temperature, followed by
enhanced chemiluminescence development (GE Healthcare Life
Sciences, Chalfont, UK). The final data were subjected to grayscale
scanning and semi-quantitative analysis using Quantity One 4.62
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Nuclear proteins were extracted using a Nuclear
Extraction Kit (KA1346; Abnova, Taipei City, Taiwan), according to
the manufacturer's protocol (28,29). The
nuclear protein extract was analyzed by western blotting using
antibodies against β-catenin (1:5,000; ab32572; Abcam). Anti-lamin
(1:3,000; ab16048; Abcam) was used as a nuclear internal control,
while anti-α-tubulin (1:4,000; ab15246; Abcam) was used to rule out
contamination of cytosolic β-catenin.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Statistical analyses were performed using Student's
t-tests or non-parametric Mann-Whitney U tests (for the
results of western blot, RT-qPCR and IHC analyses). One-way
analysis of variance was used to compare the differences among the
three subgroups. Correlation analyses were performed using the
Pearson rank-sum test. P<0.05 was considered to indicate a
statistically significant difference. SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA) was used for statistical
analyses.
Results
sFRP2 is downregulated in aggressive
NFPAs
To evaluate the expression of sFRP2 in NFPAs and
normal pituitary tissues, quantitative measurements of its mRNAs
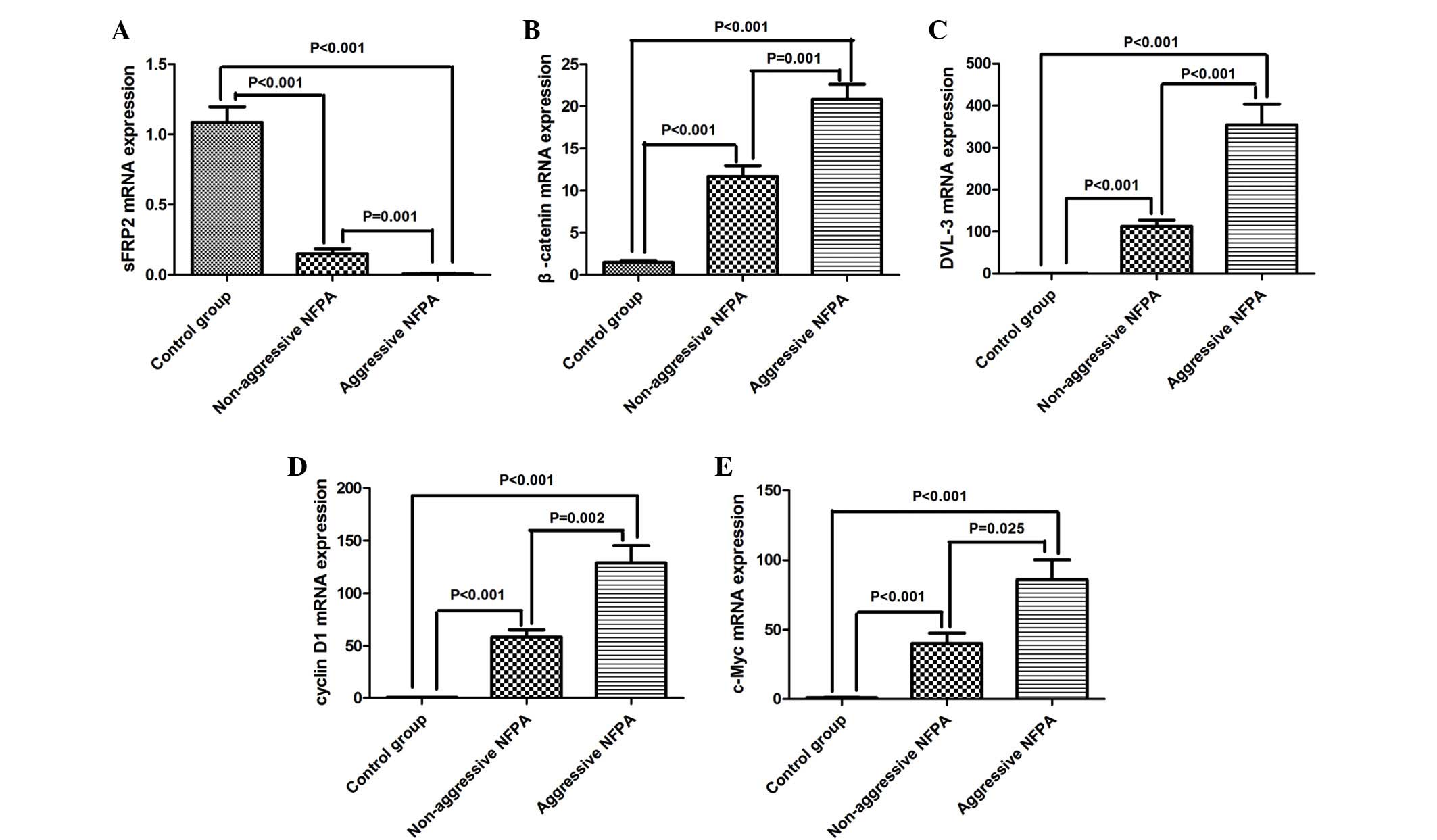
levels by RT-qPCR were performed (Fig.
