Introduction
Pancreatic cancer accounts for ~5% of
cancer-associated mortalities, and ranks the eighth in terms of
cancer incidence worldwide (1). Due
to the difficulty in early diagnosis of pancreatic cancer, the
majority of patients present an advanced stage of the disease when
the first symptoms appear (2). The
standard treatment for advanced pancreatic cancer is chemotherapy
(3). However, the median survival of
patients treated with gemcitabine is not satisfactory (4). A number of studies have compared the
efficacy of gemcitabine alone with gemcitabine-based combinations,
including 5-fluorouracil, capecitabine, cisplatin, docetaxel,
irinotecan, oxaliplatin and pemetrexed, for the treatment of
pancreatic cancer, but no clear survival benefit has been
demonstrated thus far (5). Therefore,
current research is focused on the development of novel treatments
for inoperable pancreatic cancer (6,7).
Arsenite is a natural substance with a reported
medicinal use for >2,400 years (8). However, its use in recent years has been
limited due to the toxicity and potential carcinogenicity of
chronic arsenic administration (9).
Arsenic therapy gained popularity during the 1970s, when Chinese
physicians started to use arsenic trioxide as part of the treatment
for acute promyelocytic leukemia (APL) (8). The results of those studies indicated
that a stable solution of arsenic trioxide administered by
intravenous infusion was remarkably safe and effective in patients
with newly diagnosed, refractory or relapsed APL (8). The molecular mechanism of action of
arsenic derivatives against APL involves induction of cell
apoptosis, inhibition of cell proliferation and inhibition of
angiogenesis (8), although the exact
mechanistic details remain to be fully understood.
In patients with advanced pancreatic cancer, cell
invasion into adjacent tissues is a major prognostic factor
(10). Abnormal cell migration leads
to pathological states such as invasion and metastasis of cancer
(10). It has been reported that
actin stress fibers generate contractile forces by pulling against
focal adhesions in order to induce retraction of the rear cell
membrane, which suggests that stress fibers may be important for
cell migration (11). Cytoskeletal
proteins such as vinculin and actinin, and several non-receptor
protein tyrosine kinases, including focal adhesion kinase and
members of the Src family, are involved in the organization of
focal adhesion complexes (12,13).
Platelet-derived growth factors (PDGFs) are known to participate in
the pathogenesis, invasion and distant metastasis of human solid
tumors, and their expression levels are correlated with poor
prognosis (14,15).
In the present study, the effect of arsenite on
pancreatic cancer cell migration, proliferation and apoptosis was
investigated. The results demonstrated that arsenite strongly
suppressed PDGF-BB-induced cell migration by suppressing the Akt
signaling pathway in AsPC-1 cells.
Materials and methods
Materials
Recombinant human PDGF-BB (catalog no. 220-BB) was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The
phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002, and the
Akt and glycogen synthase kinase-3 beta (GSK3β) inhibitors were
obtained from Merck & Co., Inc. (Kenilworth, NJ, USA). Goat
polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
catalog no. sc-48166) antibody was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit polyclonal
anti-proteolytically cleaved poly(adenosine diphosphate-ribose)
polymerase (PARP; catalog no. 9542), rabbit monoclonal anti-cyclin
D1 (catalog no. 2978), rabbit polyclonal anti-phosphorylated
(phospho)-retinoblastoma protein (Rb) (catalog no. 9301), mouse
monoclonal anti-phospho-p44/p42 mitogen-activated protein kinase
(MAPK) (catalog no. 9106), rabbit monoclonal anti-p44/p42 MAPK
(catalog no. 4695), rabbit monoclonal anti-phospho-Akt (catalog no.
5012) and rabbit polyclonal Akt (catalog no. 9272) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Amersham ECL Western Blotting Detection Reagent was used for
western blotting visualization and was purchased from GE Healthcare
Life Sciences (Chalfont, UK). Arsenite and Tween 20 were purchased
from Sigma-Aldrich (St. Louis, MO, USA)
Cell culture
AsPC-1 and BxPC-3 pancreatic cancer cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and were grown in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal calf serum (FCS), penicillin (100 U/ml) and
streptomycin (100 µg/ml) (Invitrogen; Thermo Fisher Scientific,
Inc.) in a humidified incubator at 37°C with 5% CO2.
Primary normal human pancreatic epithelial (PE) cells were
purchased from DS Pharma Biomedical Co., Ltd. (Osaka, Japan) and
maintained in CSC medium (Cell Systems Corporation, Kirkland, WA,
USA). Unless otherwise indicated, cells were incubated in
serum-free medium for 24 h prior to be used in the corresponding
experiments, as previously described (16).