1). The mRNA levels of sFRP2 were significantly lower in
non-aggressive NFPAs compared with those in normal pituitary
tissues (0.150±0.035 vs. 1.090±0.110; n=30 vs. 10; P<0.001). The
sFRP2 mRNA levels in the aggressive NFPA group was even lower,
compared with those in non-aggressive NFPAs (0.006±0.003 vs.
0.150±0.035; n=20 vs. 30; P=0.001) (Fig.
1A).
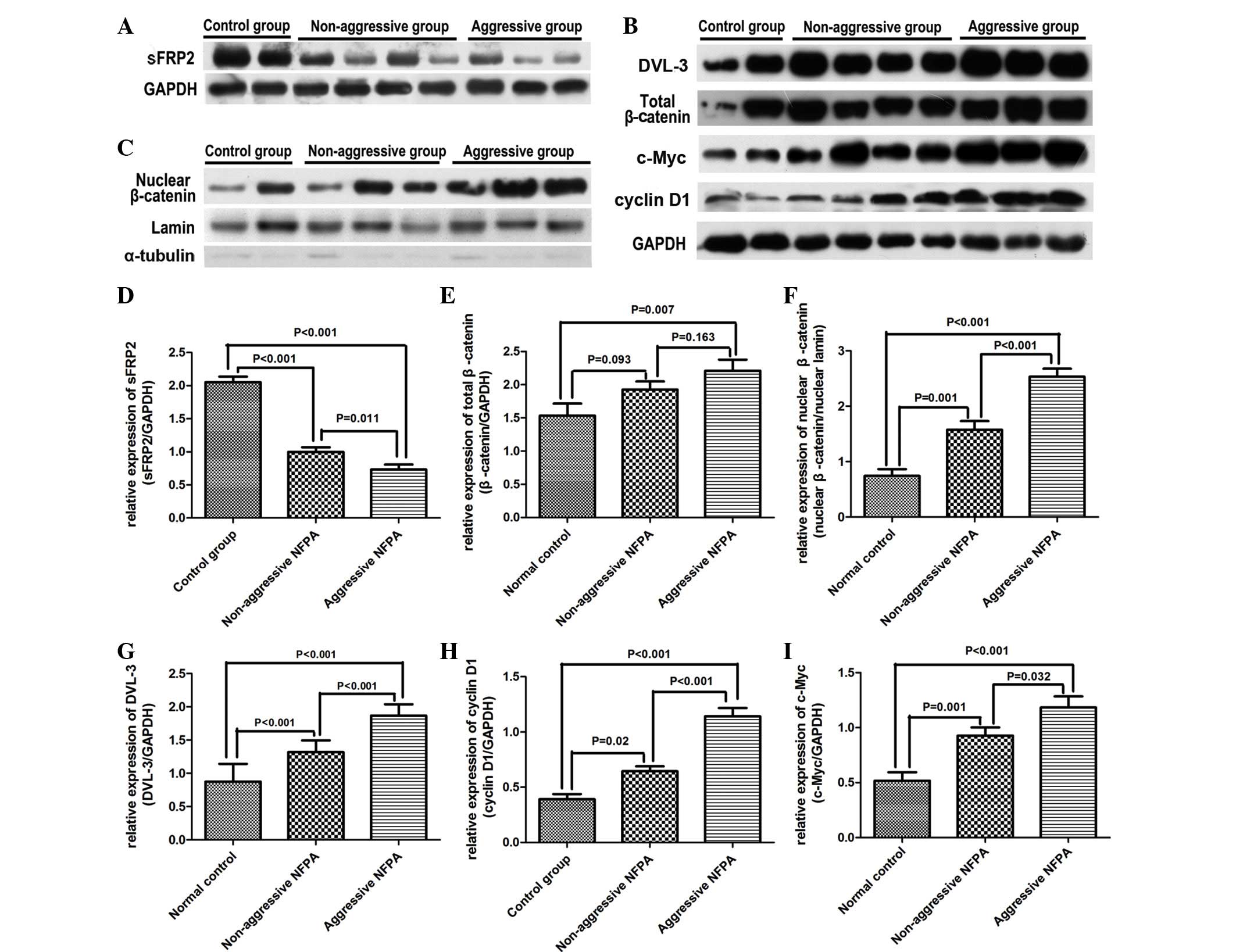
The protein levels of sFRP2 in the three groups were
also evaluated by western blotting (Fig.
2). Aggressive NFPAs exhibited downregulation of sFRP2 protein
(Fig. 2A) compared with normal
pituitary tissues (0.73±0.08 vs. 2.05±0.08; n=15 vs. 10;
P<0.001) and non-aggressive NFPAs (0.73±0.08 vs. 1.00±0.07; n=15
vs. 20; P=0.011). The relative protein expression of sFRP2 in
non-aggressive NFPAs was significantly lower than that in normal
pituitary tissues (1.00±0.07 vs. 2.05±0.08; n=20 vs. 10;
P<0.001), as indicated in Fig.
2D.
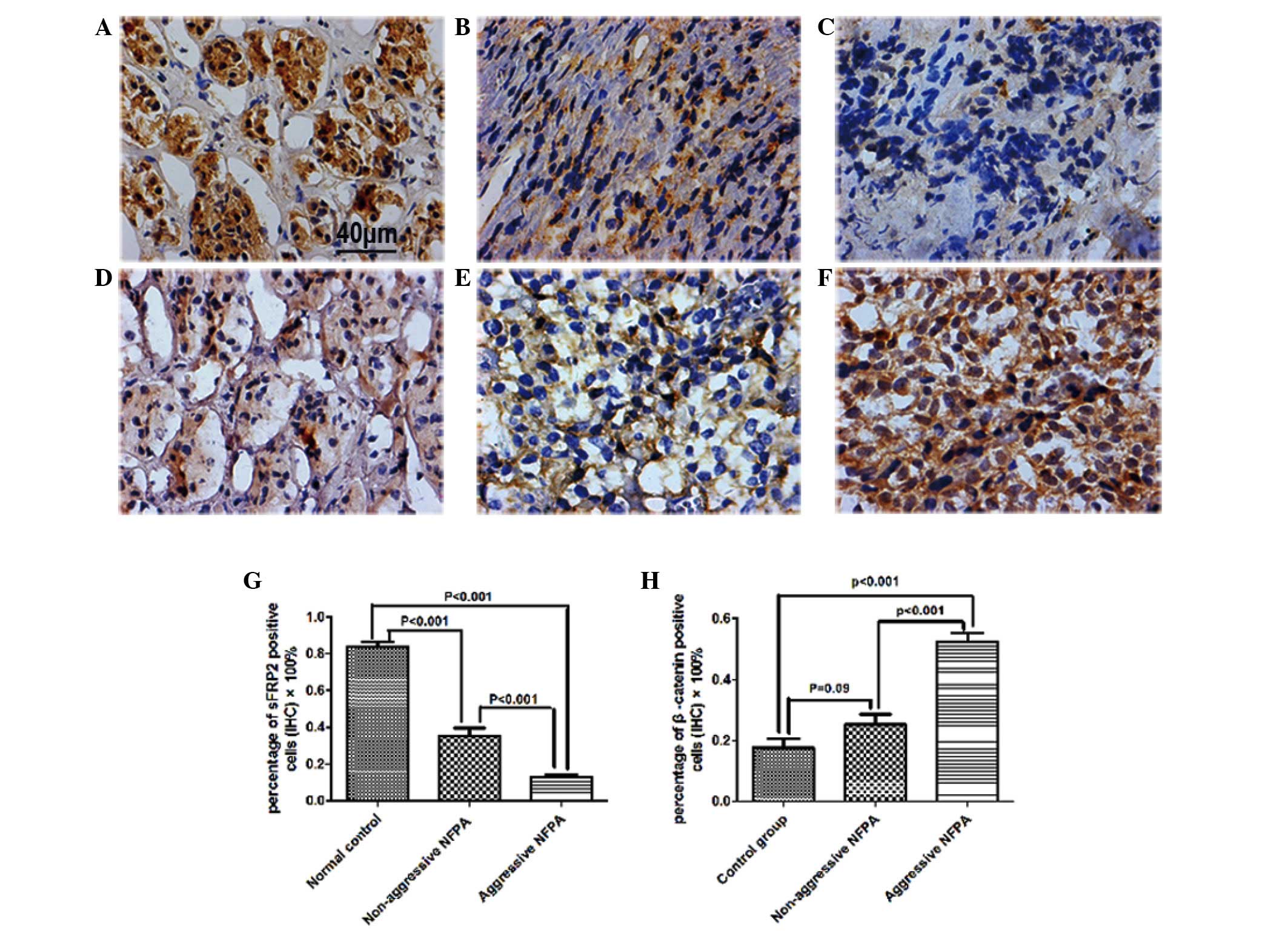
 | Figure 2.Protein expression in normal
pituitary tissues, non-aggressive NFPAs and aggressive NFPAs was
detected by western blotting. Expression of the following proteins
was measured: (A) Secreted frizzled-related protein 2, (B) total
β-catenin, dishevelled segment polarity protein 3, cyclin D1, c-Myc
and (C) nuclear β-catenin. Quantitative analyses of the western
blot results are shown in images D-I. Data are presented as the
mean ± standard error of the mean. sFRP2, secreted frizzled-related
protein 2; mRNA, messenger RNA; DVL-3, dishevelled segment polarity
protein 3; NFPA, nonfunctioning pituitary adenoma; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Downregulation of sFRP2 protein levels in NFPAs was
also confirmed by IHC staining (Fig.