Cell migration assay
Cell migration was assessed using a Boyden
Transwell® chamber of 8 µm pores for AsPC-1 and BxPC-3
cells, and 3 µm pores for PE cells (Costar; Corning Incorporated,
Corning, NY, USA). Cells were exposed to arsenite (10–30 µM) or the
aforementioned inhibitors and incubated for 24 h. Next, cells
(5×104 cells/well) were seeded onto the upper chamber in
RPMI medium containing 10% FCS. Following 16-h incubation at 37°C,
cells were treated with PDGF-BB at the indicated concentrations for
36 h. Cells were then fixed and stained with 1 ml clonogenic
reagent (50% ethanol and 0.25% 1, 9-dimethyl-methylene blue;
Sigma-Aldrich) for 30 min. Subsequently, the cells on the upper
surface of the membrane were mechanically removed, while the cells
that had migrated to the lower surface of the membrane were
observed under a microscope (BZ-9000 BioRevo; Keyence Corporation,
Osaka, Japan). The average number of migrated cells from five
randomly selected fields on the lower surface of the membrane was
counted. Data were obtained from ≥3 independent experiments.
Western blot analysis
Western blot analyses were performed as described
previously (17) using the
Mini-PROTEAN® Electrophoresis system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). In brief, the cells were
lysed in lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA,