3A–C). The percentage of sFRP2+ cells in non-aggressive NFPAs
was significantly lower than that in normal controls (Fig. 3D–F) (35.5±4.1% vs. 83.7±2.8%; n=10;
P<0.001). The percentage of sFRP2+ cells in aggressive NFPAs was
only 12.5±1.8% (n=10), which was significantly lower than that in
normal pituitary tissues (P<0.001) and non-aggressive NFPAs
(P<0.001) (Fig. 3G).
Total β-catenin is only upregulated in
aggressive NFPAs, but nuclear β-catenin protein levels increase
progressively in aggressive and non-aggressive NFPAs
The accumulation of β-catenin and its nuclear
translocation are two hallmarks of canonical Wnt signaling
(11). The sFRP family is an
antagonist of the Wnt canonical signaling pathway (16). The present RT-qPCR results revealed
that the mRNA levels of β-catenin were significantly upregulated in
aggressive and non-aggressive NFPAs, compared with those in normal
controls (20.82±8.06 vs. 1.49±0.24; n=20 vs. 10; P<0.001; and
11.68±1.30 vs. 1.49±0.24; n=30 vs. 10; P<0.001, respectively)
(Fig. 1B), and the difference between
non-aggressive and aggressive NFPAs was significant (11.68±1.30 vs.
20.82±8.06; n=30 vs. 20; P=0.001) (Fig.
1B).
Total β-catenin protein levels in cell lysates were
measured by western blotting (Fig.
2B). Consistent with the RT-qPCR results, total β-catenin
protein expression in aggressive NFPAs was higher than that in
normal pituitary tissues (2.21±0.17 vs. 1.54±0.18; n=15 vs. 10;
P=0.007). However, no difference in β-catenin protein levels
between non-aggressive NFPAs and normal pituitary tissues
(1.93±0.12 vs. 1.54±0.18; n=20 vs. 10; P=0.093), or between
non-aggressive and aggressive NFPAs (1.93±0.12 vs. 2.21±0.17; n=20
vs. 15; P=0.163), was observed (Fig.
2E).
To investigate the nuclear translocation of
β-catenin, the protein levels of nuclear β-catenin were assessed by
western blotting (Fig. 2C). The
results revealed that the protein levels of nuclear β-catenin were
higher in non-aggressive NFPAs than in normal pituitary tissues
(1.57±0.16 vs. 0.74±0.12; n=20 vs. 10; P=0.001), and the expression
levels were even higher in aggressive NFPAs compared with those in
non-aggressive NFPAs (2.53±0.15 vs. 1.57±0.16; n=15 vs. 20;
P<0.001), as represented in Fig.
2F.
Protein expression of β-catenin was also detected by
IHC. The percentage of β-catenin+ cells in non-aggressive NFPAs was
higher than that in normal control tissues, but this difference was
not significant (25.20±3.34% vs. 17.50±3.00%; n=10; P=0.090). The
percentage of β-catenin+ cells in aggressive NFPAs was 52.20±2.95%
(n=10), which was significantly higher than that in normal
pituitary tissues and non-aggressive NFPAs (P<0.001) (Fig. 3H).