1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 50 mM NaF,
50 mM HEPES, 1 mM Na3VO4 and 2 mM PMSF] and
scraped from the Petri dishes. The protein lysates (5 µg) were
fractionated on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto an Immun-Blot®
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
Membranes were blocked with 5% skimmed dry milk in
phosphate-buffered saline containing 0.1% Tween 20 for 30 min,
prior to be incubated at 25°C overnight with the indicated primary
antibodies, which were diluted to 1:2,000. Donkey anti-goat
(catalog no. sc-2020), goat anti-mouse (catalog no. sc-2005) and
goat anti-rabbit (catalog no. sc-2004) IgG horseradish
peroxidase-labeled antibodies (Santa Cruz Biotechnology, Inc.) were
used as secondary antibodies and were incubated at 25°C for 1 h at
a dilution of 1:5,000. The peroxidase activity on the membrane was
visualized on X-ray films (Fujifilm Co., Tokyo, Japan) by ECL
detection.
Densitometry analysis
Densitometry analysis was performed using a scanner
and a software package for image analysis (ImageJ version 1.32;
National Institutes of Health, Bethesda, MD, USA). The respective
bands were manually selected, and the intensities were measured
using the ‘measure’ function, following automatic quantification.
The background-subtracted signal intensity of each protein band was
normalized to the respective controls. Data were obtained from ≥3
independent experiments.
Statistical analysis
Data were analyzed by analysis of variance, followed
by the Bonferroni method between the indicated pairs for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
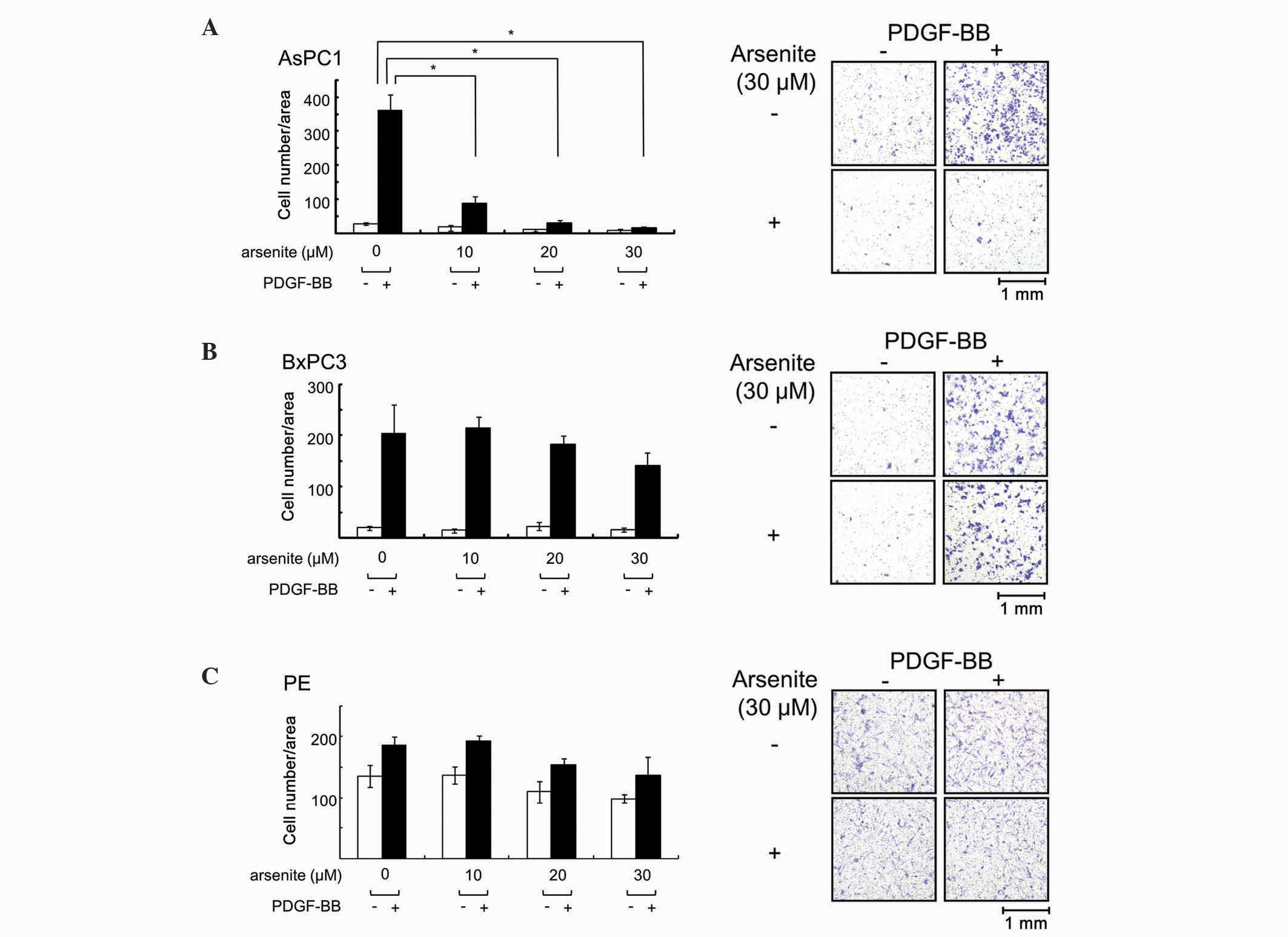
Arsenite suppressed PDGF-BB-induced
migration in AsPC-1 cells, but not in BxPC-3 or PE cells
PDGF and its receptor are known to participate in
pathogenesis, invasion and distant metastasis of human solid
tumors, and their expression levels are correlated with poor
prognosis (14,15). Therefore, the effect of arsenite on
PDGF-BB-induced migration in AsPC-1, BxPC-3 and PE cells was
examined in the present study. When cells were pretreated with
increasing doses of arsenite for 24 h and then exposed to PDGF-BB
for 36 h, the number of migrated cells was clearly reduced in
AsPC-1 cells (Fig. 1A). However,
arsenite had little effect on PDGF-BB-induced cell migration in
BxPC-3 cells (Fig. 1B) and PE cells
(Fig. 1C). These results indicate
that arsenite exerts suppressive effects on cell migration in a
particular type of pancreatic cancer cells.
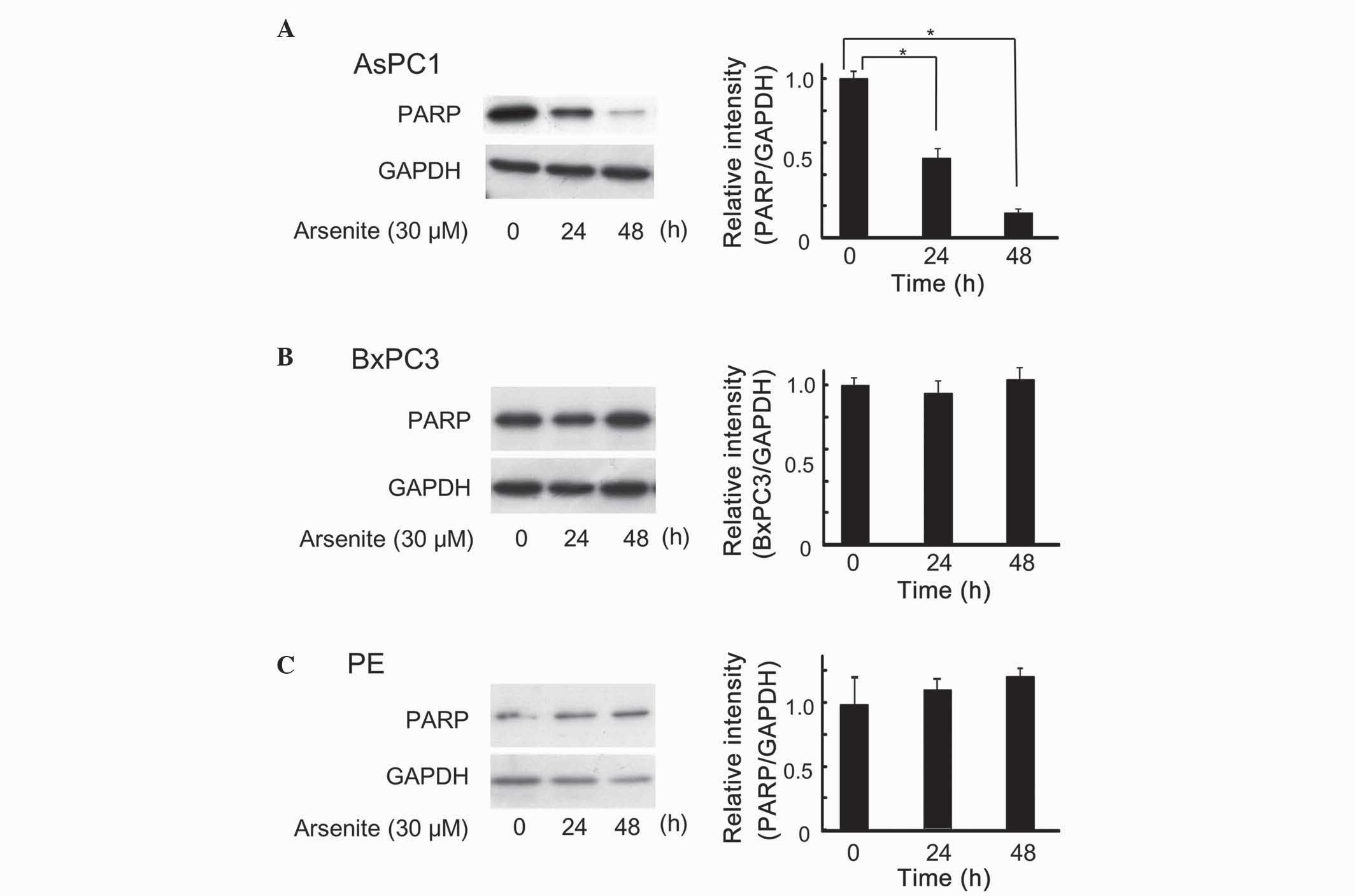
Arsenite induced apoptosis in AsPC-1
cells, but not in BxPC-3 or PE cells
Accumulating evidence indicates that arsenic
compounds induce apoptosis in APL, multiple myeloma and human
hepatoma cells (18–20). Therefore, the effect of arsenite on