Overexpression of DVL-3, cyclin D1 and
c-Myc in aggressive NFPAs
The expression of components of the Wnt canonical
signaling pathway in NFPAs was next measured by RT-qPCR and western
blotting. RT-qPCR demonstrated that the mRNA levels of DVL-3,
cyclin D1 and c-Myc were significantly increased in non-aggressive
NFPAs compared with those in normal pituitary tissues (112.85±14.79
vs. 1.25±0.29; n=30 vs. 10; P<0.001; 58.61±6.86 vs. 0.58±0.13;
n=30 vs. 10; P<0.001; and 40.25±7.57 vs. 0.94±0.27; n=30 vs. 10;
P<0.001, respectively). The levels were even higher in
aggressive NFPAs compared with those in non-aggressive NFPAs
(354.45±49.09 vs. 112.85±14.79; n=20 vs. 30; P<0.001;
128.90±16.30 vs. 58.61±6.86; n=20 vs. 30; P=0.002; and 85.98±14.30
vs. 40.25±7.57; n=20 vs. 30; P=0.025, respectively) (Fig. 1C–E).
The expression of DVL-3, cyclin D1 and c-Myc was
also evaluated by western blotting (Fig.
2B). The protein expression levels of DVL-3, cyclin D1 and
c-Myc were significantly increased in non-aggressive NFPAs compared
with those in normal NFPAs (1.32±0.04 vs. 0.88±0.08; n=20 vs. 10;
P<0.001; 0.64±0.04 vs. 0.39±0.05; n=20 vs. 10; P=0.020; and
0.93±0.08 vs. 0.48±0.08; n=20 vs. 10; P=0.001, respectively). The
expression levels of DVL-3 and cyclin D1 were even higher in
aggressive NFPAs compared with those in non-aggressive NFPAs
(1.87±0.04 vs. 1.32±0.04; n=15 vs. 20; P<0.001; and 1.14±0.08
vs. 0.64±0.04; n=15 vs. 20; P<0.001, respectively) (Fig. 2G–I). In addition, a significant
difference in c-Myc expression between non-aggressive and
aggressive NFPAs was observed (0.93±0.08 vs. 1.18±0.10; n=20 vs.
15; P=0.032).
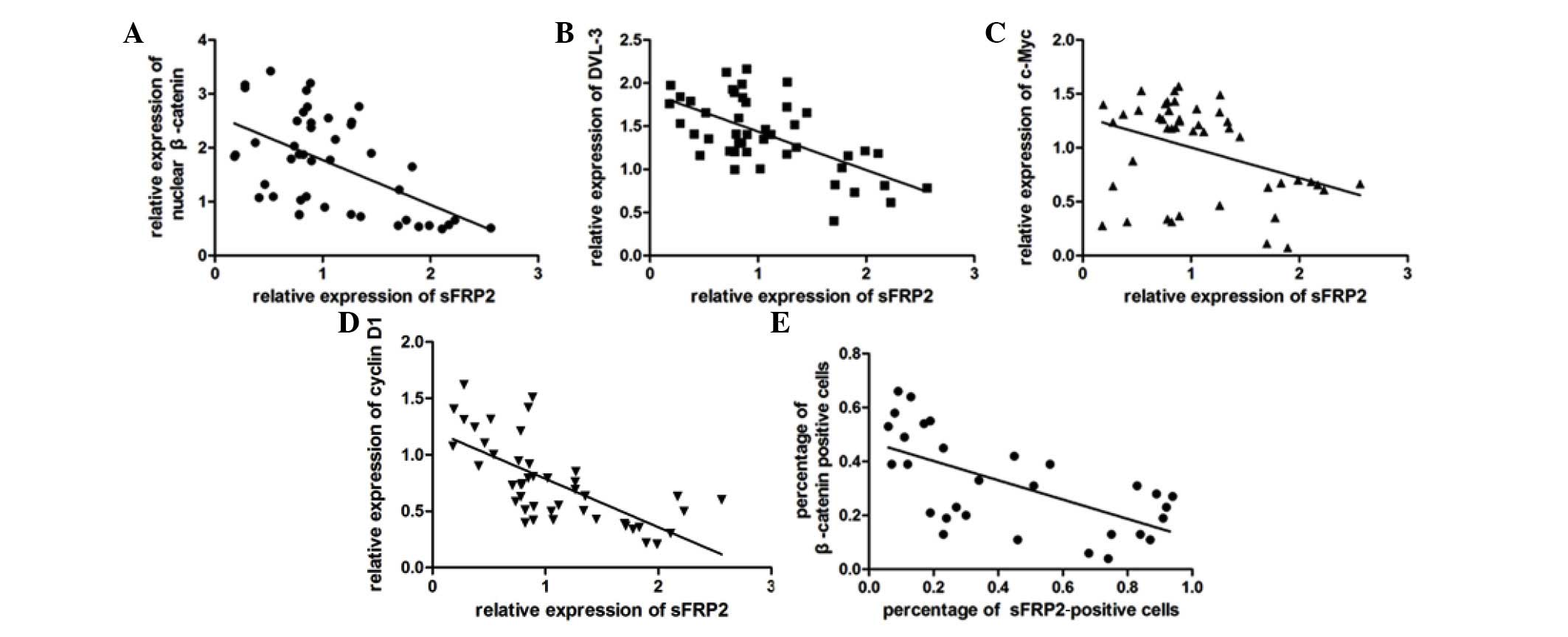
Inverse correlation of sFRP2
expression with expression of components of the Wnt canonical
signaling pathway in normal pituitary tissues and NFPAs
Western blot analysis of nuclear β-catenin and
components of the Wnt canonical signaling pathway protein revealed
that, in contrast to sFRP2, the three groups analyzed exhibited
progressively elevated levels of nuclear β-catenin and components
of the Wnt canonical signaling pathway. Linear regression analysis
demonstrated a significant negative correlation between the
expression of sFRP2 and that of various components of the Wnt
canonical signaling pathway (P<0.001; Fig. 4A–D and Table
I). Similarly, an inverse correlation was also observed between
the percentages of sFRP2+ and β-catenin+ cells by IHC (Pearson's
correlation coefficient r=−0.633; n=45; 2-tailed P<0.001)
(Fig. 4E).