apoptosis in human pancreatic cells was next examined. PARP enables
cells to maintain their viability, and cleavage of PARP results in
apoptosis (21); thus, PARP cleavage
is usually observed in cells undergoing apoptosis. As represented
in Fig. 2, arsenite decreased the
levels of full-length PARP in AsPC-1 cells, but not in BxPC-3 or PE
cells, similarly to the results shown in Fig. 1.
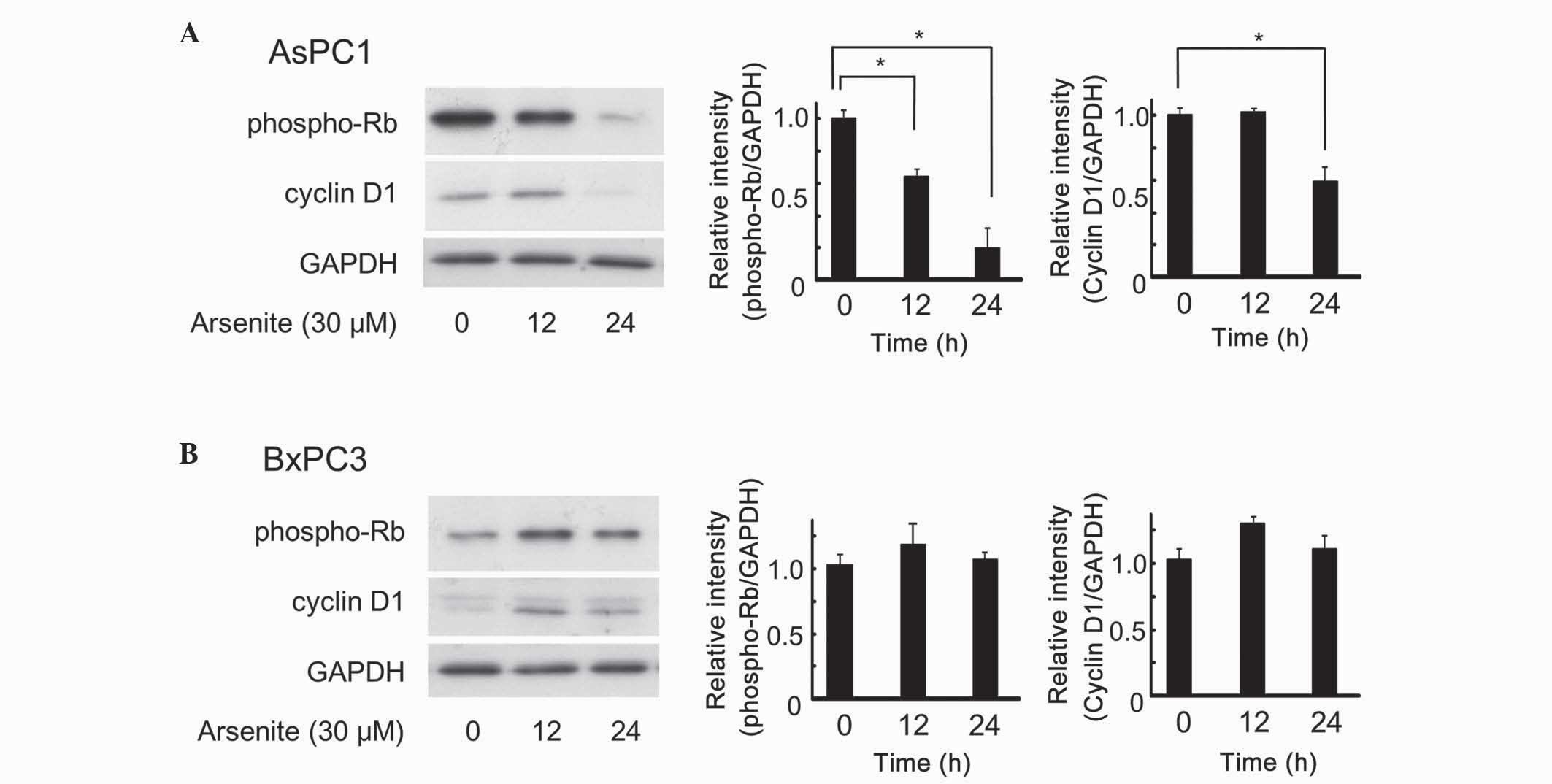
Arsenite inhibited cell proliferation
of AsPC-1 cells, but not of BxPC-3 cells
The effect of arsenite on cell proliferation was
next investigated. The ternary complex of cyclin D1,
cyclin-dependent kinase (CDK)4 and p27Kip1 requires extracellular
mitogenic stimuli for the release and degradation of p27Kip1 and
concomitant increase in the levels of cyclin D1, which affects the
progression and phospho-Rb-dependent entry into the S phase of the
cell cycle (22). Thus, increased
levels of cyclin D1 and phospho-Rb promote cell cycle transition,
resulting in cell proliferation. As depicted in Fig. 3, arsenite clearly decreased the levels
of phospho-Rb and cyclin D1 in AsPC-1 cells (Fig. 3A). However, arsenite did not decrease,
but transiently increased instead, the levels of these proteins in
BxPC-3 cells (Fig. 3B). Overall,
these findings strongly suggest that arsenite exhibits anticancer
effects in certain types of pancreatic cancer cells. PE cells were
not investigated, since the expression level of cyclin D1 and Rb
were too low to be detected using western blot analysis (data not
shown).
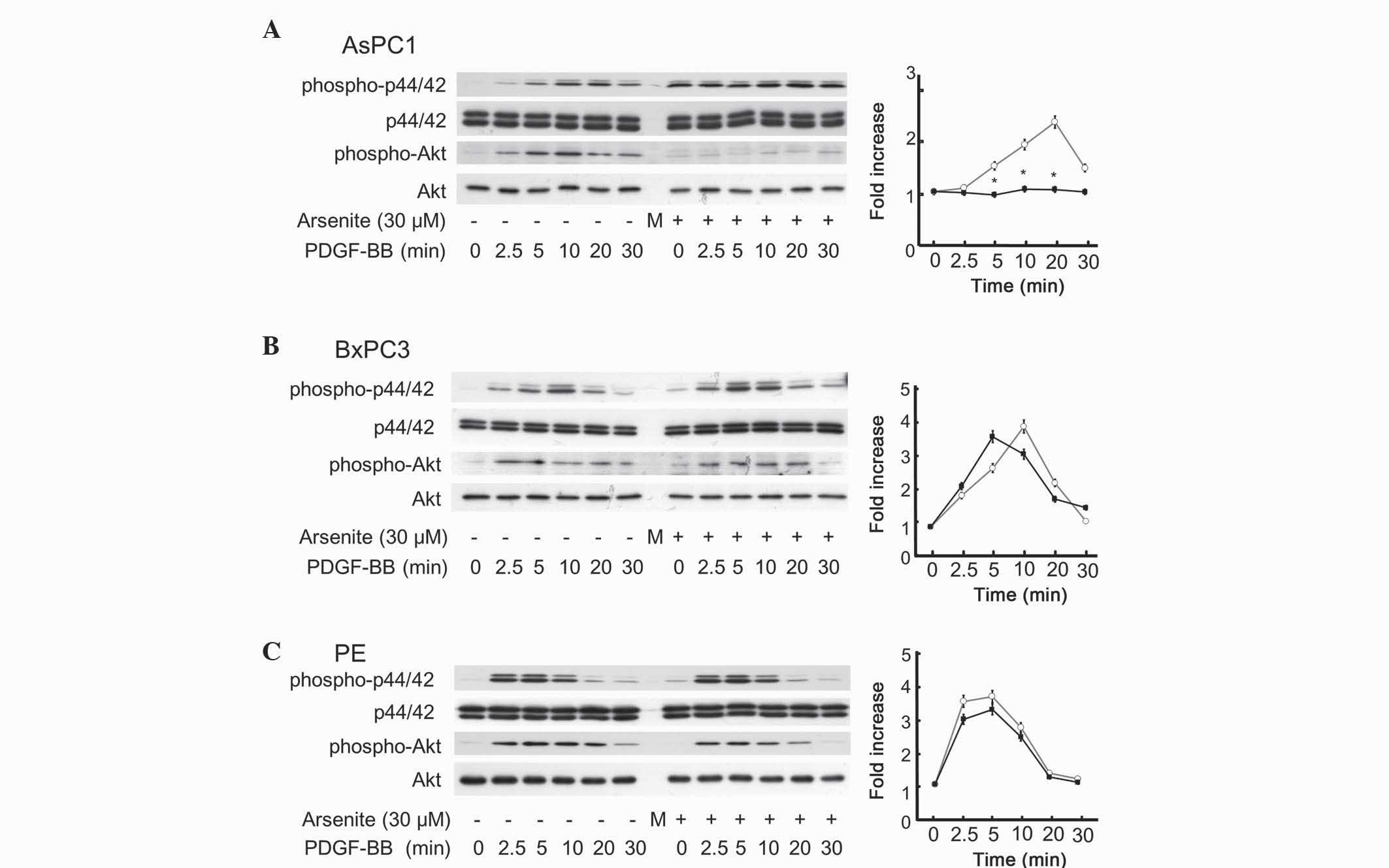
Arsenite suppressed PDGF-BB-induced
Akt activation in AsPC-1 cells, but not in BxPC-3 or PE cells
The survival ability of cancer cells is largely
dependent on growth factors such as epidermal growth factor
(23) and PDGF-BB (14,15).
Through binding to their corresponding cell surface receptor, the
above growth factors activate an extensive network of signal
transduction pathways that include activation of the Ras/p44/p42
MAPK and PI3K/Akt pathways (23). In
order to elucidate the mechanism underlying the suppressive effect
of arsenite on the migration of AsPC-1 cells, but not on that of
BxPC-3 or PE cells, western blotting was performed to examine the
effect of arsenite on PDGF-BB-induced phosphorylation of p44/p42
MAPK and Akt. Arsenite had negligible effect on p44/p42 MAPK in
AsPC-1, BxPC-3 and PE cells (Fig. 4).
By contrast, arsenite significantly inhibited PDGF-BB-induced Akt
phosphorylation in AsPC-1 cells (Fig.
4A), but did not influence PDGF-BB-induced Akt phosphorylation
in BxPC-3 or PE cells (Fig. 4B and C,
respectively). These results indicate a similar trend to that
observed for cell migration, apoptosis and proliferation (Figs. 1–3).