 | Table I.Correlation analysis between sFRP2
expression and nuclear β-catenin, DVL-3, cyclin D1 and c-Myc
expression in normal pituitary tissues and nonfunctioning pituitary
adenomas. |
Table I.
Correlation analysis between sFRP2
expression and nuclear β-catenin, DVL-3, cyclin D1 and c-Myc
expression in normal pituitary tissues and nonfunctioning pituitary
adenomas.
| sFRP2 | Nuclear
β-catenin | DVL-3 | Cyclin D1 | c-Myc |
|---|
| Pearson's
correlation coefficient r | −0.538 | −0.710 | −0.645 | −0.580 |
| P-value
(2-tailed) | <0.001 | <0.001 | <0.001 | <0.001 |
| Number of
samples | 45 | 45 | 45 | 45 |
Discussion
Although NFPA is a type of benign tumor, certain
NFPAs may present aggressive characteristics (5). Aggressive NFPAs are difficult to resect
completely, which often leads to tumor recrudescence (5). It is therefore important to identify the
aggressiveness of NFPAs for selection of the appropriate therapy
and prognostic evaluation. Recently, the Wnt signaling pathway has
been reported to be involved in tumorigenesis in several cell types
(30–34). sFRPs have been identified as possible
antagonists of the Wnt signaling pathway, and sFRP2, a member of
the sFRPs family, has been associated with the degree of tumor
malignancy and invasive ability of various types of human cancer
(20,22).
The evaluation of sFRP2 as a putative marker of
aggressive NFPAs has not been previously reported. In the present
study, the IHC results for sFRP2 revealed the highest cytoplasmic
sFRP2 expression in normal human pituitary tissues, with
strong-to-moderate sFRP2 expression in non-aggressive NFPAs and the
lowest sFRP2 expression in aggressive NFPAs. These results were
consistent with the present western blot and RT-qPCR results.
Since sFRP2 is an inhibitor of the Wnt signaling
pathway, the present study also examined the Wnt canonical
signaling pathway in order to understand the mechanism responsible
for the association between sFRP2 expression and the invasive
ability of NFPAs. Western blot analysis demonstrated that the
expression of downstream Wnt signaling proteins, including DVL-3,
c-Myc and cyclin D1, was significantly higher in non-aggressive
tumor tissues than in normal pituitary tissues, and that the
expression of these proteins in aggressive tumors was even higher
than that in non-aggressive tumors. Similar trends were also
observed at the mRNA level. In addition, the present study
conducted for the first time a comparison between the expression
levels of nuclear β-catenin in normal pituitary tissues,
non-aggressive NFPAs and aggressive NFPAs by western blotting. The
results revealed an abnormal accumulation of free β-catenin in the
nuclei of NFPAs, which is the core step for the activation of the
Wnt canonical signaling pathway (13). The expression of β-catenin was further
confirmed by IHC. The present data support the opinion that the Wnt
canonical signaling pathway is activated in NFPAs, and is
consistent with previous studies (35). However, the present western blotting
results of total β-catenin protein expression indicated that there
was no significant difference between normal control and
non-aggressive NFPA groups, or between non-aggressive and
aggressive NFPA groups. The only significant difference observed
was between normal control and aggressive NFPA groups. Therefore,
the changes in total β-catenin levels may not represent the changes
in nuclear β-catenin levels. This may explain why previous studies
failed to confirm the activation of the Wnt canonical signaling
pathway in pituitary adenomas (36).