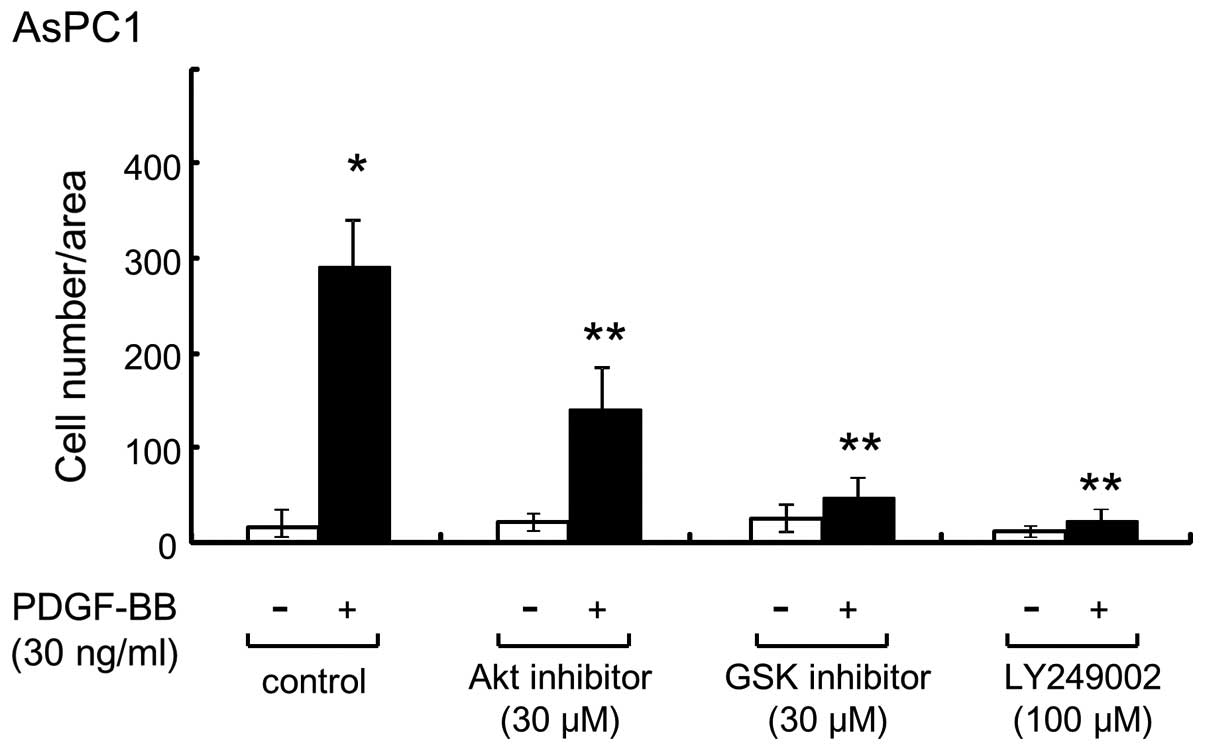
PI3K, Akt and GSK3β inhibitors
suppressed PDGF-BB-induced migration in AsPC-1 cells
Akt regulates multiple biological processes,
including cell survival, proliferation and growth (24). In addition, GSK3β is a critical
downstream element of the PI3K/Akt signaling pathway, and its
activity can be inhibited by Akt-mediated phosphorylation of GSK3β
(25,26). Since arsenite significantly suppressed
PDGF-BB-induced Akt phosphorylation in AsPC-1 cells (Fig. 4A), several inhibitors of the Akt
pathway were used to confirm that arsenite suppressed the migration
of AsPC-1 cells by inhibiting the components of this pathway. When
cells were pretreated with LY294002 (a PI3K inhibitor) or with Akt
or GSK3β inhibitors, and then exposed to PDGF-BB, PDGF-BB-induced
migration of AsPC-1 cells was significantly inhibited (Fig. 5), thus suggesting that arsenite
suppressed PDGF-BB-induced migration of AsPC-1 cells by inhibiting
the Akt signaling pathway.
Discussion
The present study demonstrated that PDGF-BB, which
is important in invasion and metastasis of various types of human
cancer (14,15), induced cell migration in AsPC-1 and
BxPC-3 pancreatic cancer cells as well as normal PE cells. Notably,
pretreatment with arsenite significantly inhibited PDGF-BB-induced
migration of AsPC-1 cells but not of BxPC-3 or PE cells. In AsPC-1
cells, exposure to arsenite induced PARP cleavage and decreased the
levels of cyclin D1 and phosphorylated Rb, indicating that arsenite
induces apoptosis and cell cycle arrest in AsPC-1 cells. Since
PDGF-BB induced phosphorylation of p44/p42 MAPK and Akt in these
cells, the involvement of these kinases in PDGF-BB-induced cell
migration was examined. The results indicated that arsenite
suppressed PDGF-BB-induced phosphorylation of Akt, but not of
p44/p42 MAPK in AsPC-1 cells. In addition, it was confirmed that
the inhibition of the Akt signaling pathway suppressed the
migration induced by PDGF-BB in these cells. Taken together, the
present results strongly suggest that arsenite inhibits
PDGF-BB-induced cell migration by suppressing the Akt signaling
pathway in certain types of pancreatic cancer cells.
The present authors previously reported that Rho
kinase, which is a downstream kinase of Rho, negatively regulates
the migration of colon cancer cells (27). In that study, a Rho kinase inhibitor
induced colon cancer cell migration by disrupting focal adhesion
formation via the Akt pathway. In the present study, it was
demonstrated that the PDGF-BB-induced cell migration mediated by
Akt activation was inhibited by arsenite. Therefore, the effect of
arsenite on focal adhesion-associated molecules should be
investigated in future studies. Although further studies are
required on the detailed mechanism of arsenite-induced suppression
of the Akt signaling pathway, arsenite may be considered an
attractive tool for the treatment of patients with certain types of
pancreatic cancer without exerting adverse effects on normal
pancreatic cells.
Acknowledgements
The present study was partly supported by a
Grant-in-Aid for Scientific Research (grant no. 24590939) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (Tokyo, Japan).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chao Y, Wu CY, Wang JP, Lee RC, Lee WP and
Li CP: A randomized controlled trial of gemcitabine plus cisplatin
versus gemcitabine alone in the treatment of metastatic pancreatic
cancer. Cancer Chemother. Pharmacol. 72:637–642. 2013.
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borad MJ, Reddy SG, Bahary N, Uronis HE,
Sigal D, Cohn AL, Schelman WR, Stephenson J Jr, Chiorean EG, Rosen
PJ, et al: Randomized phase II trial of gemcitabine plus TH-302
versus gemcitabine in patients with advanced pancreatic cancer. J
Clin Oncol. 33:1475–1481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CT, Chen YC, Yamaguchi H and Hung MC:
Carglumic acid promotes apoptosis and suppresses cancer cell
proliferation in vitro and in vivo. Am J Cancer Res. 5:3560–3569.
2015.PubMed/NCBI
|
|
8
|
Waxman S and Anderson KC: History of the
development of arsenic derivatives in cancer therapy. Oncologist.
6(Suppl 2): 3–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dangleben NL, Skibola CF and Smith MT:
Arsenic immunotoxicity: A review. Environ. Health. 12:732013.
|
|
10
|
Hynes RO and Lander AD: Contact and
adhesive specificities in the associations, migrations, and
targeting of cells and axons. Cell. 68:303–322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burridge K: Are stress fibres contractile?
Nature. 294:691–692. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Humphries JD, Wang P, Streuli C, Geiger B,
Humphries MJ and Ballestrem C: Vinculin controls focal adhesion
formation by direct interactions with talin and actin. J Cell Biol.
179:1043–1057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burridge K and Chrzanowska-Wodnicka M:
Focal adhesions, contractility, and signaling. Annu Rev Cell Dev
Biol. 12:463–518. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henriksen R, Funa K, Wilander E, Bäckström
T, Ridderheim M and Oberg K: Expression and prognostic significance
of platelet-derived growth factor and its receptors in epithelial
ovarian neoplasms. Cancer Res. 53:4550–4554. 1993.PubMed/NCBI
|
|
15
|
Uren A, Merchant MS, Sun CJ, Vitolo MI,
Sun Y, Tsokos M, Illei PB, Ladanyi M, Passaniti A, Mackall C and
Toretsky JA: Beta-platelet-derived growth factor receptor mediates
motility and growth of Ewing's sarcoma cells. Oncogene.
22:2334–2342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamauchi T, Adachi S, Yasuda I, Nakashima
M, Kawaguchi J, Nishii Y, Yoshioka T, Okano Y, Hirose Y, Kozawa O
and Moriwaki H: UVC radiation induces downregulation of EGF
receptor via phosphorylation at serine 1,046/1,047 in human
pancreatic cancer cells. Radiat Res. 176:565–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adachi S, Nagao T, Ingolfsson HI, Maxfield
FR, Andersen OS, Kopelovich L and Weinstein IB: The inhibitory
effect of (−)-epigallocatechin gallate on activation of the
epidermal growth factor receptor is associated with altered lipid
order in HT29 colon cancer cells. Cancer Res. 67:6493–6501. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glienke W, Chow KU, Bauer N and Bergmann
L: Down-regulation of wt1 expression in leukemia cell lines as part
of apoptotic effect in arsenic treatment using two compounds. Leuk
Lymphoma. 47:1629–1638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicolis I, Curis E, Deschamps P and
Bénazeth S: Arsenite medicinal use, metabolism, pharmacokinetics
and monitoring in human hair. Biochimie. 91:1260–1267. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao M, Dong W, Hu M, Yu M, Guo L, Qian L,
Guo N and Song L: GADD45alpha mediates arsenite-induced cell
apoptotic effect in human hepatoma cells via JNKs/AP-1-dependent
pathway. J Cell Biochem. 109:1264–1273. 2010.PubMed/NCBI
|
|
21
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of
poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson
from an uncleavable mutant. J Biol Chem. 273:33533–33539. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: Molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sutherland C, Leighton IA and Cohen P:
Inactivation of glycogen synthase kinase-3 beta by phosphorylation:
New kinase connections in insulin and growth-factor signalling.
Biochem J. 296:15–19. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima M, Adachi S, Yasuda I, Yamauchi
T, Kozawa O and Moriwaki H: Rho-kinase regulates negatively the
epidermal growth factor-stimulated colon cancer cell proliferation.
Int J Oncol. 36:585–592. 2010.PubMed/NCBI
|