Correlation analysis confirmed the existence of an inverse
correlation between sFRP2 expression and activation of the Wnt
canonical signaling pathway, including nuclear accumulation of
β-catenin and overexpression of DVL-3, c-Myc and cyclin D1. These
findings are consistent with those from other studies, which also
reported a similar inverse correlation between the above proteins
in endometrial, ovarian and breast cancer (37,38). The
present findings, together with those from previous studies,
suggest that sFRP2 may act as a tumor suppressor through its
interaction with the Wnt canonical signaling pathway by modulating
the cellular cytosolic pool of β-catenin (22). However, this causality is not
completely clear yet; thus, future in vitro experiments
where sFRP2 is knocked down are required to confirm this
causality.
The results of the present study demonstrated that
there was a progressive loss in the levels of sFRP2 from normal
pituitary tissues to non-aggressive NFPAs and aggressive NFPAs.
This sFRP2 expression pattern in normal pituitary tissues,
non-aggressive and aggressive NFPAs suggests that sFRP2 may be used
as a possible surrogate marker for NFPAs progression and prognosis
prediction. However, further analysis is required, in addition to
follow-up studies, in order to confirm these observations.
In conclusion, the present study has demonstrated
for the first time that sFRP2 expression is inversely correlated
with the aggressiveness of NFPAs. sFRP2, as a tumor suppressor, may
interact with the Wnt canonical signaling pathway by modulating the
cellular cytosolic pool of β-catenin, and the expression of sFRP2
may serve as a biomarker for NFPAs aggressiveness and
prognosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
30971005, 81272522 and 81072075).
Glossary
Abbreviations
Abbreviations:
|
sFRPs
|
secreted frizzled-related proteins
|
|
NFPAs
|
nonfunctioning pituitary adenomas
|
|
PCR
|
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Scheithauer BW, Gaffey TA, Lloyd RV, Sebo
TJ, Kovacs KT, Horvath E, Yapicier O, Young WF Jr, Meyer FB, Kuroki
T, et al: Pathobiology of pituitary adenomas and carcinomas.
Neurosurgery. 59:341–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korbonits M and Carlsen E: Recent clinical
and pathophysiological advances in non-functioning pituitary
adenomas. Horm Res. 71(Suppl 2): 123–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saeger W, Lüdecke DK, Buchfelder M,
Fahlbusch R, Quabbe HJ and Petersenn S: Pathohistological
classification of pituitary tumors: 10 years of experience with the
German Pituitary Tumor Registry. Eur J Endocrinol. 156:203–216.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colao A, Pivonello R, Di Somma C,
Savastano S, Grasso LF and Lombardi G: Medical therapy of pituitary
adenomas: Effects on tumor shrinkage. Rev Endocr Metab Disord.
10:111–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chand-Fouché ME, Colin P and Bondiau PY:
Pituitary adenomas: Multimodal management and modern irradiation
techniques. Cancer Radiother. 16(Suppl): S90–S100. 2012.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grossman AB: The 2004 World Health
Organization classification of pituitary tumors: Is it clinically
helpful? Acta Neuropathol. 111:76–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gürlek A, Karavitaki N, Ansorge O and Wass
JA: What are the markers of aggressiveness in prolactinomas?
Changes in cell biology, extracellular matrix components,
angiogenesis and genetics. Eur J Endocrinol. 156:143–153. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wierinckx A, Auger C, Devauchelle P,
Reynaud A, Chevallier P, Jan M, Perrin G, Fèvre-Montange M, Rey C,
Figarella-Branger D, et al: A diagnostic marker set for invasion,
proliferation, and aggressiveness of prolactin pituitary tumors.
Endocr Relat Cancer. 14:887–900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Shraim M and Asa SL: The 2004 World
Health Organization classification of pituitary tumors: What is
new? Acta Neuropathol. 111:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herr P, Hausmann G and Basler K: WNT
secretion and signalling in human disease. Trends Mol Med.
18:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:pii.a0080522012. View Article : Google Scholar
|
|
16
|
Cruciat CM and Niehrs C: Secreted and
transmembrane wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5:a0150812013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hrzenjak A, Tippl M, Kremser ML,
Strohmeier B, Guelly C, Neumeister D, Lax S, Moinfar F, Tabrizi AD,
Isadi-Moud N, et al: Inverse correlation of secreted
frizzled-related protein 4 and beta-catenin expression in
endometrial stromal sarcomas. J Pathol. 204:19–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Hurley G, Perry AS, O'Grady A, Loftus B,
Smyth P, O'Leary JJ, Sheils O, Fitzpatrick JM, Hewitt SM, Lawler M
and Kay EW: The role of secreted frizzled-related protein 2
expression in prostate cancer. Histopathology. 59:1240–1248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saran U, Arfuso F, Zeps N and Dharmarajan
A: Secreted frizzled-related protein 4 expression is positively
associated with responsiveness to cisplatin of ovarian cancer cell
lines in vitro and with lower tumour grade in mucinous ovarian
cancers. BMC Cell Biol. 13:252012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marsit CJ, Karagas MR, Andrew A, Liu M,
Danaee H, Schned AR, Nelson HH and Kelsey KT: Epigenetic
inactivation of SFRP genes and TP53 alteration act jointly as
markers of invasive bladder cancer. Cancer Res. 65:7081–7085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi J, Zhu YQ, Luo J and Tao WH:
Hypermethylation and expression regulation of secreted
frizzled-related protein genes in colorectal tumor. World J
Gastroenterol. 12:7113–7117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2
suppress the transformation and invasion abilities of cervical
cancer cells through Wnt signal pathway. Gynecol Oncol.
112:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vézina JL, Hardy J and Yamashita M:
Microadenomas and hypersecreting pituitary adenomas. Arq
Neuropsiquiatr. 33:119–127. 1975.(In Portuguese). PubMed/NCBI
|
|
24
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–618. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zlobec I, Terracciano L, Jass JR and Lugli
A: Value of staining intensity in the interpretation of
immunohistochemistry for tumor markers in colorectal cancer.
Virchows Arch. 451:763–769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao P, Wang H, Gao H, Li C and Zhang Y:
Reversal of multidrug resistance by magnetic chitosan-Fe3O4
nanoparticle-encapsulated MDR1 siRNA in glioblastoma cell line.
Neurol Res. 35:821–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu R, Zhong J, Li M, Guo X, Zhang H and
Chen J: PACAP induces the dimerization of PAC1 on the nucleus
associated with the cAMP increase in the nucleus. Neurosci Lett.
549:92–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuster DW, Merkus D, Jorna HJ, Dekkers DH,
Duncker DJ and Verhoeven AJ: Nuclear protein extraction from frozen
porcine myocardium. J Physiol Biochem. 67:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sano M, Driscoll DR, De Jesus-Monge WE,
Klimstra DS and Lewis BC: Activated wnt signaling in stroma
contributes to development of pancreatic mucinous cystic neoplasms.
Gastroenterology. 146:257–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Morris JP IV, Yan W, Schofield
HK, Gurney A, Simeone DM, Millar SE, Hoey T, Hebrok M and di Pasca
Magliano M: Canonical wnt signaling is required for pancreatic
carcinogenesis. Cancer Res. 73:4909–4922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chambers TJ, Giles A, Brabant G and Davis
JR: Wnt signalling in pituitary development and tumorigenesis.
Endocr Relat Cancer. 20:R101–R111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Shen K, Zhao Y, He X, Ma C, Wang L,
Wang B, Liu J and Ma J: MicroRNA-222 promotes tumorigenesis via
targeting DKK2 and activating the Wnt/β-catenin signaling pathway.
FEBS Lett. 587:1742–1748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dellinger TH, Planutis K, Tewari KS and
Holcombe RF: Role of canonical Wnt signaling in endometrial
carcinogenesis. Expert Rev Anticancer Ther. 12:51–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Howng SL, Wu CH, Cheng TS, Sy WD, Lin PC,
Wang C and Hong YR: Differential expression of Wnt genes,
beta-catenin and E-cadherin in human brain tumors. Cancer Lett.
183:95–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elston MS, Gill AJ, Conaglen JV, Clarkson
A, Shaw JM, Law AJ, Cook RJ, Little NS, Clifton-Bligh RJ, Robinson
BG and McDonald KL: Wnt pathway inhibitors are strongly
down-regulated in pituitary tumors. Endocrinology. 149:1235–1242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng YY, Yu J, Wong YP, Man EP, To KF,
Jin VX, Li J, Tao Q, Sung JJ, Chan FK and Leung WK: Frequent
epigenetic inactivation of secreted frizzled-related protein 2
(SFRP2) by promoter methylation in human gastric cancer. Br J
Cancer. 97:895–901. 2007.PubMed/NCBI
|
|
38
|
Becker G, Kocher M, Kortmann RD, Paulsen
F, Jeremic B, Müller RP and Bamberg M: Radiation therapy in the
multimodal treatment approach of pituitary adenoma. Strahlenther
Onkol. 178:173–186. 2002. View Article : Google Scholar : PubMed/NCBI
|